Abstract
Objectives
Neural networks supporting encoding of new information are affected early in the course of Alzheimer disease (AD). Functional magnetic resonance imaging (fMRI) studies in AD have reported decreased medial temporal lobe (MTL) activation when comparing novel versus repeated stimuli. It is, however, unclear whether this finding is related to a failure of normal suppression of MTL activity to repeated stimuli in AD.
Design, Setting, Participants and Measurements
Twenty-nine healthy older subjects comprising a comparison group (OC) and 15 mild AD patients underwent fMRI during an associative memory paradigm in an academic medical center. The task consisted of blocks of Novel and Repeated face-name pairs and visual Fixation. To reveal neural correlates of processing repeatedly presented stimuli, Repeated blocks were contrasted to Fixation.
Results
AD patients demonstrated greater activation during Repeated stimuli in the MTL and in prefrontal and superior parietal cortices, compared with OC. In contrast, OC showed greater parietal task-induced deactivation than AD. Increased MTL activity during Repeated was correlated with more impaired parietal deactivation and poorer performance of the postscan recognition memory test of encoding the face-name pairs.
Conclusion
Reduction of MTL activity to repeated stimuli, which become highly familiarized to healthy OC, was impaired in AD. This abnormal increased MTL activation was related to disrupted parietal deactivation and to poor recognition memory performance. These preliminary results suggest that the typical episodic memory impairment seen in mild AD may manifest as a failure of normal repetition suppression and loss of “beneficial” deactivation in the MTL-parietal memory networks.
Keywords: Alzheimer disease, fMRI, medial temporal lobe, memory, parietal cortex, repetition suppression
Neural networks supporting encoding of novel information1,2 are known to be affected by neuropathological changes early in the course of Alzheimer disease (AD).3-5 Accordingly, functional magnetic resonance imaging (fMRI) studies in clinical AD patients have found decreased medial temporal lobe (MTL) activation when contrasting fMRI responses to novel versus repeated stimuli, relative to cognitively intact older comparison subjects (OC).6-9 The decreased MTL activation in AD has been interpreted as primarily reflecting disruption of normal MTL function during encoding of novel stimuli. Interestingly, recent fMRI studies in OC and in Mild Cognitive Impairment thought to be a prodromal phase of AD, suggest another plausible explanation for the findings of decreased MTL activity in AD during encoding of novel relative to repeated stimuli. Cognitively normal OC demonstrated rapid reduction of MTL activity to repeatedly presented stimuli, indicating preserved neural repetition suppression,10-12 whereas subjects with Mild Cognitive Impairment showed a diminished fMRI response reduction to stimulus repetition.13 It remains unknown whether in addition to the decreased fMRI activation during novel encoding, the characteristic memory failure in early AD is also manifested as continued MTL activity to repeated stimuli—i.e., failure of repetition suppression.
A consistent fMRI finding in AD has been disruption of task-induced deactivation (or, “default mode activity”) in the medial parietal association cortices.14-17 Changes in task-related MTL activation during processing of novel versus repeated stimuli have been reported to be reciprocally related to changes in parietal deactivation across the continuum from normal aging to AD.17 In other words, healthy elderly subjects showed significant MTL activation and parietal deactivation during an fMRI memory task, whereas both MTL activation and parietal deactivation were diminished in patients with clinical AD.17 There is also recent evidence in young and elderly healthy subjects to suggest that greater fMRI task-induced deactivation predicts successful memory formation for novel stimuli.18,19 The relationship between the brain’s capability for “beneficial” parietal deactivation and MTL response suppression to repeatedly presented stimuli and how this fMRI response pattern correlates with task performance in OC and AD remains to be explored.
The goal of this study was to investigate fMRI task-induced activation and deactivation responses during repeated presentations of face-name pairs versus visual fixation in healthy OC and mild AD patients. We expected to find evidence of impaired MTL repetition suppression in AD, manifesting as continued activation to highly familiar stimuli, compared with OC. We hypothesized that OC would demonstrate greater parietal deactivation than AD, and that the magnitude of MTL activation to repeated stimuli would be related to the magnitude of parietal deactivation. Finally, we wanted to investigate whether fMRI activity during repeated stimuli correlated with performance on the postscan recognition memory test for novel associative encoding.
SUBJECTS AND METHODS
Subjects
Forty-four elderly individuals participated in the study. All subjects provided informed consent in accordance with the Human Research Committee guidelines of the Massachusetts General Hospital and Brigham and Women’s Hospital (Boston, MA). There were 29 healthy OC and 15 mild AD patients (Table 1). Thirty-four of the participants (OC: N = 29; AD: N = 5) were recruited from a longitudinal study examining preclinical predictors of AD. The remaining 10 subjects (AD: N = 10) were recruited from memory disorders clinics. All subjects were required to be free of significant underlying medical, neurologic, or psychiatric conditions. A subset of these subjects has been previously reported in one study8 using anatomically defined regions of interest (ROI) limited to the MTL (N = 20) and in another study17 using independent component analysis (N = 25). Healthy OC had a clinical dementia rating scale = 0.020 and were followed up longitudinally for at least 1 year with no evidence of cognitive decline. The AD patients met the National Institute of Neurological and Communicative Disorders and Stroke–AD and Related Disorders Association criteria for probable AD21 and had mild dementia severity as characterized by an overall clinical dementia rating = 1.0. All AD patients had either been off cholinesterase inhibitors for at least 30 days before scanning or had never taken these medications.
TABLE 1.
Demographic and Cognitive Characteristics
| Healthy Older Subjects (N = 29) | Alzheimer Patients (N = 15) | |
|---|---|---|
| Age | 74.2 ± 5.6 | 78.3 ± 6.9a |
| (Range) | (66-90) | (57-85) |
| Education | 15.6 ± 2.6 | 13.3 ± 3.2a |
| (Range) | (12-21) | (8-20) |
| Female/Male | 19/10 | 8/7 |
| % of female | 66 | 53 |
| Mini-Mental State Examination | 29.7 ± 0.5 | 23.3 ± 4.2a |
| (Range) | (29-30) | (15-30) |
| Face-Name Recognition (%) | 87.8 ± 9.4 | 65.7 ± 11.7a |
| (Range) | (64-100) | (50-86) |
Note: Results are means ± standard deviations.
Significant difference between healthy older subjects and patients with Alzheimer disease (p <0.05, Mann-Whitney U test).
fMRI Data Acquisition
Subjects were scanned using a Siemens Trio 3.0-T scanner (Siemens Medical Systems, Iselin, NJ) equipped for echo-planar imaging (EPI). High resolution structural images were acquired using a T1-weighted 3-D Magnetization Prepared Rapid Acquisition Gradient Echo sequence with the following parameters: repetition time (TR) = 2,530 milliseconds, echo time (TE) = 3.45 milliseconds, inversion time = 1,100 milliseconds, flip angle = 7 degrees, field of view (FOV) = 256 mm, matrix 192 × 256, slice thickness = 1.33 mm, 128 sagittal slices. A T1-weighted EPI sequence was used to acquire low resolution structural images with the same slice positioning than for the functional images: TR = 30,000 milliseconds, TE = 39 milliseconds, inversion time = 1200 milliseconds, FOV = 200 mm, matrix 64 × 64. fMRI data were acquired using a T2*-weighted gradient-echo EPI sequence sensitive to blood-oxygen-level-dependent (BOLD) signal with the following parameters: TR = 2,500 milliseconds, TE = 30 milliseconds, flip angle = 90 degrees, FOV = 200 mm, matrix 64 × 64, resulting in an in-plane resolution of 3.125 × 3.125 mm2. Twenty-eight oblique coronal slices with a thickness of 5.0 mm and an interslice gap of 1.0 mm were scanned, oriented perpendicular to the anterior-posterior commissural line. Scanning time for each functional run was 4 minutes 15 seconds resulting in a total functional scanning time of 25 minutes 30 seconds.
fMRI Activation Task
The fMRI activation task consisted of blocks of Novel and Repeated face-name pairs alternating with simple visual Fixation.7,22 For the Novel and Repeated activation conditions, the participants were instructed to try i) to remember the name associated with each face, and ii) to indicate with a button press whether or not they thought the name “fit” the face. For the Fixation baseline, they were instructed to focus their attention on a white cross-hair presented on the black background. Each of the six fMRI runs consisted of two Novel blocks (7 face-name pairs per block, each shown for 5 seconds) and two repeated blocks of identical length, separated by 25-second periods of Fixation. During the Repeated blocks over the entire fMRI experiment, one male and one female face-name pair was presented 42 times each. Before the scanning session, all subjects were carefully familiarized with the task, using two practice runs, which included the Repeated face-name pairs. Visual stimuli were presented using MacStim 2.5 software (WhiteAnt Occasional Publishing, West Melbourne). After the scanning session all subjects underwent a forced-choice associative recognition memory test. During this postscan memory test, a set of 12 Novel faces seen during the experiment and the two repeated faces were presented on a computer screen. Each face was shown with two names printed underneath: the correct name that was paired with the face during scanning and an incorrect name that was previously paired with a different face during scanning. The subjects were instructed to indicate the correct name by pointing to it on the computer monitor.
fMRI Data Analysis
fMRI data analysis was carried out using FMRI Expert Analysis Tool version 5.63, part of FSL (FMRIB’s Software Library, www.fMRIb.ox.ac.uk/fsl). The following prestatistical processing was applied: motion correction, removal of nonbrain structures, spatial smoothing using a Gaussian kernel of full width at half maximum of 5 mm, mean-based intensity normalization of all volumes by the same factor, and high-pass temporal filtering with a frequency cutoff point of 140.0 seconds. Time-series statistical analysis was carried out using FMRIB’s Improved Linear Model with local autocorrelation correction. Processing of Repeated face-name pairs was contrasted to visual Fixation. Both fMRI task-induced activation and deactivation (i.e., increases and decreases in BOLD fMRI signal, correspondingly) responses were examined. fMRI images were registered to high resolution structural images and to the Montreal, Que., Canada, Neurological Institute (MNI) standard brain via the low resolution structural T1-weighted EPI images using FMRIB’s Linear Image Registration Tool and 12 parameter affine registration. Registration success was visually checked in each subject using the registration report output of the first-level data analysis.
Higher-level analysis within and between study groups was carried out using FMRIB’s Local Analysis of Mixed Effects.23 FSL mixed-effects modeling exploits one-sample t tests for within-group and two-sample unpaired t tests for between-group analyses. The resulting Z statistic images (i.e., Gaussianised T statistic images) were corrected for multiple comparisons using cluster thresholding.24 To perform cluster thresholding, the voxelwise Z statistic threshold was set to Z > 2.3 and the cluster probability threshold to p <0.025. In the Table 2 reporting fMRI results, we list the peak Z-values of each significant (de)activation cluster as well as the corresponding uncorrected two-tailed p values, MNI (x, y, z) coordinates and the approximate Brodmann area (BA). FSL whole-brain analyses between the groups of OC and AD were also performed including age and education as covariates. The mean percent signal change of all voxels within hippocampal and medial parietal regions of interest (ROIs) during Repeated face-name pairs compared with Fixation was extracted using FSL Featquery. The ROIs (see Fig. 3A) were delineated manually on the average brain of the 44 study subjects in standard space. The medial parietal ROI included the precuneus (medial extent of BA 7), retrosplenial cortex (BA 29 and 30), and posterior cingulate cortex (BA 23 and 31). Differences between groups in hippocampal and parietal fMRI responses were tested using unpaired t test and correlations using the Pearson’s correlation. Furthermore, the covariance of the fMRI activation and deactivation responses with the postscan memory test performance within the hippocampal and parietal ROIs was examined using the recognition memory test scores as covariates of interest in FSL.
TABLE 2.
Differences in Brain Activation and Deactivation During Repeated Stimuli Versus Fixation (RvF) Between Healthy Older Subjects and Patients With Alzheimer Disease
| Brain Region | BA | x | y | z | Peak Z | Peak p |
|---|---|---|---|---|---|---|
| RvF activation in healthy older subjects > Alzheimer patients | ||||||
| No significant activation areas | ||||||
| RvF activation in Alzheimer patients > healthy older subjects | ||||||
| R middle frontal gyrus | 9, 46 | 42 | 36 | 26 | 4.51 | 0.000007 |
| R inferior frontal gyrus | 44 | 38 | 16 | 28 | 3.33 | 0.0009 |
| L intraparietal sulcus | 7, 39 | −36 | −50 | 40 | 4.06 | 0.00005 |
| L supramarginal gyrus | 39 | −46 | −44 | 36 | 3.88 | 0.0001 |
| L superior parietal lobule | 7 | −24 | −62 | 42 | 3.36 | 0.0008 |
| L hippocampus | −22 | −4 | −26 | 3.38 | 0.0008 | |
| RvF deactivation in healthy older subjects > Alzheimer patients | ||||||
| R posterior cingulate | 23, 30, 31 | 16 | −50 | 24 | 3.41 | 0.0007 |
| R precuneus | 7 | 6 | −68 | 40 | 3.62 | 0.0003 |
| L precuneus | 7 | −4 | −72 | 40 | 4.15 | 0.00004 |
| L angular gyrus | 39 | −36 | −70 | 36 | 3.62 | 0.0003 |
| RvF deactivation in Alzheimer patients > healthy older subjects | ||||||
| No significant deactivation areas |
Notes: L = left; R = right. Peak Z-values, corresponding uncorrected two-tailed p values, MNI coordinates (x, y, z) and Brodmann areas (BA) of significant brain (de)activation regions are reported. Activation areas exceeding the corrected cluster-level threshold of p <0.025 in FSL mixed-effects analyses (two-sample unpaired t test, df = 42) were considered significant. Healthy older subjects: N = 29; Alzheimer patients: N = 15.
FIGURE 3. Greater Hippocampal Activation During Processing of Repeated Stimuli (i.e., Impaired Repetition Suppression) is Related to Failure of Parietal Deactivation in Patients With Alzheimer Disease.
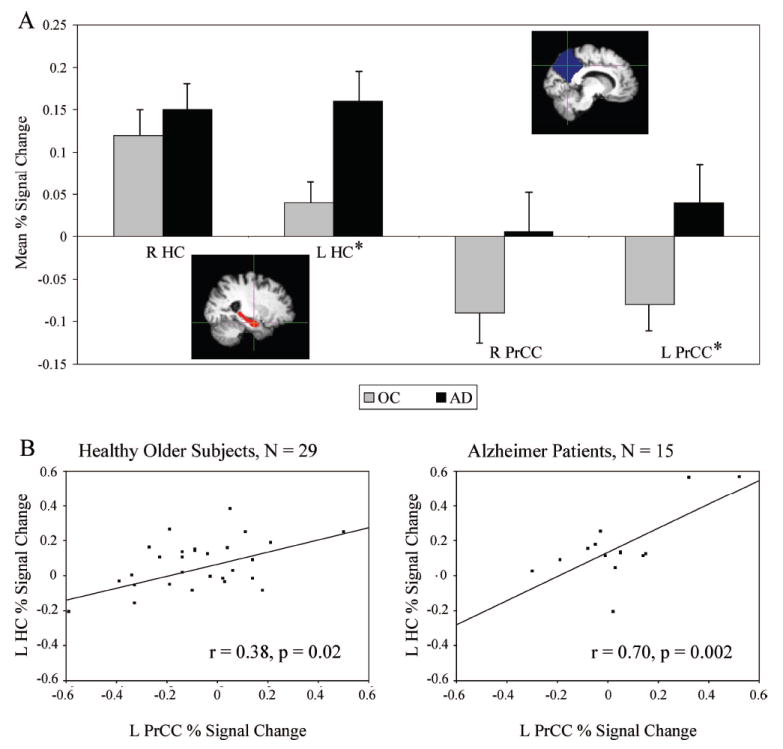
Notes: [A] The mean percent fMRI signal change for the healthy older subjects (gray) and Alzheimer patients (black) in the right (R) and left (L) hippocampal (HC; red) and precuneal/posterior cingulate cortical (PrCC; blue) regions. *Significant difference between healthy older subjects and Alzheimer patients (p <0.05, unpaired t test, df = 42). Vertical lines present the standard error of the means. [B] The mean percent signal change within the L PrCC is presented on the x axis and of the L HC on the y axis.
Demographic and Cognitive Data Analysis
Statistical analysis of demographic, neuropsychological and behavioral data were conducted with SPSS 11.5 (SPSS Inc., Chicago, IL). Differences in age, education, Mini-Mental State Examination, and postscan memory performance were tested using the non-parametric Mann-Whitney U test. The level of statistically significant differences was set at p <0.05.
RESULTS
Demographic and Cognitive Data
The group data for OC and AD are summarized in Table 1. AD patients were older, had less education, lower Mini-Mental State Examination, and poorer postscan memory test performance than the OC.
fMRI Activation Areas in Healthy Older Subjects and Alzheimer Patients
Both OC and AD patient groups demonstrated large brain activation areas in the Repeated versus Fixation (RvF) contrast bilaterally in occipital unimodal and heteromodal visual areas, parietal, anterior cingulate, and insular and prefrontal cortices. Similarly, both OC and AD showed activation in the middle and posterior parts of the MTL. Interestingly, only the AD group showed a significant activation cluster in the left anterior MTL during processing of repeated stimuli (peak location: MNI coordinate = −24, −4, −28; Z = 4.14; corresponding uncorrected p value = 0.00004, one-sample t test, df = 14).
fMRI Deactivation Areas in Healthy Older Subjects and Alzheimer Patients
The OC demonstrated significant task-induced deactivation in RvF bilaterally in posterior cingulate and precuneal cortices and in left lateral parietal cortex. We found no areas of significant fMRI deactivation in AD patients.
Differences in fMRI Activation Between Healthy Older Subjects and Alzheimer Patients
Next, we explored group differences for the RvF brain activation between OC and AD patients. We found no significant fMRI activation clusters greater in OC compared with AD. In contrast, AD patients showed significantly more activation during processing of repeated stimuli in the middle and inferior prefrontal gyri corresponding to BA 9, 44, and 46, and left superior parietal lobule, intraparietal sulcus and supramarginal gyrus (BA 7 and 39; Fig. 1A, Table 2). We found greater left MTL activation in AD than in OC in the head of the hippocampus, which extended to neighboring entorhinal or perirhinal cortices (Fig. 1B, Table 2). These analyses were also performed including age and education as covariates in the FSL higher-level contrasts with no change in the results.
FIGURE 1. Greater Brain Activation Areas During Processing of Repeated Face-Name Pairs in Patients With Alzheimer Disease Compared With Healthy Older Subjects.
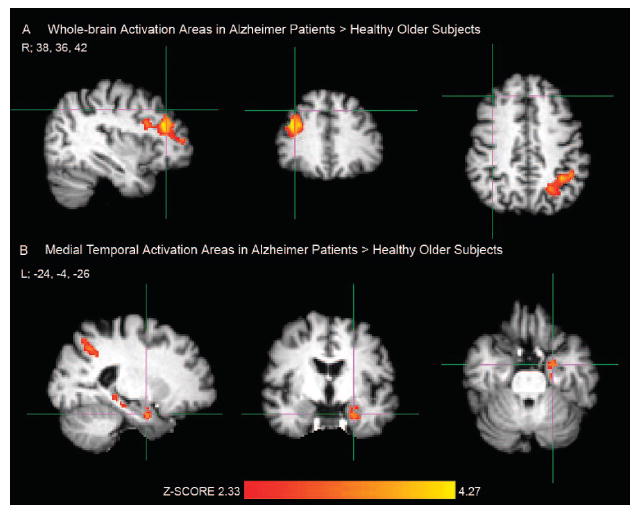
Notes: [A] Crosshair in the right (R) prefrontal cortex, MNI coordinate: 38, 36, 42; [B] Crosshair in the left (L) anterior hippocampus, MNI coordinate: − 24, −4, −26. Activation areas exceeding the corrected cluster-level threshold of p <0.025 (two-sample unpaired t test, df = 42) were considered significant in FSL mixed-effects analyses. Healthy older subjects: N = 29; Alzheimer patients: N = 15.
Differences in fMRI Deactivation Between Healthy Older Subjects and Alzheimer Patients
As the within-group results suggested, OC demonstrated significantly greater task-induced deactivation in the RvF contrast in bilateral precuneal, right posterior cingulate, and left lateral parietal cortices compared with AD patients (Fig. 2, Table 2). We found no significant fMRI deactivation areas greater in AD than in OC.
FIGURE 2. Greater Brain Deactivation Areas During Processing of Repeated Face-Name Pairs in Healthy Older Subjects compared With Patients With Alzheimer Disease.
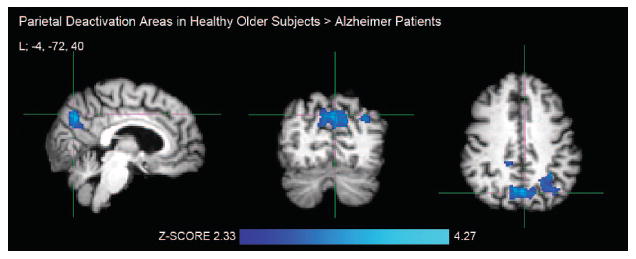
Notes: Crosshair in the left (L) precuneus, MNI coordinate: −4, −72, 40. Activation areas exceeding the corrected cluster-level threshold of p <0.025 (two-sample unpaired t test, df = 42) were considered significant in FSL mixed-effects analyses. Healthy older subjects: N = 29; Alzheimer patients: N = 15.
Distinct Medial Temporal Activation and Parietal Deactivation Patterns in Healthy Older Subjects and Alzheimer Patients
The results above for within- and between-group analyses demonstrated increased left MTL activation and decreased parietal deactivation during processing of Repeated face-name pairs in AD compared with OC. To investigate this finding in more detail, we extracted the mean percent signal change within right and left hippocampal and medial parietal ROIs (Fig. 3A). In this ROI analysis, the OC showed significantly less positive fMRI signal (i.e., more suppression of BOLD response during repeated stimuli) in the left hippocampus (t = 2.3, p = 0.03, unpaired t test, df = 42) and significantly greater negative fMRI signal (i.e., more deactivation) in the left medial parietal cortices (t = 2.3, p = 0.03, unpaired t test, df = 42) than AD patients. In other words, the AD patients demonstrated greater hippocampal activation in RvF (i.e., impaired repetition suppression), and no task-induced deactivation in the midline parietal regions compared with OC. In AD (Fig. 3B), greater hippocampal fMRI signal was related to failure of the medial parietal deactivation, or in fact paradoxical activation of the areas deactivated in healthy OC, within each hemisphere (right: r = 0.64, p = 0.01; left: r = 0.70, p = 0.004; Pearson’s correlation, AD: N = 15). Within OC (Fig. 3B), the magnitude of hippocampal activation in RvF was weakly correlated with decreasing deactivation (right: r = 0.39, p = 0.04; left: r = 0.38, p = 0.04; Pearson’s correlation, OC: N = 29). These ROI analyses confirmed our between-group map-level results demonstrating increased left hippocampal activation and decreased left medial parietal deactivation in AD compared with OC.
When using the postscan recognition memory test scores as a covariate of interest in FSL group-level analyses, we found a negative correlation between the associative recognition memory performance and MTL activation to repeated stimuli (peak location in the left hippocampus: −28, −30, −12; Z = 2.37; corresponding uncorrected p = 0.02, df = 42). In other words, impaired reduction of MTL activation during repeated stimuli was related to poor postscan memory test performance. In contrast, recognition memory test scores correlated positively with right (6, −78, 44; Z = 3.41, p = 0.0007, df = 42) and left (−12, −72, 34; Z = 2.78, p = 0.006, df = 42) precuneal deactivation. Greater deactivation was related to better postscan memory test performance.
DISCUSSION
This study provides evidence that the normal suppression of MTL response to repeated stimuli that are highly familiarized in OC is impaired in AD patients. In addition to increased MTL activation, AD patients showed greater activation than OC in prefrontal and parietal cortical areas of the “novelty detection network” during repeated stimuli. Consistent with previous functional imaging studies, task-induced deactivation was observed in the medial parietal regions in OC but not in AD. Interestingly, impaired MTL repetition suppression (i.e., greater activation during repeated stimuli) was related to failure of parietal deactivation in patients with AD and to poor postscan recognition memory test performance on novel associations encoded during scanning.
A network of brain areas consisting of the MTL, prefrontal and parietal cortices, which is known to support the encoding of novel information,1,2,22,25,26 demonstrated greater activation in AD than in OC during processing of Repeated face-name pairs. In previous studies, reduction of MTL responses were observed in OC by the second or third presentation of the stimuli.12,13 In this study, the two Repeated face-name pairs were familiarized to the subjects before the fMRI scanning session and presented repeatedly 42 times each during the fMRI task. The continued activity in the brain “novelty detection network” indicates that AD patients failed to recognize the familiarity of the repeated stimuli but rather continued to process these stimuli as novel. This notion is also supported by Golby et al.9 who found a trend toward greater MTL activation during processing of repeated scenes in seven AD patients compared with OC.
Multiple fMRI studies have reported that MTL responses to novel stimuli, compared either with fixation or with repeated stimuli, are compromised in the AD brain.6-9,17,27,28 Our current preliminary findings, taken together with previous work, demonstrate that the MTL responses in AD patients are decreased to novel but increased to familiar stimuli. Therefore, fMRI paradigms comparing novel to repeated stimuli25 seem to be particularly valuable in investigating emerging disturbances in the MTL function early in the course of AD. Similar to AD, MTL responses during novel picture encoding have been reported to be altered in elderly patients with chronic schizophrenia compared with demographically matched healthy subjects.29 However, to expand the use of fMRI from a research tool, to investigate abnormal brain function in neuropsychiatric disorders, and to clinical diagnostic purposes of, for example, early AD, more research at the level of individual patients with longitudinal clinical follow-up is needed.
The anterior region of the MTL demonstrating the failure of repetition suppression in AD is consistent with studies reporting increased anterior MTL responses during processing of novel stimuli22,26,30 and during successful memory formation for novel associations in particular.31 MTL activity has also been related to better fMRI task performance during an episodic memory paradigm in elderly individuals ranging from cognitively high-performing subjects to Alzheimer patients.32 In this study, increased anterior MTL activity during repeated stimuli was actually related to worse performance in the postscan recognition memory test, providing evidence that failure of repetition suppression is indicative of impaired MTL encoding function. This finding is well supported by recent studies in young and older subjects demonstrating that intact MTL repetition suppression is part of the successful encoding process and is related to later recognition memory strength.12,33 Theories of the neural mechanisms underlying repetition suppression suggest that repeated presentations of identical stimuli may change the synaptic efficacy of the connections between neocortical and MTL regions11 and thus support encoding and later remembering.33 AD has been recently described as a failure of synaptic function,5 which may provide a link between the failure of MTL response reduction and poor recognition memory performance in AD found in this study.
Previous fMRI studies reporting decreased MTL activation in AD6-9,17,27,28 have raised the question of the contribution of structural atrophy to functional changes. In this study, we found, however, increased anterior MTL activation to repeated stimuli in AD, which is unlikely to be explained by atrophy. Regarding the anatomic localization accuracy of MTL activation findings in OC and AD patients, it is possible that brain atrophy in AD could lead to less accurate coregistration of the structural and functional images into the MNI reference brain. In this study, coregistration success was visually evaluated in each individual study subject. Nevertheless, coregistration still remains a challenging issue in functional imaging research of AD in general. Another potential confounding factor regarding our results was the significant difference in age and education between the groups of OC and AD. Our results were, however, unchanged when covarying age and education in the fMRI data analyses. In addition, older age, if anything, has been typically associated with decreased MTL activation, and we actually found greater MTL activation to repeated stimuli in AD. Thus, it seems unlikely that the group age or education differences account for the present fMRI findings.
Consistent with previous fMRI deactivation studies,14-17 we found failure of medial parietal task-induced deactivation in AD compared with OC, even during the “low-level” condition of repeated presentation of face-name pairs compared with visual fixation. As suggested by studies in healthy controls,18,19 decreased precuneal deactivation was related to a worse postscan recognition memory test performance. Interestingly, failure of the “beneficial” deactivation in AD patients or, in fact, paradoxical fMRI activation of the medial parietal cortices,14 was also correlated with greater impairment in the MTL repetition suppression. Our results are in accordance with other studies demonstrating parallel alterations in both the MTL and parietal regions early in the course of AD.17,34 Instead of the beneficial medial parietal deactivation, the AD patients showed increased activation in superior parietal regions, suggestive of continued attention to the Repeated face-name pairs which became highly familiar to OC.
This study reveals new perspectives to the dysfunction of the MTL and anatomically connected cortical brain structures in AD. In addition to advancing our understanding of the pathophysiology of neuropsychiatric disorders in the elderly, fMRI holds potential in the evaluation of novel pharmacological strategies.35,36 FMRI paradigms comparing novel to repeated stimuli such as in the present study may be particularly suitable to detect pharmacological effects because brain responses to both novel and repeated stimuli are significantly altered early in the course of AD but not in healthy aging.12,13 Furthermore, imaging of alterations in medial parietal deactivation responses using cognitive paradims or resting state fMRI,15 is of potential clinical significance given the consistent finding of hypometabolism in the same regions in [18F]fluorodeoxyglucose positron emission tomography studies in AD.37
In summary, we found that AD patients demonstrated failure of repetition suppression in the MTL, as well as loss of beneficial medial parietal task-induced deactivation. These fMRI findings were related to poor associative recognition memory of novel face-name pairs, suggesting that impaired repetition suppression is indicative of impaired MTL encoding mechanisms and loss of relative MTL sensitivity to novel information. Our results provide evidence that episodic memory impairment typically seen in mild AD may manifest as an abnormal increased activation in MTL memory structures in addition to previously documented decreased activity to novel information. Results of this study support the hypothesis that the normal function of a distributed neural network including both MTL and parietal memory systems is disrupted in clinical AD.
Acknowledgments
The authors thank the staff of the Massachusetts General Hospital Gerontology Research Unit and Brigham and Women’s Hospital Memory Disorders Unit Clinical Research for assistance with subject recruitment, evaluation, and data management, as well as Mary Foley, Larry White, and the Athinoula A. Martinos Center staff for assistance with MRI data collection. The authors express special gratitude to the subjects who participated in this study.
This work was supported by NIA R01-AG027435, NIA PO1-AG04953, NIA P50-AG00513421, and Academy of Finland.
References
- 1.Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- 2.Hasselmo ME, Stern CE. Mechanisms underlying working memory for novel information. Trends Cogn Sci. 2006;10:487–493. doi: 10.1016/j.tics.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyman BT, Van Hoesen GW, Damasio AR, et al. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 5.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 6.Rombouts SA, Barkhof F, Veltman DJ, et al. Functional MR imaging in Alzheimer’s disease during memory encoding. Am J Neuroradiol. 2000;21:1869–1875. [PMC free article] [PubMed] [Google Scholar]
- 7.Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickerson BC, Salat DH, Greve DN, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golby A, Silverberg G, Race E, et al. Memory encoding in Alzheimer’s disease: an fMRI study of explicit and implicit memory. Brain. 2005;128:773–787. doi: 10.1093/brain/awh400. [DOI] [PubMed] [Google Scholar]
- 10.Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 11.Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Rand-Giovannetti E, Chua EF, Driscoll AE, et al. Hippocampal and neocortical activation during repetitive encoding in older persons. Neurobiol Aging. 2006;27:173–182. doi: 10.1016/j.neurobiolaging.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Johnson SC, Baxter LC, Susskind-Wilder L, et al. Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia. 2004;42:980–989. doi: 10.1016/j.neuropsychologia.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Lustig C, Snyder AZ, Bhakta M, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greicius MD, Srivastava G, Reiss AL, et al. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rombouts SA, Barkhof F, Goekoop R, et al. Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: an fMRI study. Hum Brain Mapp. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celone KA, Calhoun VD, Dickerson BC, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Grady CL, Springer MV, Hongwanishkul D, et al. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC, Ernesto C, Schafer K, et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer’s Disease Cooperative Study experience. Neurology. 1997;48:1508–1510. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.Sperling RA, Bates J, Cocchiarella A, et al. Encoding novel face-name associations: a functional MRI study. Hum Brain Mapp. 2001;14:129–139. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beckmann C, Jenkinson M, Smith SM. General multi-level linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 24.Worsley KJ, Evans AC, Marrett S, et al. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 25.Stern CE, Corkin S, Gonzalez RG, et al. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pihlajamäki M, Tanila H, Hänninen T, et al. Encoding of novel picture pairs activates the perirhinal cortex: an fMRI study. Hippocampus. 2003;13:67–80. doi: 10.1002/hipo.10049. [DOI] [PubMed] [Google Scholar]
- 27.Small SA, Perera GM, DeLaPaz R, et al. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer’s disease. Ann Neurol. 1999;45:466–472. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 28.Hämäläinen A, Pihlajamäki M, Tanila H, et al. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging. 2006;28:1889–1903. doi: 10.1016/j.neurobiolaging.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Zorrilla LT, Jeste DV, Brown GG. Functional MRI and novel picture-learning among older patients with chronic schizophrenia: abnormal correlations between recognition memory and medial temporal brain response. Am J Geriatr Psychiatry. 2002;10:52–61. [PubMed] [Google Scholar]
- 30.Pihlajamäki M, Tanila H, Könönen M, et al. Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe subareas in humans. Eur J Neurosci. 2004;19:1939–1949. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- 31.Sperling R, Chua E, Cocchiarella A, et al. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grön G, Riepe MW. Neural basis for the cognitive continuum in episodic memory from health to Alzheimer disease. Am J Geriatr Psychiatry. 2004;12:648–652. doi: 10.1176/appi.ajgp.12.6.648. [DOI] [PubMed] [Google Scholar]
- 33.Gonsalves BD, Kahn I, Curran T, et al. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–761. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Kogure D, Matsuda H, Ohnishi T, et al. Longitudinal evaluation of early Alzheimer’s disease using brain perfusion SPECT. J Nucl Med. 2000;41:1155–1162. [PubMed] [Google Scholar]
- 35.Kircher TT, Erb M, Grodd W, et al. Cortical activation during cholinesterase-inhibitor treatment in Alzheimer disease: preliminary findings from a pharmaco-fMRI study. Am J Geriatr Psychiatry. 2005;13:1006–1013. doi: 10.1176/appi.ajgp.13.11.1006. [DOI] [PubMed] [Google Scholar]
- 36.Matthews B, Siemers ER, Mozley PD. Imaging-based measures of disease progression in clinical trials of disease-modifying drugs for Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:146–159. [PubMed] [Google Scholar]
- 37.Herholz K, Salmon E, Perani D, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17:302–316. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]


