Abstract
A unifying feature of mammalian and insect olfactory systems is that olfactory sensory neurons (OSNs) expressing the same unique odorant receptor gene converge onto the same glomeruli in the brain (1–7). Most odorants activate a combination of receptors and thus distinct patterns of glomeruli, forming a proposed combinatorial spatial code that could support discrimination between a large number of odorants (8–11). OSNs also exhibit odor-evoked responses with complex temporal dynamics (11), but the contribution of this activity to behavioral odor discrimination has received little attention (12). Here we investigated the importance of spatial encoding in the relatively simple Drosophila antennal lobe. We show that Drosophila can learn to discriminate between two odorants with one functional class of Or83b-expressing OSNs. Furthermore, these flies encode one odorant from a mixture, and cross-adapt to odorants that activate the relevant OSN class, demonstrating that they discriminate odorants using the same OSNs. Lastly, flies with a single class of Or83b-expressing OSNs recognize a specific odorant across a range of concentration indicating that they encode odorant identity. Therefore flies can distinguish odorants without discrete spatial codes in the antennal lobe, implying an important role for odorant-evoked temporal dynamics in behavioral odorant discrimination.
In fruit flies, specific odorants interact with unique combinations of olfactory sensory neurons giving rise to a putative topographic odor code of activated glomeruli in the antennal lobe. To test the requirement of differential spatial encoding in odorant discrimination we reduced olfactory input complexity using Or83b2 null mutant flies (13). OR83b is an essential subunit of odorant receptor (OR) containing odorant-gated cation channels (13–16). Most fruit fly OSNs co-express Or83b with a single unique (OR) gene and all those housed in basiconic and trichoid sensillae, with the exception of a highly specialized class that detect CO2, require Or83b for function (13, 16–18). Or83b is also co-expressed with Or35a in a broadly tuned class of coeloconic OSNs, but the remaining OSNs in coeloconic sensillae, specialized to select volatiles including small amines, have not been reported to express Or83b, Or or Gr genes (6, 7, 19). Therefore, Or83b2 mutant flies are anosmic to odorants sensed by basiconic and trichoid sensillae. Importantly, OSNs wire to the appropriate glomeruli in Or83b mutant flies and one can restore function to a single OSN class by expressing a uas-Or83b transgene using Or-specific GAL4 control (20, 21). Using this technique others demonstrated that larvae with a single OSN chemotax toward odorants that attract wild-type larvae (20, 21). While clearly establishing a role for single OSNs, these studies did not investigate whether odorant-evoked activity through a single class of OSN can be decoded as a discrete odor percept. One way to do this is to assign value to an arbitrary odorant with associative conditioning and demonstrate that flies choose appropriately between odorants. If discrete spatial patterns of glomerular activation are essential for encoding odorant identity, flies with one OSN class will fail to discriminate odorants, because the glomerulus activated by all odorants is the same in these flies. Odorant discrimination with one class of OSNs would challenge a spatial encoding model.
We used an olfactory conditioning paradigm where flies associate one of two odorants with electric shock punishment and then choose between the two odorants (22). Trained flies preferentially avoid the T-maze arm with the conditioned odorant. A different population of the same genotype of flies is subsequently taught to associate the other odorant with punishment and a single learning score represents the average of the two reciprocal experiments. This design provides a rigorous test of odorant discrimination and controls against innate odorant bias.
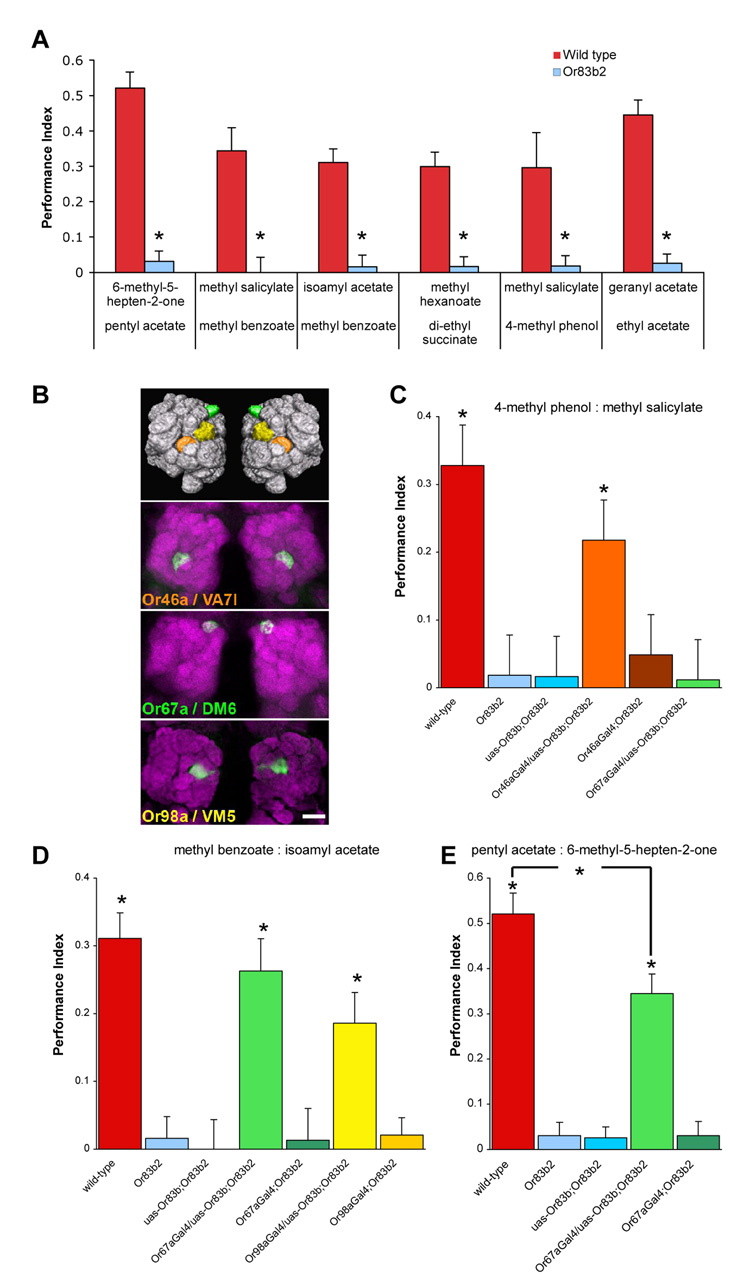
The electrophysiological response to a large panel of odorants has been reported for most Drosophila ORs (11), allowing us to select and test OSNs and their cognate odorants. We first determined whether Or83b2 mutant flies can learn to discriminate between six pairs of odorants (6-methyl-5-hepten-2-one versus pentyl acetate, methyl salicylate versus methyl benzoate, isoamyl acetate versus methyl benzoate, methyl hexanoate versus di-ethyl succinate, methyl salicylate versus 4-methyl phenol and geranyl acetate versus ethyl acetate) selected because they activate defined ORs (Fig. 1A). As expected, wild-type flies showed robust learned discrimination with all six odorant pairs whereas Or83b2 mutant flies did not. Therefore, Or83b expressing OSNs are required to learn to discriminate between the chosen odorants and residual responses in Or83b2 mutant flies are not sufficient to support learned odorant discrimination.
Figure 1. Or83b2 flies with functional Or46a, Or67a or Or98a-expressing neurons learn to discriminate between odorants that activate these receptors.
(A) Or83b2 mutant flies cannot learn to discriminate between odors. Wild-type flies can learn to discriminate between six pairs of odorants whereas Or83b2 mutant flies cannot. Asterisks indicate no significant difference to zero (all P>0.1, Mann Whitney U-test). (B) Upper panel, volume rendering of the fly antennal lobes highlighting the relative position of the VA7l (orange), DM6 (green) and VM5 (yellow) glomeruli innervated by Or46a, Or67a and Or98a expressing OSNs. Lower panels show corresponding confocal stack projections through the antennal lobes of flies expressing uas-n-syb::GFP driven by Or46a-GAL4, Or67a-GAL4 or Or98a-GAL4. N-syb::GFP is stained with anti-GFP (green) and neuropil is visualized with nc82 antibody (magenta) staining. Scale bar is 20µm and refers to all micrographs. (C) Flies with only functional OR46a neurons can learn to discriminate between 4-methyl phenol and methyl salicylate but flies with only OR67a neurons cannot. Asterisks indicate significant difference (all P<0.04, ANOVA) between the marked groups and all others. (D) Flies with only functional OR67a or OR98a neurons can learn to discriminate between methyl benzoate and isoamyl acetate. Asterisks indicate significant difference (all P<0.005, ANOVA) between the marked groups and all others. (E) Flies with only functional OR67a neurons can learn to discriminate between pentyl acetate and 6-methyl-5-hepten-2-one. Asterisks indicate significant difference (all P<0.005, ANOVA) between the marked groups and all others. Data are mean ± s.e.m.
We next tested flies in which the function of Or46a, Or67a or Or98a-expressing OSNs were restored individually. These OSNs are housed in different sensory sensilla (pb2, ab10 and ab7a) in the maxillary palp or antenna, project their axons to the spatially discrete VA7l, DM6 and VM5 glomeruli (Fig. 1B), and respond to a subset of the odorant pairs used in Fig. 1A (6, 7, 11, 23). Furthermore, these receptors are not co-expressed with other functional ORs (6, 7, 24). We first used Or46a-GAL4 to express uas-Or83b in an otherwise Or83b2 mutant fly and tested whether these flies could discriminate between two odorants reported to activate OR46a; 4-methyl phenol and methyl salicylate (23). Re-establishing OR46a OSN function in this way faithfully restored odor-evoked responses to those approximating wild-type OR46a neurons (23). We assayed wild-type, Or83b2 mutant, Or46a-GAL4; Or83b2 and uas-Or83b; Or83b2 mutant flies in parallel for comparison (Fig. 1C). All flies without functional Or83b-expressing OSNs did not learn whereas flies with restored OR46a neurons learned to discriminate between 4-methyl phenol and methyl salicylate. As an indicator of specificity, we tested flies with restored OR67a neurons. OR67a is broadly tuned but apparently does not respond to 4-methyl phenol and methyl salicylate (11, 23). Consistent with this, OR67a restored flies did not learn with these odorants (Fig. 1C). Therefore flies with a single class of functional Or83b-expressing OSNs can discriminate between two odorants if they activate the relevant OR.
The odor-tuning curve of OR67a partially overlaps with that of OR98a (11). We therefore tested flies with restored OR67a or OR98a neurons for learned discrimination between methyl benzoate and isoamyl acetate (Fig. 1D). As in the previous experiments, all flies without functional Or83b-expressing OSNs did not learn but robust learning was evident in flies with restored OR67a or OR98a neurons. Therefore Or83b2 mutant flies can use either OR67a or OR98a-restored OSNs to discriminate between methyl benzoate and isoamyl acetate.
OR67a also responds to pentyl acetate and 6-methyl-5-hepten-2-one (11). We therefore tested whether flies with restored OR67a neurons could learn to discriminate between these two odorants (Fig. 1E). All flies without functional Or83b-expressing OSNs did not exhibit learning, whereas flies with restored OR67a OSNs learned. Therefore OR67a restored flies can learn to discriminate between at least two pairs of different odorants that activate OR67a. The preceding experiments demonstrate that flies can employ a single class of Or83b-expressing OSNs to learn to discriminate between two odorants that activate that OSN class, consistent with the notion that they can use neural activity in the same class of OSNs to differentially represent odorants.
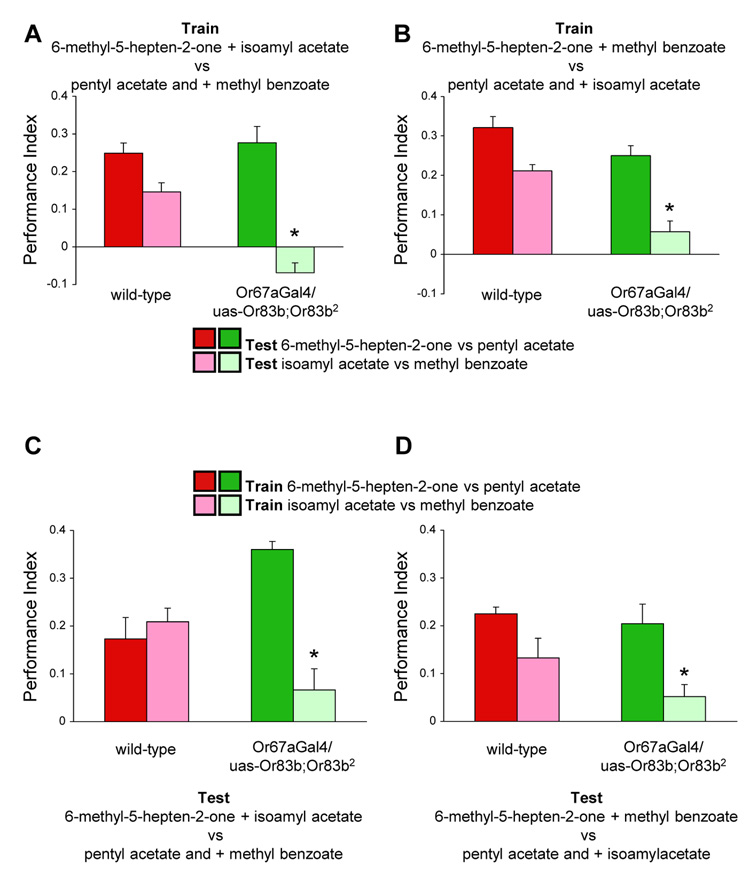
Our findings using Or83b2 mutant flies suggest that Or83b-independent OSNs are not sufficient for learned discrimination with multiple odorant combinations (Fig. 1). Nevertheless, we further tested whether flies with restored OR67a neurons had other relevant OSNs by testing whether flies could simultaneously encode multiple odorant components, like wild-type flies. We combined the four odorants that flies with restored OR67a neurons can discriminate between; methyl benzoate, isoamyl acetate, pentyl acetate and 6-methyl-5-hepten-2-one, into two binary mixtures, trained the flies with these mixtures (Fig. 2A and B) and tested for discrimination between the component odorants. Whereas wild-type flies exhibited learned discrimination for all four component odorants, regardless of the mixture combination used during training, learned discrimination was only observed for the 6-methyl-5-hepten-2-one and pentyl acetate components in OR67a restored flies. These data suggest that OR67a restored flies encode one odor component at a time, consistent with the notion that these odorants activate the same OSNs.
Fig. 2. Limitations in learned behavior in Or83b2 flies with functional Or67a-expressing neurons.
(A) Flies with only functional OR67a neurons learn one component of a binary blend. Flies were trained with 6-methyl-5-hepten-2-one + isoamyl acetate versus methyl benzoate + pentyl acetate mixtures. Wild-type flies show learning when tested with all components alone whereas flies with only functional OR67a neurons exclusively show learned discrimination for the 6-methyl-5-hepten-2-one and pentyl acetate components. (B) Wild-type flies learn both components of a different binary blend but OR67a restored flies still only learn one. Flies were trained with 6-methyl-5-hepten-2-one + methyl benzoate versus pentyl acetate + isoamyl acetate mixtures. Wild-type flies learn all components whereas flies with restored OR67a neurons again only show learned discrimination for the 6-methyl-5-hepten-2-one and pentyl acetate components. (C) Flies with restored OR67a neurons do not show learned discrimination of isoamyl acetate and methyl benzoate when 6-methyl-5-hepten-2-one and pentyl acetate are also present during test. Wild-type flies trained with either set of single components show learned discrimination when tested with the additional complexity of binary mixtures, but flies with OR67a neurons only show robust performance if trained with 6-methyl-5-hepten-2-one versus pentyl acetate. (D) Flies with functional OR67a neurons do not show learned discrimination of isoamyl acetate and methyl benzoate when tested with a different composition of odorant mixtures. Wild-type flies trained with either set of single components, 6-methyl-5-hepten-2-one versus pentyl acetate or isoamyl acetate versus methyl benzoate, show learned discrimination when tested with binary mixtures but flies with OR67a neurons only show robust performance if trained with 6-methyl-5-hepten-2-one versus pentyl acetate. Individual odor concentrations in the blends were the same as those used separately in Fig.2 C and 2D and when tested for component learning (Fig.3A and 3B). Asterisks denote no significant difference to zero (all P>0.5, Mann Whitney U-test).
To further test the model that odorants compete for ORs in OR67a restored flies, we trained flies with single odorants and tested discrimination with binary mixtures (Fig. 2C and D). We reasoned that a competing odorant in a mixture during testing, would mask learned behavior for the other odorant. Training wild-type flies with either 6-methyl-5-hepten-2-one versus pentyl acetate or isoamyl acetate versus methyl benzoate and testing with 6-methyl-5-hepten-2-one + isoamyl acetate versus pentyl acetate + methyl benzoate revealed learned discrimination in both cases (Fig. 2C). However, in flies with OR67a restored neurons, robust learned discrimination was only observed following training with 6-methyl-5-hepten-2-one versus pentyl acetate and not with isoamyl acetate versus methyl benzoate. We also tested learned discrimination with a different odorant combination; 6-methyl-5-hepten-2-one + methyl benzoate versus pentyl acetate + isoamyl acetate (Fig. 2D). Wild-type flies showed learned discrimination in both cases, whereas OR67a restored flies only showed learned discrimination when trained with 6-methyl-5-hepten-2-one versus pentyl acetate. Therefore these data are consistent with the notion that odorants compete at the OSN level in OR67a restored flies, providing further support that these flies have a single relevant OSN class (Fig. S1 and S2).
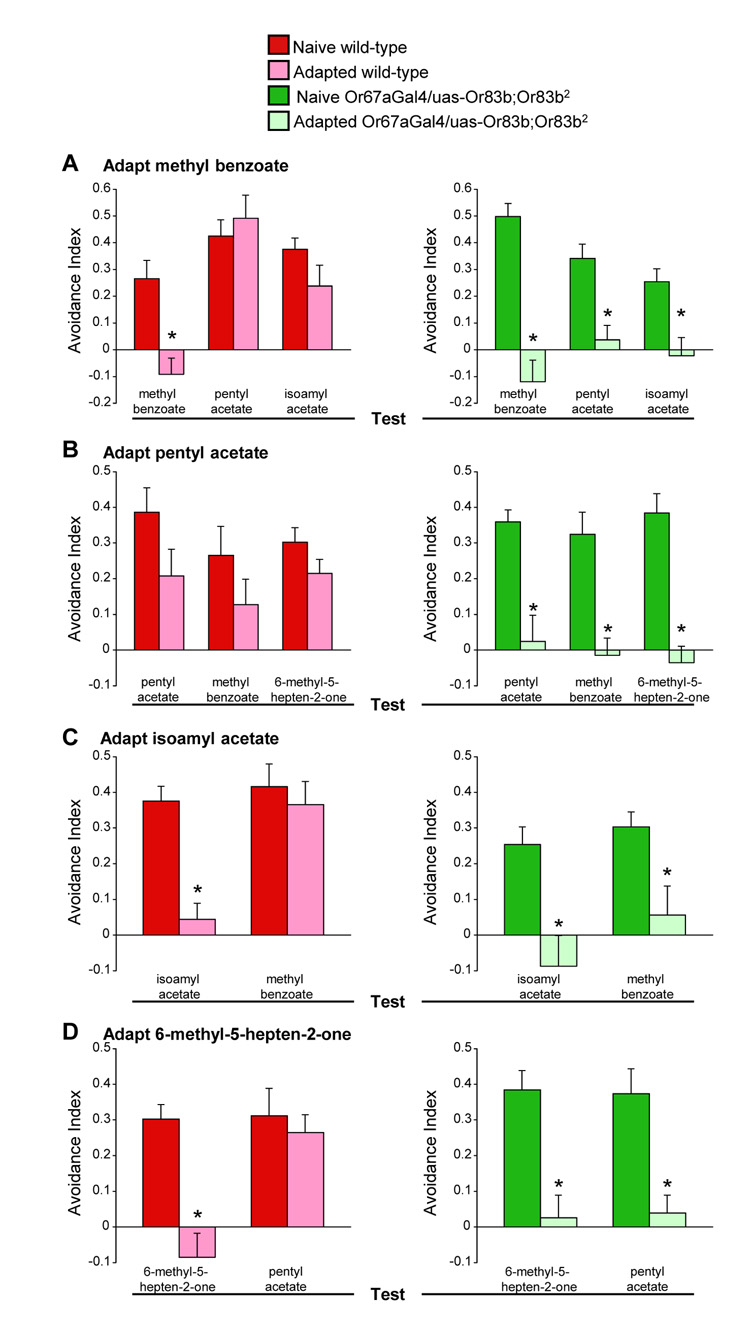
We also tested the contribution of Or83b-independent OSNs using a cross-adaptation assay that does not require learning. We predicted OR67a restored flies would cross-adapt to odorants that activate OR67a OSNs if these odorants activated the same OSNs. We first used methyl benzoate and pentyl acetate because odorant mixture experiments suggested these odorants compete for OR67a neurons (Fig. 3A and B). Wild-type and OR67a restored flies were adapted by pre-exposure to methyl benzoate for 30 minutes and tested for methyl benzoate or pentyl acetate avoidance behavior. Naive wild-type and OR67a restored flies avoided methyl benzoate but avoidance was abolished in both genotypes following adaptation (Fig.3A), demonstrating the efficacy of the adaptation protocol. For cross-adaptation we tested separate groups of methyl benzoate adapted flies for pentyl acetate avoidance (Fig. 3A). Wild-type flies adapted with methyl benzoate avoided pentyl acetate indicating that pentyl acetate activates additional OSNs in wild-type flies that do not respond to methyl benzoate. However, OR67a restored flies adapted with methyl benzoate also adapted their behavioral response to pentyl acetate, suggesting that pentyl acetate activates the same OSNs in these flies that respond to methyl benzoate. We also performed reciprocal cross-adaptation experiments where flies were adapted to pentyl acetate and tested for pentyl acetate or methyl benzoate avoidance behavior (Fig. 3B). OR67a restored flies adapted to pentyl acetate also lost their response to methyl benzoate. In contrast, the same pentyl acetate pre-exposure partially altered the pentyl acetate and methyl benzoate response in wild-type flies. Therefore methyl benzoate and pentyl acetate activate overlapping OSNs in OR67a restored flies, again supporting the notion that odorants compete for a single relevant class of functional OSNs in OR67a restored flies.
Figure 3. OR67a restored flies cross-adapt to odorants that activate OR67a neurons.
(A) Adaptation of innate odor avoidance behavior in wild-type and OR67a restored flies. Pre-exposing wild-type flies and those with restored OR67a neurons to methyl benzoate adapts methyl benzoate avoidance behavior. Flies with OR67a restored neurons, but not wild-type flies, cross-adapt to methyl benzoate, pentyl acetate and isoamyl acetate. Asterisk indicates significant difference (P<0.002, ANOVA) (B) Pre-exposure to pentyl acetate significantly adapts pentyl acetate avoidance behavior of flies with restored OR67a neurons (P<0.002, ANOVA) but does not significantly adapt wild-type flies (P>0.1, ANOVA). Pre-exposure to pentyl acetate cross-adapts methyl benzoate and 6-methyl-5-hepten-2-one avoidance in flies with OR67a restored neurons (both P<0.001, ANOVA) but not in wild-type flies (P>0.2, ANOVA). (C) Pre-exposure to isoamyl acetate cross-adapts the methyl benzoate avoidance behavior of flies with restored OR67a neurons (P<0.002, ANOVA) but does not significantly adapt wild-type flies (P>0.1, ANOVA). (D) Pre-exposure to 6-methyl-5-hepten-2-one cross-adapts pentyl acetate avoidance behavior of flies with restored OR67a neurons (P<0.002, ANOVA) but does not significantly adapt wild-type flies (P>0.1, ANOVA). Data are mean ± s.e.m.
We also used cross-adaptation to test whether odorants that can be discriminated by OR67a restored flies, activate overlapping OSNs. Indeed, Or67a restored flies displayed reciprocal cross-adaptation to methyl benzoate and isoamyl acetate (Fig. 3A and C) and to pentyl acetate and 6-methyl-5-hepten-2-one (Fig. 3B and D). In addition, we demonstrated that OR67a restored flies discriminate between methyl benzoate and pentyl acetate (Fig. S3), two other odorants that they cross-adapt to. These data present further evidence that flies can discriminate between odorants using the same, and very likely a single class of, OSNs. Importantly, a purely spatial model for odorant encoding cannot account for discrimination between two odorants that activate the same class(es) of OSNs.
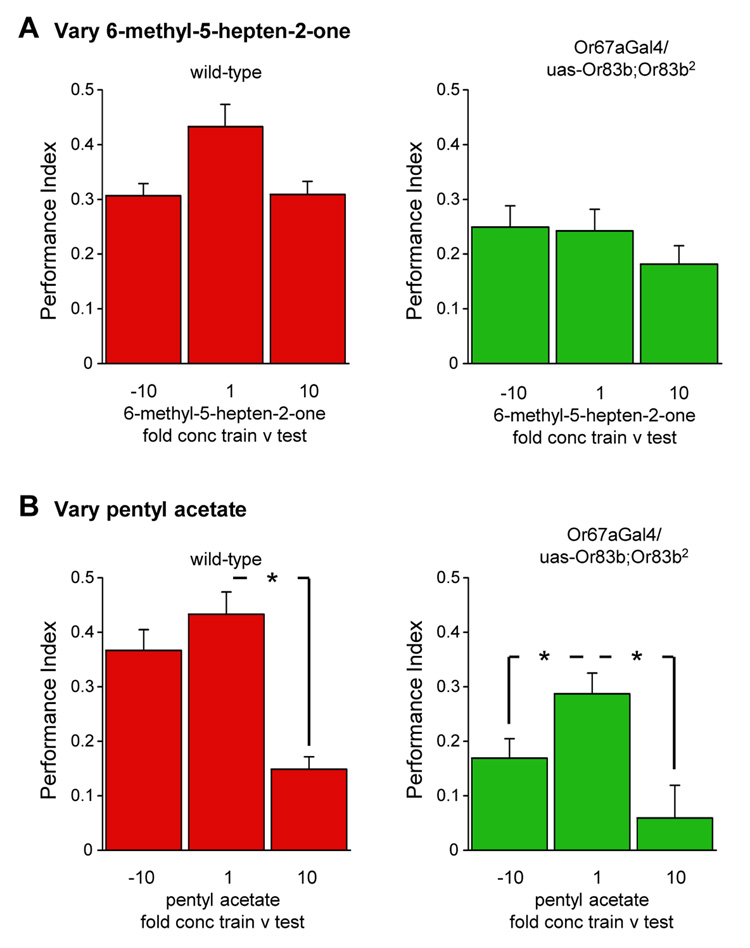
Flies with a single class of functional Or83b-expressing OSNs could discriminate between odorants using odorant intensity (relative concentration) and/or identity (chemical structure) information. We therefore tested whether OR67a restored flies only coded odorant intensity by altering the concentration of one of the two odorants between training and testing discrimination. These manipulations simultaneously changed absolute concentration of one of the odorants and the relationship between odorants. We used pentyl acetate and 6-methyl-5-hepten-2-one because the odor-evoked firing rate of Or67a-expressing OSNs to these odorants has been reported to vary between 10−2 and 10−4 dilutions (11). We first trained flies with either 10−2, 10−3 or 10−4 dilutions of 6-methyl-5-hepten-2-one versus a constant 10−3 dilution of pentyl acetate and tested all groups for discrimination between 10−3 6-methyl-5-hepten-2-one versus 10−3 pentyl acetate (Fig. 4A). Learned discrimination scores varied little for wild-type and OR67a rescued flies with changing 6-methyl-5-hepten-2-one concentration demonstrating that both wild-type and OR67a rescued flies identify 6-methyl-5-hepten-2-one, despite a change in absolute and relative odorant intensity. We similarly manipulated pentyl acetate concentration between training and testing. In this case, learned discrimination in wild-type and OR67a restored flies was robust when training concentration was lower, or the same, as that at test (Fig. 4B). Both wild-type and OR67a restored flies performed most poorly when training concentration was higher than that at test. Since flies with restored OR67a neurons distinguish the appropriate odorant across changing concentration, they cannot be only coding absolute odorant intensity. Furthermore, since our experimental design also changed relative odorant intensity between training and testing, the flies do not utilize this parameter to discriminate odorants. Instead, these data suggest flies with restored OR67a neurons encode odorant identity.
Figure 4. Or83b2 flies with functional OR67a neurons discriminate odorants across changing concentration.
(A) Wild-type flies and OR67a restored flies were trained with 6-methyl-5-hepten-2-one concentrations that were 10X less, the same or 10X more than they were tested with, while pentyl acetate concentrations were kept constant. (B) Wild-type flies and those with restored OR67a neurons were trained with pentyl acetate concentrations that were 10X less, the same or 10X more than they were tested with, while 6-methyl-5-hepten-2-one concentrations were kept constant. Asterisks indicate significant difference (all P<0.01, ANOVA). Data are mean ± s.e.m.
In conclusion, multiple classes of OSNs are not required for flies to discriminate odorants. Although flies without functional Or83b-expressing neurons cannot learn to discriminate between a number of chemically distinct odorants, providing a single class of Or83b-expressing OSNs restores learned discrimination between two odorants that activate that particular OSN class. These flies cross-adapt to odorants that activate the restored OSNs demonstrating that the relevant OSNs are the same, thus challenging a requirement for discrete spatial codes for odorants in the antennal lobe. As expected, flies with one class of Or83b-expressing OSNs have limitations and can apparently only encode one odorant that activates the appropriate receptor at a time. These data suggest a benefit of having multiple classes of OSNs is the ability to identify certain odorants present within a more complex milieu. Importantly, Or83b2 mutant flies with one functional class of Or83b-expressing OSNs choose appropriately between two odorants even though the absolute and relative concentration is changed between training and testing, implying that they encode odorant identity, and do not only rely on encoding odorant intensity.
Finding that distinct combinatorial spatial patterns of OSN activation in the antennal lobe are not essential to represent odorant information implies an important role for odorant-evoked temporal dynamics. Previous studies in insects and vertebrates have documented considerable temporal complexity in odor-evoked activity at successive layers of the olfactory system (12, 25–32) but few have investigated the behavioral relevance (12). Recent work has shown that excitatory and inhibitory lateral connectivity in the Drosophila antennal lobe can shape projection neuron responses (21, 33–37), therefore we expect different temporal signals in the same OSNs to generate distinct temporal, and perhaps spatial, patterns of projection neuron activity. However, since flies with a single functional class of Or83b-expressing OSNs lack the lateral input driven by additional classes of OSN, it will be important to determine how lateral connectivity within the antennal lobe contributes to odorant discrimination in Drosophila.
Supplementary Material
Acknowledgements
We thank Uwe Homberg for valuable discussion and Benjamin Leung, Michael Krashes, Alex Keene and Jena Pitman for discussion and critical comments on the manuscript. Supported by National Institute of Mental Health grant MH09883.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 2.Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 3.Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 4.Gao Q, Yuan B, Chess A. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurosci. 2000;3:780–785. doi: 10.1038/77680. [DOI] [PubMed] [Google Scholar]
- 5.Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- 6.Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 7.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 8.Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- 9.Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 10.Ng M, Roorda RD, Lima SQ, Zemelman BV, Morcillo P, Miesenbock G. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–474. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 11.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 12.Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- 13.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 15.Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 16.Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 18.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci U S A. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao CA, Ignell R, Carlson JR. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J Neurosci. 2005;25:8359–8367. doi: 10.1523/JNEUROSCI.2432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fishilevich E, Domingos AI, Asahina K, Naef F, Vosshall LB, Louis M. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr Biol. 2005;15:2086–2096. doi: 10.1016/j.cub.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Louis M, Huber T, Benton R, Sakmar TP, Vosshall LB. Bilateral olfactory sensory input enhances chemotaxis behavior. Nat Neurosci. 2008;11:187–199. doi: 10.1038/nn2031. [DOI] [PubMed] [Google Scholar]
- 22.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol [A] 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 23.Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2007;54:89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldman AL, Van der Goes van Naters W, Lessing D, Warr CG, Carlson JR. Coexpression of two functional odor receptors in one neuron. Neuron. 2005;45:661–666. doi: 10.1016/j.neuron.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 25.Wehr M, Laurent G. Odour encoding by temporal sequences of firing in oscillating neural assemblies. Nature. 1996;384:162–166. doi: 10.1038/384162a0. [DOI] [PubMed] [Google Scholar]
- 26.Friedrich RW, Laurent G. Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science. 2001;291:889–894. doi: 10.1126/science.291.5505.889. [DOI] [PubMed] [Google Scholar]
- 27.Muller D, Abel R, Brandt R, Zockler M, Menzel R. Differential parallel processing of olfactory information in the honeybee, Apis mellifera L. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002;188:359–370. doi: 10.1007/s00359-002-0310-1. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- 29.Lei H, Christensen TA, Hildebrand JG. Spatial and temporal organization of ensemble representations for different odor classes in the moth antennal lobe. J Neurosci. 2004;24:11108–11119. doi: 10.1523/JNEUROSCI.3677-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedrich RW, Habermann CJ, Laurent G. Multiplexing using synchrony in the zebrafish olfactory bulb. Nat Neurosci. 2004;7:862–871. doi: 10.1038/nn1292. [DOI] [PubMed] [Google Scholar]
- 31.Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- 32.Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron. 2008;57:586–598. doi: 10.1016/j.neuron.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenbock G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kazama H, Wilson RI. Homeostatic matching and nonlinear amplification at identified central synapses. Neuron. 2008;58:401–413. doi: 10.1016/j.neuron.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Root CM, Masuyama K, Green DS, Enell LE, Nässel DR, Lee CH, Wang JW. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Estes PS, Ho GL, Narayanan R, Ramaswami M. Synaptic localization and restricted diffusion of a Drosophila neuronal synaptobrevin--green fluorescent protein chimera in vivo. J Neurogenet. 2000;13:233–255. doi: 10.3109/01677060009084496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.