Abstract
In conjunction with an AOAC Presidential Task Force on Dietary Supplements, a method was validated for measurement of 3 plant sterols (phytosterols) in saw palmetto raw materials, extracts, and dietary supplements. AOAC Official Method 994.10, “Cholesterol in Foods,” was modified for purposes of this validation. Test samples were saponified at high temperature with ethanolic potassium hydroxide solution. The unsaponifiable fraction containing phytosterols (campesterol, stigmasterol, and beta-sitosterol) was extracted with toluene. Phytosterols were derivatized to trimethylsilyl ethers and then quantified by gas Chromatography with a hydrogen flame ionization detector. The presence of the phytosterols was detected at concentrations greater than or equal to 1.00 mg/100 g based on 2–3 g of sample. The standard curve range for this assay was 0.00250 to 0.200 mg/mL. The calibration curves for all phytosterols had correlation coefficients greater than or equal to 0.995. Precision studies produced relative standard deviation values of 1.52 to 7.27% for campesterol, 1.62 to 6.48% for stigmasterol, and 1.39 to 10.5% for beta-sitosterol. Recoveries for samples fortified at 100% of the inherent values averaged 98.5 to 105% for campesterol, 95.0 to 108% for stigmasterol, and 85.0 to 103% for beta-sitosterol.
Saw palmetto (Serenoa repens) is a native plant of North America, most commonly found in Florida. The berries of the saw palmetto plant have been used for medicinal purposes for centuries. Both berry raw material and extract are found in dietary supplements and are most commonly used to treat symptoms related to benign prostatic hyperplasia (BPH). In conjunction with an AOAC Task Force on Dietary Supplements, a method was validated for measurement of 3 plant sterols (phytosterols) in saw palmetto raw materials, extracts, and dietary supplements. AOAC Official Method 994.10, “Cholesterol in Foods,” was modified for purposes of this validation. The phytosterols campesterol, stigmasterol, and beta-sitosterol are chemically very similar to cholesterol. They extract and chromatograph very comparably to cholesterol. Due to the similarities of these compounds, a number of laboratories have successfully extended AOAC Official Method 994.10 to measure not only cholesterol, but these phytosterols as well. The following test materials were used in this validation: powdered saw palmetto berry, saw palmetto (Serenoa repens) dried fruit CO2 extracts, saw palmetto 45% powdered extract, ProstActive® Once Daily, Prostasan™ Prostrate Capsules, Liquid Herbal Extract: saw palmetto, alcohol-free saw palmetto, ProstActive Plus saw palmetto combined with nettle root extract, pygeum and saw palmetto standardized herbal extracts, and saw palmetto pygeum lycopene complex tablets.
Experimental
Apparatus
Centrifuge tubes.—Pyrex No. 13, 15 mL, silanized. Tubes were silanized using a commercial silinizing reagent (Surfasil™ Siliconizing Fluid, No. 42801, Pierce Chemical Co., Rockford, IL). Manufacturer’s instructions were followed for silanization. Before each reuse, tubes were cleaned with water, ethanol, hexane, and acetone and oven-dried at 100°C.
Plastic stoppers.
Gas Chromatography (GC).—With 2-ramp oven temperature programming (Hewlett Packard Model 5890A is suitable) and hydrogen flame ionization detector (FID; Agilent Technologies, Palo Alto, CA).
Column.—Capillary, split-mode, 25 m × 0.32 mm × 0.17 μm film thickness, cross-linked 5% phenyl-methyl silicone or methyl silicone gum (e.g., Hewlett Packard No. HP-5, Ultra 2, or HP-1; Agilent Technologies).
Deactivated split inlet liner filled with glass wool.
Rotary evaporator.—With glass condenser flask between the concentration flask and metal shaft.
Magnetic stirrer-hot plate.—With variable speed and heat controls; Model PC320, Corning (Corning, NY).
Micropipets.—Delivering 100 and 200 μL.
Vortex mixer.—Model M37615, Type 37600 Mixer: Thermolyne (Dubuque, IA).
Balance.—Analytical, weighing to 0.0001 g.
Centrifuge.—Model K; International Equipment Co. (Needham Heights, MA).
(Note: Equivalent apparatus may be substituted. All glassware is class A.)
Reagents
Dimethylformamide (DMF).—Spectrophotometry grade; J.T. Baker (Phillipsburg, NJ).
Hexamethyldisilane (HMDS).—Derivatization grade, No. 3-3011; Supelco (Bellefonte, PA).
Potassium hydroxide solutions.—50% KOH (w/w), 1 M KOH, and 0.5 M KOH. (1) 50% KOH (w/w).—500 g KOH (Certified A.C.S. Pellets; Fisher Scientific, Fairlawn, NJ) was dissolved in 500 g water; (2) 1 M KOH.—56 g KOH was dissolved in ca 800 mL water with cooling and diluted to the mark in a 1 L volumetric flask; (3) 0.5 M KOH.—One part of 1 M KOH solution was diluted with 1 part water.
Trimethylchlorosilane (TMCS).—No. 88530; Pierce Chemical Co.
Toluene.—Distilled in glass, HPLC grade (Fisher Scientific).
Sodium sulfate.—Anhydrous, certified A.C.S. grade (Fisher Scientific).
Glass wool.—Fiberglass, 8 micron (Corning).
Acetone.—HPLC grade (Fisher Scientific).
95% Ethanol.—Remet Alcohol Division (La Mirada, CA).
n-Heptane.—HPLC grade, No. 34873-4L: Sigma-Aldrich Co. (St. Louis, MO).
Water.—Prepared with a Milli-Q® purification system; Millipore Corp. (Bedford, MA).
(Note: Equivalent reagents may be substituted.)
Reference Standards
5α-Cholestane internal standard (IS) solution.—No. C-8003; Sigma-Aldrich Co.
Campesterol (CAS No. 474-62-4).—No. 03072-641, >95% pure; Chromadex (Santa Ana, CA).
Stigmasterol (CASNo. 83-48-7).—No. S-2424, >95% pure; Sigma-Aldrich Co.
beta-Sitosterol (CAS No. 83-46-5).—No. S-9889, >98% pure; Sigma-Aldrich Co.
(Note: Equivalent reference materials may be substituted.)
Preparation of Standards
(a) IS
Ca 0.0100 g 5α-cholestane was weighed into a 100 mL volumetric flask and diluted to mark with n-heptane to make a solution with a concentration of 0.100 mg/mL. Solutions were stored protected from light at room temperature when not in use and prepared fresh as needed.
(b) GC calibration standards
In a 100 mL volumetric flask, campesterol, Stigmasterol, and beta-sitosterol were accurately weighed to the nearest 0.1 mg so that the concentration was ca 0.200 mg/mL after dilution to volume with DMF. The solution was mixed by inverting the flask numerous times. Then the standard was diluted as necessary to obtain 5 additional standards ranging in concentration from ca 0.00250 to 0.200 mg/mL. Solutions were stored protected from light at room temperature when not in use. Standards were shown to be stable for at least 1 year.
(c) Mixed standards
Prepared by appropriate dilution to the concentrations listed in Table 1.
Table 1.
Preparation of mixed standards
| Standard No. | Intermediate volume, mL | Final volume, mL | Concentration, mg/mL |
|---|---|---|---|
| 1 | NAa | 50 | 0.200 |
| 2 | 25 | 50 | 0.100 |
| 3 | 25 | 50 | 0.0500 |
| 4 | 10 | 50 | 0.0100 |
| 5 | 25 | 50 | 0.00500 |
| 6 | 25 | 50 | 0.00250 |
NA = Not applicable.
Preparation of Samples
(a) Saponification
An appropriate amount of homogenous product was weighed (2.00 to 3.00 g to nearest 0.01 g) into a 250 or 300 mL Erlenmeyer flask. Amagnetic stir bar was placed into the flask, and 40 mL of 95% ethanol and 8 mL of 50% KOH solution were added to the flask.
The flask was placed on a magnetic stirrer hot plate with a condenser attached, and the contents were refluxed for 80 ± 10 min. To ensure complete saponification, test portions were occasionally visually checked, and any clumps were dispersed with a glass rod or by adding KOH solution to the test portion while stirring.
After refluxing, the heat was turned off and 60 mL of 95% ethanol was added through the top of the condenser while stirring the solution. The 95% ethanol was added carefully to avoid splashing of alcohol from the top of the condenser. After being stirred for ca 15 min, the flask was removed from the condenser and closed with a stopper, and the solution was cooled to room temperature. Test solution was stable for up to 1 week if tightly sealed.
(b) Extraction
100 mL toluene (V1) was added to the saponified test portion while stirring on a magnetic stirrer. The solution was poured into a 500 mL separatory funnel without rinsing, and 110 mL of 1 M KOH solution was added and shaken vigorously for at least 20 s. The layers were allowed to separate and the aqueous lower layer, which was turbid, was discarded. KOH solution (40 mL of 0.5 M) was added to the separatory funnel. The funnel was inverted, and the contents were gently swirled for at least 10 s. The aqueous lower layer was discarded.
The toluene layer was washed with 40 mL water by gently rotating the separatory funnel. The layers were allowed to separate, and the aqueous phase was discarded. The water wash was repeated at least 3 times, shaken more vigorously each time. If emulsification occurred, a small amount of 95% ethanol was added, the contents of funnel were swirled, the layers were allowed to separate, and the water washes were continued. After the final wash, the toluene layer was crystal clear.
The toluene layer was poured from the top of the separatory funnel through a glass funnel containing a plug of glass wool and ca 20 g Na2SO4 into a 125 mL Erlenmeyer flask containing ca 2 g Na2SO4. The flask was stoppered, and the contents were swirled. The mixture was allowed to stand for at least 15 min. Test solutions were held up to 24 h if tightly sealed.
The toluene extract (V2; 25 mL) was pipetted into a 125 mL flat-bottom boiling flask, and the contents were evaporated to dryness on a rotary evaporator set at 55°C. Ca 3 mL acetone was added, and the contents were evaporated to dryness again. The residue was dissolved in 3.0 mL DMF (V3). The final concentration of campesterol, stigmasterol, and beta-sitosterol in DMF needed to be within range of working standard solutions. If, after quantification by GC, the test portion concentration fell outside of the standard curve, the amount of V2 evaporated was changed so that the final concentration of campesterol, stigmasterol, and beta-sitosterol in DMF fell within the range of the standards.
(c) Derivatization
Aliquots (1.0 mL) of working standard solutions and test solution were pipetted into separate 15 mL centrifuge tubes. To each tube, 0.2 mL HMDS and 0.1 mL TMCS were added. The tubes were stoppered and shaken vigorously on a Vortex mixer for 30 s. The solution was allowed to stand undisturbed for 15 min. To each tube, 1.0 mL 5α-cholestane IS solution and 10 mL water were added. The tubes were stoppered, shaken vigorously for 30 s, and centrifuged for ca 2 min.
A sufficient portion of heptane (upper) layer was transferred to an injection vial, with ensurance that no aqueous layer was transferred. Derivatized standards and test solutions were analyzed within 24 h.
Determination
Standards and samples were analyzed using the instrumental conditions shown in Table 2. At least one set of GC calibration standards was injected at the beginning of the run and one at the end of the run. A standard was run in between each sample to avoid possible analyte carryover. When using standards that had carryover in the standard curve, standard peaks were integrated from valley to valley.
Table 2.
Gas chromatography conditions
| Column | Capillary column, split mode (25 m × 0.32 mm) cross-linked 5% phenyl-methyl silicone or methyl silicone gum |
| Film thickness | 0.17 μm |
| Detector | Hydrogen flame ionization detector (FID) |
|
| |
| Temperatures
| |
| Column | 190°C, hold 2 min; increase 20°/min to 230°C, hold 3 min; increase 40°/min to 255°C, hold 25 min |
| Injector | 250°C |
| Detector | 300°C |
|
| |
| Flow rates
| |
| Carrier | 2 mL/min, split vent ca 30 mL/min, purge vent ca 3 mL/min helium |
| Makeup | 20 mL/min helium |
| Hydrogen | 35 mL/min |
| Air | 280 mL/min |
| Injection volume | 1 μL |
Calculations
A standard calibration curve was generated by using the ratio of the analyte peak area versus the area of the internal standard peak for each concentration level. A calibration curve was produced for each analyte. High-level test solutions were diluted to fall within the standard range. Weighting (1/x) was necessary to obtain acceptable linearity at lower standard concentrations:
where y = relative peak area (area of analyte/area of IS), m = slope of the line generated by a standard curve, x = concentration of analyte found (mg/mL), and b = y-intercept of the line generated by the standard curve.
Grams of test portion/mL derivatized was calculated as follows:
where W1 = weight of test portion (g), V1 = volume of toluene used in extraction (mL), V2 = aliquot of extract taken to dryness (mL), and V3 = volume of DMF used to dissolve residue (mL).
The content of each phytosterol component in test portions was calculated as follows:
Results and Discussion
The limit of quantification (LOQ) for this method was found to be 1.00 mg/100 g. Calibration curves were generated with each data set, on each day during the course of this validation. The calibration range encompassed the expected concentration of each extracted and diluted test material range. Calibration standards (consisting of 6 concentration levels ranging from 0.00250 to 0.200 mg/mL) were analyzed at a minimum before and after each sample set with another set interspersed throughout the run. Each test sample was placed between the interspersed standards to avoid possible carryover. The response ratio of the analyte (peak area of analyte/peak area of IS) versus concentration was used to construct the calibration curve using a 1/x weighted linear regression method. The calibration curves had correlation coefficients (r) of greater than or equal to 0.995.
Precision of the method was evaluated by having 6 replicates of each test material analyzed by the same analyst on 2 separate days. If the initial sample extract fell above the highest standard of the curve, as indicated above, the extract was diluted to fit on the curve. For the majority of the precision results, the RSDrS were either below or within the target RSDr ranges with a few exceeding this range. The HorRat values were calculated, and all precision results (Day 1, Day 2, and Days 1 and 2 combined) for all test materials had values below 0.3 or between 0.3 and 1.3. Based on the acceptable HorRat values for this single laboratory validation (SLV), the collaborative study should be successful. The target RSDr and HorRat values were calculated as follows (1):
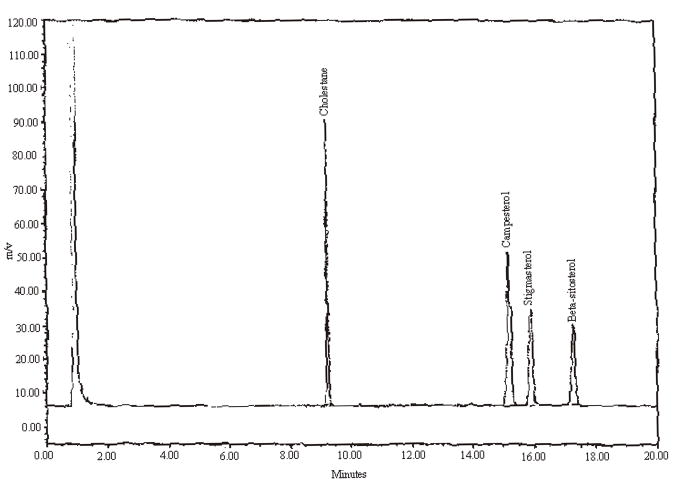
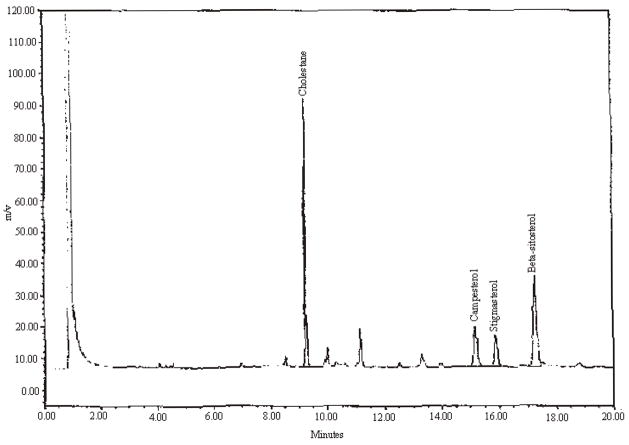
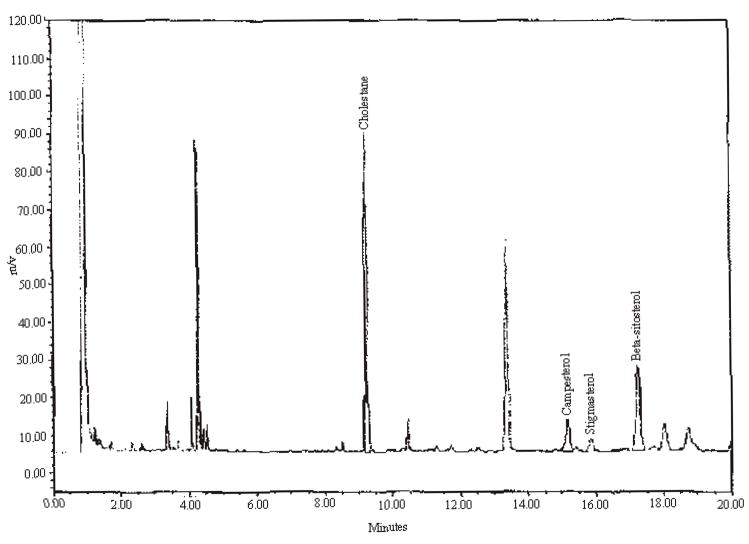
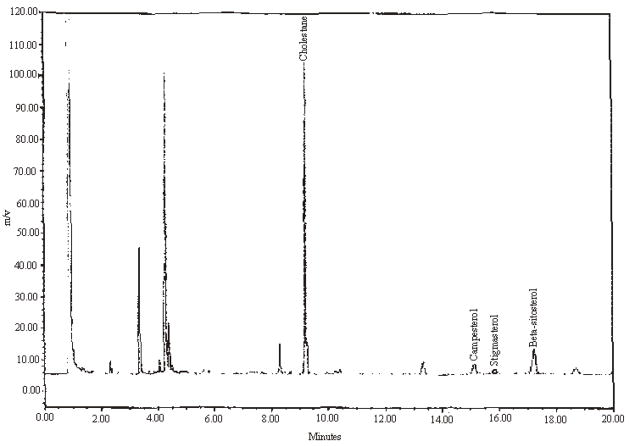
Precision results are presented in Table 3. Example chromatograms of a mixed standard and test materials with varying amounts of campesterol, stigmasterol, and beta-sitosterol are presented in Figures 1 through 4.
Table 3.
Precision data
| Day 1 precision mg/100 g
|
Day 2 precision mg/100 g
|
Days 1 and 2 precision combined, mg/100 g
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Test material identification/replicates | Campesterol | Stigmasterol | beta-Sitosterol | Campesterol | Stigmasterol | beta-Sitosterol | Campesterol | Stigmasterol | beta-Sitosterol |
| Saw palmetto 45% powdered extract
| |||||||||
| 1 | 34.9 | 16.8 | 114 | 34.8 | 17.4 | 105 | |||
| 2 | 35.2 | 16.7 | 116 | 34.5 | 16.9 | 101 | |||
| 3 | 34.5 | 16.8 | 115 | 35.2 | 17.5 | 103 | |||
| 4 | 36.7 | 17.2 | 116 | 34.7 | 17.6 | 102 | |||
| 5 | 34.8 | 16.5 | 115 | 35.3 | 17.4 | 103 | |||
| 6 | 34.6 | 16.7 | 114 | 34.7 | 16.8 | 102 | |||
| Mean | 35.1 | 16.8 | 115 | 34.9 | 17.3 | 103 | 35.0 | 17.0 | 109 |
| SDa | 0.813 | 0.232 | 0.894 | 0.314 | 0.333 | 1.37 | 0.602 | 0.372 | 6.53 |
| RSDr, %b | 2.32 | 1.38 | 0.777 | 0.900 | 1.92 | 1.33 | 1.72 | 2.19 | 5.99 |
| Target RSDr lowb | 3 | 4 | 3 | 3 | 4 | 3 | 3 | 4 | 3 |
| Target RSDr highc | 4 | 5 | 4 | 4 | 5 | 4 | 4 | 5 | 4 |
| PRSDRc | 6.60 | 7.37 | 5.52 | 6.60 | 7.33 | 5.61 | 6.60 | 7.35 | 5.56 |
| HorRatd | 0.4 | 0.2 | 0.1 | 0.1 | 0.3 | 0.2 | 0.3 | 0.3 | 1.0 |
|
| |||||||||
| Alcohol-free saw palmetto
| |||||||||
| 1 | 2.50 | 1.13 | 7.34 | 2.37 | 1.27 | 6.06 | |||
| 2 | 2.52 | 1.19 | 7.67 | 2.50 | 1.35 | 6.37 | |||
| 3 | 2.57 | 1.23 | 7.61 | 2.51 | 1.35 | 6.39 | |||
| 4 | 2.61 | 1.24 | 7.81 | 2.44 | 1.34 | 6.28 | |||
| 5 | 2.60 | 1.22 | 7.70 | 2.37 | 1.26 | 6.10 | |||
| 6 | 2.52 | 1.20 | 7.53 | 2.50 | 1.33 | 6.26 | |||
| Mean | 2.55 | 1.20 | 7.61 | 2.45 | 1.32 | 6.24 | 2.50 | 1.26 | 6.93 |
| SD | 0.0463 | 0.0397 | 0.162 | 0.0655 | 0.0408 | 0.137 | 0.0770 | 0.0713 | 0.728 |
| RSDr, % | 1.82 | 3.31 | 2.13 | 2.67 | 3.09 | 2.20 | 3.08 | 5.66 | 10.5 |
| Target RSDr low | 5 | 5 | 4 | 5 | 5 | 4 | 5 | 5 | 4 |
| Target RSDr high | 7 | 7 | 6 | 7 | 7 | 6 | 7 | 7 | 6 |
| PRSDR | 9.77 | 10.9 | 8.30 | 9.83 | 10.8 | 8.55 | 9.80 | 10.9 | 8.41 |
| HorRat | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.5 | 1.0 |
|
| |||||||||
| Liquid Herbal Extract: saw palmetto
| |||||||||
| 1 | 1.98 | 1.34 | 7.17 | 1.85 | 1.47 | 7.50 | |||
| 2 | 2.04 | 1.34 | 7.37 | 1.83 | 1.52 | 7.84 | |||
| 3 | 1.98 | 1.35 | 7.23 | 1.82 | 1.57 | 8.00 | |||
| 4 | 1.95 | 1.33 | 7.11 | 1.88 | 1.44 | 7.77 | |||
| 5 | 1.97 | 1.40 | 7.33 | 1.87 | 1.50 | 7.80 | |||
| 6 | 2.29 | 1.54 | 7.78 | 1.84 | 1.49 | 7.90 | |||
| Mean | 2.04 | 1.38 | 7.33 | 1.85 | 1.50 | 7.80 | 1.94 | 1.44 | 7.57 |
| SD | 0.128 | 0.0807 | 0.240 | 0.0232 | 0.0445 | 0.169 | 0.131 | 0.0864 | 0.315 |
| RSDr, % | 6.27 | 5.85 | 3.27 | 1.25 | 2.97 | 2.17 | 6.75 | 6.00 | 4.16 |
| Target RSDr low | 5 | 5 | 4 | 5 | 5 | 4 | 5 | 5 | 4 |
| Target RSDr high | 7 | 7 | 6 | 7 | 7 | 6 | 7 | 7 | 6 |
| PRSDR | 10.1 | 10.7 | 8.34 | 10.3 | 10.6 | 8.26 | 10.2 | 10.6 | 8.30 |
| HorRat | 0.6 | 0.5 | 0.4 | 0.1 | 0.3 | 0.03 | 0.7 | 0.6 | 0.5 |
|
| |||||||||
| Powdered saw palmetto berry
| |||||||||
| 1 | 5.28 | 3.93 | 30.9 | 5.01 | 3.95 | 33.4 | |||
| 2 | 6.12 | 3.90 | 30.3 | 4.99 | 3.91 | 33.6 | |||
| 3 | 5.30 | 3.82 | 30.4 | 5.01 | 4.05 | 33.9 | |||
| 4 | 5.89 | 4.50 | 31.8 | 4.97 | 3.82 | 33.3 | |||
| 5 | 5.67 | 4.28 | 30.4 | 5.33 | 4.18 | 34.3 | |||
| 6 | 5.63 | 4.01 | 30.9 | 5.05 | 3.93 | 33.6 | |||
| Mean | 5.65 | 4.07 | 30.8 | 5.06 | 3.97 | 33.7 | 5.35 | 4.02 | 32.2 |
| SD | 0.328 | 0.262 | 0.564 | 0.135 | 0.125 | 0.366 | 0.389 | 0.203 | 1.58 |
| RSDr, % | 5.81 | 6.44 | 1.83 | 2.67 | 3.15 | 1.09 | 7.27 | 5.05 | 4.91 |
| Target RSDr low | 4 | 5 | 3 | 4 | 5 | 3 | 4 | 5 | 3 |
| Target RSDr high | 6 | 6 | 4 | 6 | 6 | 4 | 6 | 6 | 4 |
| PRSDR | 8.67 | 9.11 | 6.73 | 8.82 | 9.15 | 6.64 | 8.75 | 9.13 | 6.68 |
| HorRat | 0.7 | 0.7 | 0.3 | 0.3 | 0.3 | 0.2 | 0.8 | 0.6 | 0.7 |
|
| |||||||||
| ProstActive Once Daily
| |||||||||
| 1 | 38.3 | 19.9 | 117 | 35.6 | 18.0 | 101 | |||
| 2 | 35.3 | 18.5 | 109 | 35.0 | 18.1 | 100 | |||
| 3 | 36.7 | 19.9 | 115 | 36.6 | 19.5 | 103 | |||
| 4 | 34.8 | 18.6 | 108 | 36.3 | 19.2 | 102 | |||
| 5 | 39.6 | 22.6 | 119 | 36.2 | 19.3 | 102 | |||
| 6 | 35.3 | 18.2 | 109 | 36.0 | 19.3 | 100 | |||
| Mean | 36.7 | 19.6 | 113 | 36.0 | 18.9 | 101 | 36.3 | 19.3 | 107 |
| SD | 1.92 | 1.63 | 4.75 | 0.572 | 0.666 | 1.21 | 1.40 | 1.25 | 6.86 |
| RSDr, % | 5.23 | 8.32 | 4.20 | 1.59 | 3.52 | 1.20 | 3.86 | 6.48 | 6.41 |
| Target RSDr low | 3 | 4 | 3 | 3 | 4 | 3 | 3 | 4 | 3 |
| Target RSDr high | 4 | 5 | 4 | 4 | 5 | 4 | 4 | 5 | 4 |
| PRSDR | 6.55 | 7.20 | 5.53 | 6.57 | 7.24 | 5.63 | 6.56 | 7.21 | 5.58 |
| HorRat | 0.8 | 1.0 | 0.8 | 0.2 | 0.5 | 0.2 | 0.6 | 0.9 | 1.0 |
|
| |||||||||
| ProstActive Plus saw palmetto combined with nettle root extract
| |||||||||
| 1 | 31.8 | 10.4 | 129 | 34.4 | 9.42 | 117 | |||
| 2 | 31.5 | 9.25 | 128 | 36.0 | 10.2 | 119 | |||
| 3 | 32.0 | 10.1 | 130 | 35.6 | 10.1 | 119 | |||
| 4 | 31.3 | 9.17 | 121 | 34.5 | 9.90 | 116 | |||
| 5 | 30.3 | 9.37 | 119 | 36.5 | 10.5 | 120 | |||
| 6 | 32.3 | 10.5 | 126 | 34.1 | 10.0 | 115 | |||
| Mean | 31.5 | 9.80 | 126 | 35.2 | 10.0 | 118 | 33.4 | 9.91 | 1.22 |
| SD | 0.700 | 0.604 | 4.51 | 0.983 | 0.359 | 1.97 | 2.07 | 0.488 | 5.26 |
| RSDr, % | 2.22 | 6.16 | 3.58 | 2.79 | 3.59 | 1.67 | 6.20 | 4.92 | 4.31 |
| Target RSDr low | 3 | 4 | 3 | 3 | 4 | 3 | 3 | 4 | 3 |
| Target RSDr high | 4 | 5 | 4 | 4 | 5 | 4 | 4 | 5 | 4 |
| PRSDR | 6.70 | 7.99 | 5.44 | 6.59 | 7.96 | 5.50 | 6.64 | 7.97 | 5.47 |
| HorRat | 0.3 | 0.8 | 0.7 | 0.4 | 0.5 | 0.3 | 0.9 | 0.6 | 0.8 |
|
| |||||||||
| Prostasan Prostrate Capsules
| |||||||||
| 1 | 39.8 | 20.2 | 116 | 40.4 | 20.7 | 108 | |||
| 2 | 39.6 | 19.3 | 117 | 40.2 | 20.8 | 108 | |||
| 3 | 37.8 | 19.6 | 113 | 40.1 | 20.4 | 110 | |||
| 4 | 38.6 | 19.6 | 117 | 39.6 | 20.3 | 107 | |||
| 5 | 37.7 | 19.4 | 115 | 41.5 | 20.4 | 108 | |||
| 6 | 39.2 | 19.9 | 118 | 39.8 | 20.8 | 108 | |||
| Mean | 38.8 | 19.7 | 116 | 40.3 | 20.6 | 108 | 39.5 | 20.1 | 112 |
| SD | 0.900 | 0.333 | 1.79 | 0.668 | 0.225 | 0.983 | 1.08 | 0.542 | 4.32 |
| RSDr, % | 2.32 | 1.69 | 1.54 | 1.66 | 1.09 | 0.910 | 2.73 | 2.7 | 3.86 |
| Target RSDr low | 3 | 4 | 3 | 3 | 4 | 3 | 3 | 4 | 3 |
| Target RSDr high | 4 | 5 | 4 | 4 | 5 | 4 | 4 | 5 | 4 |
| PRSDR | 6.50 | 7.19 | 5.51 | 6.46 | 7.14 | 5.57 | 6.48 | 7.17 | 5.54 |
| HorRat | 0.4 | 0.2 | 0.3 | 0.3 | 0.2 | 0.2 | 0.4 | 0.4 | 0.7 |
|
| |||||||||
| Pygeum and saw palmetto standardized herbal extracts
| |||||||||
| 1 | 21.5 | 8.07 | 264 | 22.3 | 8.26 | 278 | |||
| 2 | 21.8 | 8.91 | 266 | 22.9 | 8.68 | 277 | |||
| 3 | 23.3 | 9.76 | 281 | 21.8 | 8.47 | 274 | |||
| 4 | 22.5 | 9.13 | 269 | 22.9 | 8.53 | 278 | |||
| 5 | 21.9 | 8.91 | 270 | 22.1 | 8.35 | 273 | |||
| 6 | 22.2 | 8.32 | 267 | 22.0 | 8.39 | 276 | |||
| Mean | 22.2 | 8.85 | 270 | 22.3 | 8.45 | 276 | 22.3 | 8.65 | 273 |
| SD | 0.639 | 0.600 | 6.02 | 0.468 | 0.148 | 2.10 | 0.538 | 0.467 | 5.48 |
| RSDr, % | 2.88 | 6.78 | 2.23 | 2.10 | 1.75 | 0.761 | 2.41 | 5.40 | 2.01 |
| Target RSDr low | 4 | 4 | 2 | 4 | 4 | 2 | 4 | 4 | 2 |
| Target RSDr high | 5 | 5 | 3 | 5 | 5 | 3 | 5 | 5 | 3 |
| PRSDR | 7.06 | 8.11 | 4.86 | 7.06 | 8.17 | 4.84 | 7.06 | 8.14 | 4.85 |
| HorRat | 0.4 | 0.8 | 0.5 | 0.3 | 0.2 | 0.2 | 0.3 | 0.7 | 0.4 |
|
| |||||||||
| Saw palmetto pygeum lycopene complex tablets
| |||||||||
| 1 | 112 | 100 | 268 | 114 | 102 | 269 | |||
| 2 | 113 | 101 | 268 | 129 | 115 | 300 | |||
| 3 | 114 | 101 | 268 | 115 | 102 | 271 | |||
| 4 | 116 | 103 | 274 | 117 | 103 | 274 | |||
| 5 | 109 | 98.1 | 263 | 121 | 107 | 280 | |||
| 6 | 118 | 104 | 279 | 125 | 112 | 293 | |||
| Mean | 114 | 101 | 270 | 120 | 107 | 281 | 117 | 104 | 276 |
| SD | 3.14 | 2.11 | 5.62 | 5.95 | 5.56 | 12.6 | 5.66 | 4.98 | 11.0 |
| RSDr, % | 2.75 | 2.09 | 2.08 | 4.96 | 5.20 | 4.48 | 4.84 | 4.79 | 3.99 |
| Target RSDr low | 3 | 3 | 2 | 3 | 3 | 2 | 3 | 3 | 2 |
| Target RSDr high | 4 | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 3 |
| PRSDR | 5.53 | 5.63 | 4.86 | 5.48 | 5.58 | 4.83 | 5.51 | 5.60 | 4.84 |
| HorRat | 0.5 | 0.4 | 0.4 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.8 |
|
| |||||||||
| Saw palmetto (Serenoa repens) dried fruit CO2 extracts
| |||||||||
| 1 | 55.5 | 28.7 | 176 | 56.6 | 28.9 | 174 | |||
| 2 | 55.1 | 29.1 | 178 | 55.5 | 28.7 | 172 | |||
| 3 | 55.6 | 28.7 | 178 | 55.9 | 28.3 | 175 | |||
| 4 | 55.4 | 29.1 | 176 | 55.9 | 28.6 | 173 | |||
| 5 | 53.8 | 27.8 | 171 | 56.3 | 28.7 | 174 | |||
| 6 | 53.9 | 27.6 | 171 | 55.1 | 28.5 | 173 | |||
| Mean | 54.9 | 28.5 | 175 | 55.9 | 28.6 | 174 | 55.4 | 28.6 | 1.74 |
| SD | 0.818 | 0.648 | 3.22 | 0.538 | 0.204 | 1.05 | 0.842 | 0.462 | 2.42 |
| RSDr, % | 1.49 | 2.27 | 1.84 | 0.962 | 0.713 | 0.603 | 1.52 | 1.62 | 1.39 |
| Target RSDr low | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Target RSDr high | 4 | 5 | 3 | 4 | 5 | 3 | 4 | 5 | 3 |
| PRSDR | 6.17 | 6.80 | 5.18 | 6.15 | 6.80 | 5.19 | 6.16 | 6.80 | 5.19 |
| HorRat | 0.2 | 0.3 | 0.4 | 0.2 | 0.1 | 0.1 | 0.2 | 0.2 | 0.3 |
SD = Standard deviation.
RSDr = Intralaboratory relative standard deviation.
PRSDR = 2 C−0.15, where C is the mean concentration expressed as a mass fraction.
HorRat = RSD/PRSDR.
Figure 1.
An example chromatogram of a mixed standard, 0.100 mg/mL.
Figure 4.
An example chromatogram for saw palmetto pygeum lycopene complex tablets.
For this SLV, a negative control was not used. In order to determine accuracy, the powdered saw palmetto sample was fortified 7 times with the mixed reference standard at 0.5, 1, and 2 times the amount detected, or at 3 levels that bracketed the amounts detected. In addition, a single midpoint fortification was conducted on the remaining 9 test materials in replicates of 6. Recovery was conducted on 2 separate days. The Days 1 and 2 combined averages recovery for each phytosterol was within 80–120%. On each individual day, average recoveries also met this criteria except for beta-sitosterol in 2 test materials. Furthermore, beta-sitosterol appears to have a lower recovery than the other phytosterol components. Accuracy results are shown in Table 4.
Table 4.
Accuracy data (percent)
| Day 1 accuracy
|
Day 2 accuracy
|
Days 1 and 2 accuracy combined
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Test material identification/replicates | Campesterol | Stigmasterol | beta-Sitosterol | Campesterol | Stigmasterol | beta-Sitosterol | Campesterol | Stigmasterol | beta-Sitosterol |
| Spiking level | 1X | 1X | 1X | ||||||
| Saw palmetto 45% powdered extract
| |||||||||
| 1 | 82.9 | 89.7 | 85.2 | 103 | 98.4 | 101 | |||
| 2 | 100 | 101 | 107 | 103 | 101 | 102 | |||
| 3 | 100 | 95.7 | 101 | 103 | 99.9 | 102 | |||
| 4 | 112 | 101 | 113 | 104 | 103 | 101 | |||
| 5 | 98.0 | 104 | 94.2 | 102 | 102 | 99.1 | |||
| 6 | 105 | 103 | 109 | 101 | 102 | 98.6 | |||
| Mean | 99.7 | 99.1 | 102 | 103 | 101 | 101 | 101 | 100 | 101 |
| SDa | 9.64 | 5.41 | 10.4 | 1.03 | 1.67 | 1.45 | 6.72 | 3.96 | 7.08 |
| RSDr, %b | 9.67 | 5.46 | 10.2 | 1.00 | 1.65 | 1.44 | 6.65 | 3.96 | 7.01 |
|
| |||||||||
| Alcohol-free saw palmetto
| |||||||||
| 1 | 101 | 107 | 113 | 99.8 | 101 | 100 | |||
| 2 | 98.0 | 102 | 109 | 101 | 96.2 | 103 | |||
| 3 | 99.4 | 111 | 108 | 101 | 93.8 | 102 | |||
| 4 | 96.9 | 101 | 101 | 99.9 | 7.34c | 101 | |||
| 5 | 94.9 | 106 | 95.0 | 100 | 101 | 101 | |||
| 6 | 97.3 | 102 | 101 | 102 | 102 | 100 | |||
| Mean | 97.9 | 105 | 105 | 101 | 98.8 | 101 | 99.3 | 102 | 103 |
| SD | 2.11 | 3.87 | 6.63 | 0.868 | 3.59 | 1.17 | 2.09 | 4.75 | 4.86 |
| RSDr, % | 2.16 | 3.69 | 6.31 | 0.859 | 3.63 | 1.16 | 2.10 | 4.66 | 4.72 |
|
| |||||||||
| Liquid Herbal Extract: saw palmetto
| |||||||||
| 1 | 99.9 | 101 | 96.1 | 101 | 100 | 100 | |||
| 2 | 98.8 | 100 | 93.9 | 101 | 101 | 101 | |||
| 3 | 99.8 | 101 | 98.3 | 102 | 104 | 101 | |||
| 4 | 99.5 | 95.5 | 97.5 | 101 | 102 | 102 | |||
| 5 | 101 | 113 | 103 | 101 | 101 | 97.5 | |||
| 6 | 98.4 | 114 | 102 | 101 | 100 | 97.5 | |||
| Mean | 99.6 | 104 | 98.5 | 101 | 101 | 99.8 | 100 | 103 | 99.2 |
| SD | 0.914 | 7.58 | 3.48 | 0.408 | 1.51 | 1.91 | 1.07 | 5.40 | 2.77 |
| RSDr, % | 0.918 | 7.29 | 3.53 | 0.404 | 1.50 | 1.91 | 1.07 | 5.24 | 2.79 |
|
| |||||||||
| ProstActive Once Daily
| |||||||||
| 1 | 129 | 143 | 120 | 100 | 95.6 | 93.3 | |||
| 2 | 105 | 86.7 | 97.4 | 103 | 98.2 | 99.7 | |||
| 3 | 110 | 116 | 110 | 102 | 95.7 | 98.2 | |||
| 4 | 103 | 98.8 | 96.8 | 100 | 96.2 | 96.5 | |||
| 5 | 107 | 94.5 | 104 | 99.4 | 95.7 | 95.6 | |||
| 6 | 109 | 113 | 104 | 98.3 | 94.8 | 94.6 | |||
| Mean | 111 | 109 | 105 | 100 | 96.0 | 96.3 | 105 | 102 | 101 |
| SD | 9.42 | 20.2 | 8.67 | 1.73 | 1.15 | 2.35 | 8.32 | 15.1 | 7.68 |
| RSDr, % | 8.49 | 18.5 | 8.26 | 1.73 | 1.20 | 2.44 | 7.92 | 14.8 | 7.60 |
|
| |||||||||
| ProstActive plus saw palmetto combined with nettle root extract
| |||||||||
| 1 | 105 | 103 | 104 | 104 | 103 | 94.5 | |||
| 2 | 106 | 99.1 | 105 | 105 | 99.5 | 89.3 | |||
| 3 | 99.9 | 99.7 | 105 | 107 | 104 | 94.7 | |||
| 4 | 101 | 96.0 | 97.2 | 94.9 | 99.7 | 77.5 | |||
| 5 | 103 | 98.6 | 106 | 102 | 103 | 85.7 | |||
| 6 | 101 | 104 | 99.8 | 104 | 104 | 97.2 | |||
| Mean | 103 | 100 | 103 | 103 | 102 | 89.8 | 103 | 101 | 96.3 |
| SD | 2.44 | 2.96 | 3.51 | 4.20 | 2.06 | 7.34 | 3.28 | 2.68 | 8.73 |
| RSDr, % | 2.37 | 2.96 | 3.41 | 4.08 | 2.02 | 8.17 | 3.18 | 2.65 | 9.07 |
|
| |||||||||
| Prostasan Prostrate Capsules
| |||||||||
| 1 | 108 | 99.5 | 108 | 100 | 99.3 | 97.3 | |||
| 2 | 98.6 | 95.2 | 105 | 98.5 | 95.4 | 92.8 | |||
| 3 | 95.2 | 92.3 | 101 | 97.0 | 93.9 | 92.6 | |||
| 4 | 106 | 95.8 | 108 | 97.7 | 94.8 | 93.5 | |||
| 5 | 88.5 | 87.3 | 93.4 | 99.2 | 95.7 | 94.3 | |||
| 6 | 103 | 94.8 | 107 | 99.3 | 95.9 | 96.1 | |||
| Mean | 99.9 | 94.2 | 104 | 98.6 | 95.8 | 94.4 | 99.3 | 95.0 | 99.1 |
| SD | 7.30 | 4.08 | 5.71 | 1.11 | 1.85 | 1.89 | 5.02 | 3.14 | 6.33 |
| RSDr, % | 7.31 | 4.33 | 5.49 | 1.13 | 1.93 | 2.00 | 5.06 | 3.31 | 6.39 |
|
| |||||||||
| Pygeum and saw palmetto standardized herbal extracts
| |||||||||
| 1 | 91.2 | 89.6 | 78.7 | 101 | 103 | 77.9 | |||
| 2 | 115 | 84.7 | 104 | 106 | 103 | 81.7 | |||
| 3 | 118 | 83.6 | 95.5 | 104 | 102 | 84.2 | |||
| 4 | 112 | 100 | 84.8 | 90.4 | 102 | 73.9 | |||
| 5 | 96.5 | 111 | 85.4 | 103 | 108 | 86.5 | |||
| 6 | 119 | 85.5 | 97.1 | 104 | 103 | 73.1 | |||
| Mean | 109 | 92.4 | 90.9 | 101 | 104 | 79.6 | 105 | 98.0 | 85.2 |
| SD | 11.8 | 10.9 | 9.46 | 5.63 | 2.26 | 5.49 | 9.60 | 9.49 | 9.47 |
| RSDr, % | 10.8 | 11.8 | 10.4 | 5.57 | 2.17 | 6.90 | 9.14 | 9.68 | 11.1 |
|
| |||||||||
| Saw palmetto pygeum lycopene complex tablets
| |||||||||
| 1 | 92.1 | 94.3 | 91.5 | 100 | 98.4 | 94.7 | |||
| 2 | 94.9 | 95.8 | 93.4 | 106 | 105 | 107 | |||
| 3 | 105 | 106 | 104 | 104 | 102 | 102 | |||
| 4 | 75.6 | 80.5 | 72.7 | 107 | 103 | 106 | |||
| 5 | 111 | 107 | 106 | 98.4 | 96.8 | 89.9 | |||
| 6 | 99.8 | 103 | 101 | 88.1 | 87.5 | 77.7 | |||
| Mean | 96.4 | 97.8 | 94.8 | 101 | 98.8 | 96.2 | 98.5 | 98.3 | 95.5 |
| SD | 12.3 | 9.94 | 12.2 | 6.97 | 6.30 | 11.2 | 9.76 | 7.95 | 11.2 |
| RSDr, % | 12.8 | 10.2 | 12.9 | 6.90 | 6.38 | 11.6 | 9.91 | 8.09 | 11.7 |
|
| |||||||||
| Saw palmetto (Serenoa repens) dried fruit CO2 extracts
| |||||||||
| 1 | 110 | 106 | 103 | 99.6 | 99.7 | 106 | |||
| 2 | 110 | 102 | 97.7 | 97.7 | 99.7 | 100 | |||
| 3 | 104 | 93.4 | 93.5 | 96.2 | 97.0 | 94.4 | |||
| 4 | 113 | 104 | 102 | 98.9 | 97.4 | 99.4 | |||
| 5 | 108 | 103 | 101 | 99.7 | 97.6 | 101 | |||
| 6 | 109 | 105 | 99.5 | 91.7 | 93.9 | 91.2 | |||
| Mean | 109 | 102 | 99.5 | 97.3 | 97.6 | 98.7 | 103 | 99.9 | 99.1 |
| SD | 2.97 | 4.55 | 3.46 | 3.04 | 2.14 | 5.21 | 6.75 | 4.18 | 4.24 |
| RSDr, % | 2.72 | 4.46 | 3.48 | 3.12 | 2.19 | 5.28 | 6.55 | 4.18 | 4.28 |
| Spiking level | 1X | 1/2X | 2X | ||||||
|
| |||||||||
| Powdered saw palmetto berry (Day 1 accuracy)
| |||||||||
| 1 | 104 | 96.1 | 89.6 | 108 | 89.3 | 77.8 | 108 | 97.8 | 102 |
| 2 | 97.5 | 113 | 79.5 | 90.6 | 68.1 | 43.7 | 102 | 96.8 | 98.3 |
| 3 | 108 | 110 | 91.7 | 116 | 110 | 82.4 | 105 | 100 | 100 |
| 4 | 103 | 81.0 | 86.6 | 100 | 89.9 | 66.7 | 104 | 103 | 102 |
| 5 | 103 | 125 | 84.7 | 100 | 100 | 69.1 | 104 | 93.6 | 103 |
| 6 | 105 | 101 | 90.9 | 104 | 68.9 | 68.7 | 106 | 95.1 | 98.3 |
| 7 | 96.1 | 75.4 | 78.9 | 116 | 110 | 60.4 | 108 | 98.7 | 101 |
| Mean | 102 | 100 | 86.0 | 105 | 90.9 | 67.0 | 105 | 97.9 | 101 |
| SD | 4.19 | 17.7 | 5.23 | 9.21 | 17.4 | 12.6 | 2.21 | 3.13 | 1.86 |
| RSDr, % | 4.11 | 17.7 | 6.08 | 8.77 | 19.1 | 18.8 | 2.10 | 3.20 | 1.84 |
|
| |||||||||
| Powdered saw palmetto berry (Day 2 accuracy)
| |||||||||
| 1 | 102 | 106 | 88.9 | 114 | 99.2 | 96.1 | 107 | 104 | 105 |
| 2 | 102 | 116 | 85.8 | 118 | 97.6 | 97.7 | 96.1 | 97.7 | 101 |
| 3 | 103 | 101 | 85.6 | 113 | 99.1 | 98.0 | 101 | 98.7 | 101 |
| 4 | 99.5 | 96.5 | 78.8 | 115 | 80.3 | 88.9 | 98.3 | 98.0 | 104 |
| 5 | 103 | 106 | 89.5 | 103 | 91.3 | 91.9 | 104 | 97.7 | 103 |
| 6 | 89.8 | 171 | 81.6 | 118 | 95.5 | 92.7 | 99.3 | 98.9 | 103 |
| 7 | 95.3 | 117 | 78.2 | 120 | 92.3 | 99.6 | 104 | 102 | 108 |
| Mean | 99.2 | 116 | 84.1 | 114 | 93.6 | 95.0 | 101 | 99.6 | 104 |
| SD | 4.97 | 25.3 | 4.59 | 5.62 | 6.65 | 3.89 | 3.81 | 2.46 | 2.44 |
| RSDr, % | 5.01 | 21.8 | 5.46 | 4.93 | 7.10 | 4.09 | 3.77 | 2.47 | 2.35 |
|
| |||||||||
| Powdered saw palmetto berry (Days 1 and 2 accuracy combined)
| |||||||||
| Mean | 101 | 108 | 85.0 | 110 | 92.3 | 81.0 | 103 | 98.7 | 102 |
| SD | 4.71 | 22.5 | 4.83 | 8.83 | 12.7 | 17.1 | 3.61 | 2.84 | 2.57 |
| RSDr, % | 4.66 | 20.8 | 5.68 | 8.03 | 13.8 | 21.1 | 3.50 | 2.88 | 2.52 |
SD = Standard deviation.
RSDr = Intralaboratory relative standard deviation.
Rejected based on the Dixon Test.
To demonstrate ruggedness relative to the reference method, the following were investigated as part of the SLV: change in operating conditions, second analyst, and expansion of saponified solution stability. The operating conditions in the reference method were modified in this SLV; however, it appeared that other conditions caused more chromatographic carryover between sample injections in certain test materials. Additional method ruggedness tests included using a different analyst to conduct precision over 2 days for at least 2 test materials. Furthermore, a second analyst conducted some of the fortification recovery accuracy results. Table 5 presents the second analyst’s precision data. These data showed good precision (the RSDrs are mostly within or below the target RSDr ranges), and the HorRat values were all below 0.3 or between 0.3 and 1.3. The saponified solution stability was tested and proved to be stable at room temperature up to 7 days. This was achieved by comparison to freshly saponified sample solutions. Table 6 presents the saponified solution stability results.
Table 5.
Second analyst precision (ruggedness test)
| Day 1 precision, mg/100 g
|
Day 2 precision, mg/100 g
|
Days 1 and 2 precision combined, mg/100 g
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Test material identification/replicates | Campesterol | Stigmasterol | beta-Sitosterol | Campesterol | Stigmasterol | beta-Sitosterol | Campesterol | Stigmasterol | beta-Sitosterol |
| Saw palmetto pygeum lycopene complex tablets
| |||||||||
| 1 | 117 | 108 | 316 | 122 | 119 | 328 | |||
| 2 | 122 | 112 | 329 | 131 | 121 | 348 | |||
| 3 | 132 | 121 | 351 | 122 | 117 | 329 | |||
| 4 | 116 | 108 | 315 | 125 | 117 | 335 | |||
| 5 | 124 | 116 | 324 | 119 | 109 | 320 | |||
| 6 | 122 | 115 | 328 | 125 | 118 | 337 | |||
| Mean | 122 | 113 | 327 | 124 | 117 | 333 | 123 | 115 | 330 |
| SDa | 5.74 | 5.05 | 13.1 | 4.10 | 4.12 | 9.54 | 4.85 | 4.76 | 11.3 |
| RSDr, %b | 4.70 | 4.47 | 4.01 | 3.31 | 3.52 | 2.86 | 3.94 | 4.14 | 3.42 |
| Target RSDr lowb | 3 | 3 | 2 | 3 | 3 | 2 | 3 | 3 | 2 |
| Target RSDr highb | 4 | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 3 |
| PRSDRc | 5.47 | 5.53 | 4.72 | 5.46 | 5.51 | 4.71 | 5.46 | 5.52 | 4.71 |
| HorRatd | 0.9 | 0.8 | 0.8 | 0.6 | 0.6 | 0.6 | 0.7 | 0.8 | 0.7 |
|
| |||||||||
| Saw palmetto (Serenoa repens) dried fruit CO2 extracts
| |||||||||
| 1 | 40.4 | 22.7 | 145 | 43.2 | 25.6 | 157 | |||
| 2 | 42.0 | 26.6 | 151 | 42.6 | 24.0 | 152 | |||
| 3 | 42.7 | 25.5 | 153 | 43.1 | 24.7 | 155 | |||
| 4 | 42.8 | 25.8 | 154 | 43.9 | 25.2 | 159 | |||
| 5 | 42.6 | 24.9 | 153 | 44.3 | 25.1 | 159 | |||
| 6 | 44.0 | 25.9 | 157 | 40.3 | 25.5 | 145 | |||
| Mean | 42.4 | 25.2 | 152 | 42.9 | 25.0 | 155 | 42.7 | 25.1 | 153 |
| SD | 1.18 | 1.36 | 4.02 | 1.41 | 0.591 | 5.36 | 1.27 | 1.01 | 4.68 |
| RSDr, % | 2.78 | 5.40 | 2.64 | 3.29 | 2.36 | 3.46 | 2.97 | 4.02 | 3.06 |
| Target RSDr low | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Target RSDr high | 4 | 5 | 4 | 4 | 5 | 4 | 4 | 5 | 4 |
| PRSDR | 6.41 | 6.93 | 5.29 | 6.40 | 6.94 | 5.28 | 6.40 | 6.94 | 5.29 |
| HorRat | 0.4 | 0.8 | 0.5 | 0.5 | 0.3 | 0.7 | 0.5 | 0.6 | 0.6 |
SD = Standard deviation.
RSDr = Intralaboratory relative standard deviation.
PRSDR= 2 C−0.15, where C is the mean concentration expressed as a mass fraction.
HorRat = RSDr/PRSDR.
Table 6.
Saponified solution stability (ruggedness test)
| Campesterol | Stigmasterol | beta-Sitosterol | ||
|---|---|---|---|---|
| Timepoints | mg/100g | |||
| Saw palmetto (Serenoa repens)
dried fruit CO2 extracts |
Day 1a | 42.4 | 25.2 | 152 |
| Day 3 | 40.4 | 24.1 | 146 | |
| Day 7 | 42.4 | 23.6 | 151 | |
| Mean | 41.7 | 24.3 | 150 | |
| SDb | 1.15 | 0.819 | 3.21 | |
| RSD, %c | 2.76 | 3.37 | 2.14 | |
Mean from 2nd analyst Day 1 precision.
SD = Standard deviation.
RSD = Relative standard deviation.
Conclusions
The validation process showed the precision and accuracy required for determination of campesterol, stigmasterol, and beta-sitosterol in saw palmetto raw materials and dietary supplements. The collaborative study protocol for the method has been approved by AOAC INTERNATIONAL, and a collaborative study is currently in progress.
Figure 2.
An example chromatogram for saw palmetto 45% powered extract.
Figure 3.
An example chromatogram for alcohol-free saw palmetto.
References
- 1.Horwitz W. AOAC Requirements for Single Laboratory Validation of Chemical Methods for Dietary Supplements. 2002 Draft §3.4.1. [Google Scholar]