Abstract
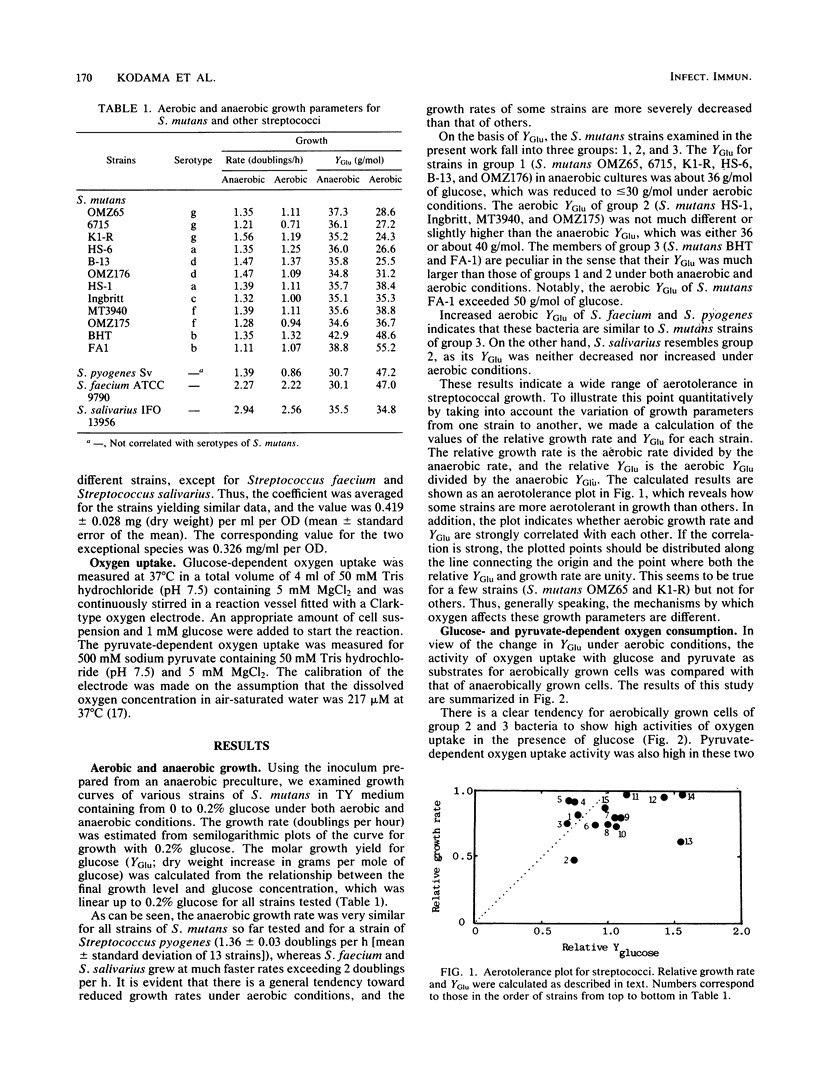
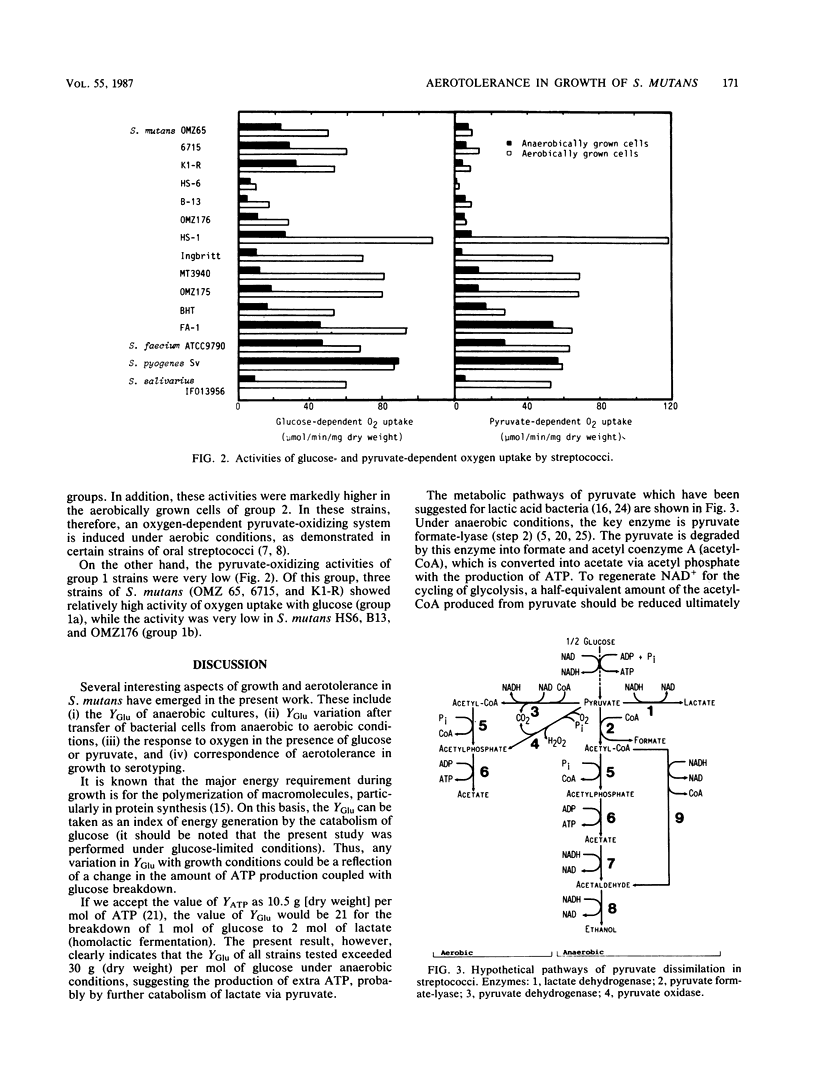
Aerotolerance in the growth of Streptococcus mutans and related streptococci was examined under glucose-limited conditions. The growth rate of all strains tested was more or less retarded when they were transferred from anaerobic to aerobic conditions. As for growth yield, however, some strains (group 1) showed reduced values for the increment of cellular dry weight change (in grams per mole of glucose), whereas others showed either unaltered (group 2) or increased (group 3) yields. The characteristic feature of strains in groups 2 and 3 was their high activity of both glucose- and pyruvate-dependent oxygen uptake when strains were grown under aerobic conditions. By contrast, the activity of pyruvate-dependent oxygen uptake by group 1 strains was negligibly low, whether grown under aerobic or anaerobic conditions. Some strains (group 1a) consumed oxygen in the presence of glucose at a much faster rate than others did (group 1b). There seems to be a good correspondence of these aerotolerance groupings to those based on serotypes of S. mutans. Thus, the groups 1a, 1b, 2, and 3 correspond to serotypes g, a+d, c+f, and b, respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbe K., Takahashi S., Yamada T. Involvement of oxygen-sensitive pyruvate formate-lyase in mixed-acid fermentation by Streptococcus mutans under strictly anaerobic conditions. J Bacteriol. 1982 Oct;152(1):175–182. doi: 10.1128/jb.152.1.175-182.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Britton L., Malinowski D. P., Fridovich I. Superoxide dismutase and oxygen metabolism in Streptococcus faecalis and comparisons with other organisms. J Bacteriol. 1978 Apr;134(1):229–236. doi: 10.1128/jb.134.1.229-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Griffith C. J. Fermentation products and bacterial yields in glucose-limited and nitrogen-limited cultures of streptococci. Arch Oral Biol. 1974 Dec;19(12):1105–1109. doi: 10.1016/0003-9969(74)90238-6. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Iwami Y., Yamada T. Hydrogen peroxide excretion by oral streptococci and effect of lactoperoxidase-thiocyanate-hydrogen peroxide. Infect Immun. 1983 Apr;40(1):70–80. doi: 10.1128/iai.40.1.70-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Kujala U., Edlund M. B. Pyruvate dehydrogenase activity in Streptococcus mutans. Infect Immun. 1985 Sep;49(3):674–678. doi: 10.1128/iai.49.3.674-678.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J. Presence of various types of non-haemolytic streptococci in dental plaque and in other sites of the oral cavity in man. Odontol Revy. 1967;18(1):55–74. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973 Jun;114(3):1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Tai S., Slade H. D. Serotype-dependent inhibition of glucan synthesis and cell adherence of Streptococcus mutans by antibody against glucosyltransferase of serotype e S. mutans. Microbiol Immunol. 1979;23(2):61–70. doi: 10.1111/j.1348-0421.1979.tb00442.x. [DOI] [PubMed] [Google Scholar]
- Higuchi M. The effect of oxygen on the growth and mannitol fermentation of Streptococcus mutants. J Gen Microbiol. 1984 Jul;130(7):1819–1826. doi: 10.1099/00221287-130-7-1819. [DOI] [PubMed] [Google Scholar]
- Kandler O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek. 1983 Sep;49(3):209–224. doi: 10.1007/BF00399499. [DOI] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Abbe K., Yamada T. Purification of pyruvate formate-lyase from Streptococcus mutans and its regulatory properties. J Bacteriol. 1982 Mar;149(3):1034–1040. doi: 10.1128/jb.149.3.1034-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Neijssel O. M. The status of YATP and maintenance energy as biologically interpretable phenomena. Annu Rev Microbiol. 1984;38:459–486. doi: 10.1146/annurev.mi.38.100184.002331. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Pera K. A. Oxygen metabolism of Streptococcus mutans: uptake of oxygen and release of superoxide and hydrogen peroxide. J Bacteriol. 1983 Jun;154(3):1236–1244. doi: 10.1128/jb.154.3.1236-1244.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance P. G., Keele B. B., Jr, Rajagopalan K. V. Superoxide dismutase from Streptococcus mutans. Isolation and characterization of two forms of the enzyme. J Biol Chem. 1972 Aug 10;247(15):4782–4786. [PubMed] [Google Scholar]
- Yamada T., Takahashi-Abbe S., Abbe K. Effects of oxygen on pyruvate formate-lyase in situ and sugar metabolism of Streptococcus mutans and Streptococcus sanguis. Infect Immun. 1985 Jan;47(1):129–134. doi: 10.1128/iai.47.1.129-134.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINNER D. D., JABLON J. M., ARAN A. P., SASLAW M. S. EXPERIMENTAL CARIES INDUCED IN ANIMALS BY STREPTOCOCCI OF HUMAN ORIGIN. Proc Soc Exp Biol Med. 1965 Mar;118:766–770. doi: 10.3181/00379727-118-29964. [DOI] [PubMed] [Google Scholar]



