Abstract
The periodontal pathogen, Porphyromonas gingivalis, is highly resistant to the bactericidal activity of human complement, which is present in the gingival crevicular fluid at 70% of serum concentration. All thirteen clinical and laboratory P. gingivalis strains tested were able to capture the human complement inhibitor, C4b-binding protein (C4BP), which may contribute to their serum resistance. Accordingly, in serum deficient of C4BP, it was found that significantly more terminal complement component C9 was deposited on P. gingivalis. Moreover, using purified proteins and various isogenic mutants, we found that the cysteine protease gingipain HRgpA is a crucial C4BP ligand on the bacterial surface. Binding of C4BP to P. gingivalis appears to be localized to two binding sites: on the complement control protein (CCP) 1 domain and CCP 6–7 domains of the α-chains. Furthermore, the bacterial binding of C4BP was found to increase with time of culture and a particularly strong binding was observed for large aggregates of bacteria that formed during culture on solid blood agar medium. Taken together, gingipains appeared to be a very significant virulence factor not only destroying complement due to proteolytic degradation as we have shown previously but was also inhibiting complement activation due to their ability to bind complement inhibitor C4BP.
Introduction
Periodontitis is an inflammatory condition with an infective etiology that leads to the loss of the supporting structures of the tooth. Together with Treponema denticola and Tannerella forsythia, Porphyromonas gingivalis is a part of the “red complex” of microorganisms most often associated with periodontitis (1). Although this periodontopathogen can be cultured occasionally from healthy sites, the bacteria proliferate in high numbers during active periodontal disease (2) despite the fact that there is a significant antibody response (3). Furthermore, several periodontal health indicators correlate inversely with the presence of P. gingivalis (4) and P. gingivalis is able to induce periodontal disease in animal models (5). More recently, there is accumulating evidence that P. gingivalis may also be associated with cardiovascular disease (6). Virulence factors produced by P. gingivalis include outer membrane vesicles, adhesins, lipopolysaccharides, hemolysins, and proteinases (7).
Amongst the proteinases, the gingipain cysteine proteinases are responsible for 85% of the general proteolytic activity displayed by the pathogen. There are three members of the gingipain family: Lys-gingipain (Kgp)3 is specific for the Lys-X peptide bond, whereas Arg-gingipains (RgpA and RgpB) are specific for the Arg-X peptide bond (8). RgpA, derived from the rgpA gene, is present in several molecular forms due to extensive posttranslational processing and glycosylation of the nascant polypeptide chain. These include the membrane-bound enzyme mt-RgpA and its two soluble forms, the 50 kDa catalytic domain alone (RgpA(cat)) and the 95kDa, non-covalent complex composed of the catalytic domain and hemagglutinin/adhesin domains (HRgpA). In contrast to rgpA, rgpB lacks the sequence encoding hemagglutinin/adhesin domains and therefore its product, RgpB, may be encountered only in two different forms: either membrane-bound (mt-RgpB) or as a soluble 50 kDa RgpB. The hemagglutinin/adhesion domain responsible for binding to fibrinogen, fibronectin and laminin as well as for hemagglutinin activity of P. gingivalis is also found in the Kgp (9). Working in concert, gingipains are able to cleave not only constituents of periodontal tissues, including basement membrane structural protein collagen, but are also able to degrade host proteins used for protection, such as antibodies and components of the complement system (10).
Complement is a major arm of the innate immune defense system and its main function is to recognize and destroy microorganisms (11). The three pathways of human complement ensure that virtually any non-host surface is recognized as hostile. The classical pathway is usually mediated by binding of the C1 complex to immunoglobulins recognizing invading pathogens. Thus complement enhances the effectiveness of the existing “natural” or specifically generated antibodies in pathogen clearance. The lectin pathway is able to recognize, via mannose-binding lectin (MBL), “foreign” polysaccharide molecules normally present only on microbial surfaces. C4 is a crucial component of both pathways as it becomes covalently attached to the surfaces that activated C1 or MBL to form a part of the C3-convertase complex (C4bC2a), which activates C3. Finally, complement can also be activated through the alternative pathway, which can be directly initiated by properdin or due to a failure to appropriately regulate the constant low-level spontaneous activation of C3 (constantly initiated due to inherent instability of this protein). All three pathways lead to opsonisation of pathogen with C3b, which enhances phagocytosis while releasing anaphylatoxins C5a and C3a to attract phagocytes. Finally, the end result of the complement cascade is formation of the membrane attack complex (MAC) and lysis of the target cell. Host cells protect themselves from bystander damage following complement activation through the expression of membrane-bound or recruitment of soluble endogenous complement inhibitors.
C4b-binding protein (C4BP) is a circulating inhibitor of the classical and the lectin pathways of complement and inhibits the formation and accelerates the decay of C3 convertase. It also serves as a cofactor to factor I in the proteolytic degradation of C4b (12) and C3b (13). C4BP is a large plasma glycoprotein that exists in several forms with varying subunit composition. The major form consists of seven identical α-chains (70-kDa each) and one β-chain (45 kDa) (14). The α- and β-chains are composed of repeating domains of ~60 amino acid residues known as complement control protein (CCP) domains with α-chain having eight while β-chain only three such domains (15). C4BP is also linked to the coagulation system since the β-chain is bound with high affinity to the vitamin K-dependent anticoagulant protein S (14).
Every successful human microbial pathogen must develop means to circumvent complement and we have found that many bacteria are able to capture either C4BP or/and factor H (FH), an inhibitor of the alternative pathway, and thereby decrease complement activation on their surface. This leads to a decrease in opsonisation and ensuing phagocytosis. For example, binding of C4BP to M proteins of Streptococcus pyogenes appears to be responsible for the resistance of these bacteria to phagocytosis (16). We have shown previously that resistance to killing by serum of Neisseria gonorrhoeae correlates with the ability of gonococci to bind C4BP (17) and that human C4BP selectively interacts with Neisseria gonorrhoeae, which results in species-specific infection (18). Pathogens known to bind C4BP include Streptococcus pyogenes (19), Moraxella catarrhalis (20), Escherichia coli strain K1 (21), Borrelia recurrentis (22), Candida albicans (23) and Haemophilus influenzae (24).
In the current study, we demonstrate that both culture collection strains and clinical isolates of P. gingivalis interact with C4BP, which contributes to the exceptional resistance to the complement system by this microorganism.
Materials and Methods
Proteins
C4BP (in complex with protein S) was purified from human plasma (25) and labeled with FITC (26) as described previously. C4b was purchased from Complement Technologies. Recombinant C4BP was expressed in eukaryotic cells and purified by affinity chromatography (27). Arginine-specific (HRgpA and RgpB) and lysine-specific (Kgp) gingipains were purified from the P. gingivalis HG66 strain culture fluid as described previously (28, 29).
Bacterial strains and culture conditions
P. gingivalis strains listed in Table I were grown in enriched tryptic soy broth medium (TSB) or in blood TSB agar at 37°C in an anaerobic chamber (Concept 400, Biotrace) with an atmosphere of 90% N2, 5% CO2 and 5% H2. For growth selection of mutants on solid media, 1 µg/ml tetracycline or 5 µg/ml erythromycin were used. Clinical strains were obtained from patients with severe periodontitis (aggressive periodontitis (n=3), chronic periodontitis (n=9)). Two paper points were inserted in each pocket for 20 s and subsequently placed in 2 ml of a transport medium (reduced buffered saline). After vigorous mixing for 30 s, the samples were serially diluted up to 10−5. Aliquots of 0.1 ml were plated on Schaedler-agar (Oxoid) supplemented with 8% sheep blood without antibiotics, on the same agar plates with 100 µg/ml kanamycin. The Schaedler-agar plates were incubated anaerobically at 37°C for 7 days. After that the total number of cfu as well as the colonies typical for P. gingivalis were counted, the identity was confirmed by a biochemical test (rapid ID 32A identification system (BioMerieux) and 16S rDNA sequence analysis. The percentage of P. gingivalis was up to 57% of the total anaerobically cultivable flora. Strain PorT was constructed exactly as described (30) and displayed the previously reported phenotype.
Table I.
Description of laboratory P. gingivalis strains used in this study.
Binding of C4BP to bacteria
P. gingivalis from six-day old agar plates (unless indicated otherwise) were harvested, washed twice in the binding buffer (10 mM HEPES, 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, pH 7.2) and adjusted to an optical density of 1.0 at 600 nm. FITC-labelled C4BP was diluted in the binding buffer to a concentration of 50 µg/ml and mixed with 6 × 105 cells followed by incubation for 60 min at RT. In competition experiments, samples also contained plasma purified C4BP (20 µg/ml), recombinant C4BP (20 µg/ml), C4b (100 µg/ml), mAb104 (100 µg/ml), mAb67 (100 µg/ml), heparin (100 µg/ml), or BSA (100 µg/ml). mAb104 and mAb67 are directed against CCP1 and CCP4 of C4BP α-chain, respectively (27). Concentration of C4BP used as competitor was chosen to give 50% inhibition on the basis of initial titration. Other competitors were added at five times higher concentrations than C4BP in order to compensate for possible avidity effects as C4BP is a polymeric molecule, able to interact with most ligands via multiple binding sites. Thereafter, the cells were washed twice in the binding buffer and finally resuspended in flow cytometry buffer (50 mM HEPES, 100 mM NaCl, 30 mM NaN3, 1% BSA; pH 7.4). Flow cytometry analysis was performed using a FACS Calibur (Beckton Dickinson).
Bacterial binding of C4BP from serum
Normal human serum (NHS) was prepared from blood taken from six healthy volunteers and pooled. P. gingivalis from six-day old agar plates (unless indicated otherwise) were harvested, washed twice in the binding buffer and adjusted to an optical density of 1.0 at 600 nm. NHS was diluted in GVB++ (5 mM veronal buffer, 140 mM NaCl, 0.1% gelatin, 1 mM MgCl2, and 0.15 mM CaCl2; pH 7.3) to a concentration of 5%, mixed with 6 × 105 cells and incubated for 75 min at 37°C with shaking. Thereafter, the bacterial cells were washed twice and incubated with monoclonal mouse anti-C4BP antibodies (mAb104, 2 µg/ml) for 1 hour at RT. Bacteria were washed twice and resuspended in goat anti-mouse FITC-conjugated polyclonal antibodies (DakoCytomation, diluted 1:1000) and incubated 1 hour RT. All washing and antibody binding steps were performed in the binding buffer. Thereafter, flow cytometry buffer was added and flow cytometry analysis was performed. An aliquot of the bacteria was stained with a standard Gram staining procedure and pictures were taken using LCD camera connected to a microscope (Nikon).
Binding of C4BP to purified gingipains by ELISA
Microtiter plates (Maxisorp; Nunc) were incubated overnight at 4°C with 50 µl of a solution containing 8 µg/ml HRgpA, RgpB or Kgp in 75 mM sodium carbonate (pH 9.6). Plates were washed four times with 50 mM Tris-HCl, 150 mM NaCl, and 0.1% Tween 20 (pH 7.5) between each of the following steps. The wells were blocked with the quenching solution (3% fish gelatin (Nordland) in the washing solution) for 1 hour at RT. C4BP was diluted in TBS and used at concentrations ranging from 3 to 200 µg/ml and was thereafter incubated for 4 hours at RT. When binding of C4BP and its mutants to HRgpA was tested, the recombinant proteins were diluted in GVB++ and used at 20 µg/ml. In the competition assay with prothrombin (purified from plasma) and fibrinogen (Sigma), C4BP was used at 15 µg/ml in TBS mixed together with up to 100 µg/ml of competitors. Deposited C4BP was detected by mouse anti-C4BP antibodies diluted in the quenching solution. Bound antibodies were detected with HRP-labeled goat anti-mouse secondary antibodies (DakoCytomation). Bound HRP-labeled pAbs were revealed using 1,2-phenylenediamine dihydrochloride (OPD) tablets (DakoCytomation) and the absorbance was measured at 490 nm using microtiter plate reader (Varian).
Deposition of C9 on bacteria
For C4BP-depletion, fresh NHS was passed through a HiTrap-column (GE Healthcare) coupled with mAb 104 (31). The flow through was analyzed by ELISA and the fractions lacking C4BP were pooled and frozen in −80°C. Plasma purified C1q was added to the depleted serum in order to compensate for C1q that bound to the antibody-coupled column. The final concentration of C1q in NHS and C4BP-depleted NHS was then verified by ELISA. C4BP-depleted serum was supplemented with physiological concentrations of purified C4BP (0.2 mg/ml) in order to control that any effect exerted by C4BP-depleted serum was due to lack of C4BP and could be corrected in replete serum. P. gingivalis from six-day old agar plate were harvested, washed twice in the binding buffer and adjusted to an optical density of 1.0 at 600 nm. NHS was diluted in GVB++ to a concentration of 5%, mixed with 6 × 105 cells (final volume of 50 µl) and incubated for 60 min at 37°C with shaking. Heat inactivated serum (56° C, 30 min) was used as a negative control. Thereafter, cells were washed twice in the binding buffer and C9 deposition was assessed by incubation of the cells for 1 h at RT with the goat anti-human C9 antibodies (Complement Technologies) diluted 1:1000 in the binding buffer. Afterwards, cells were washed twice and resuspended in rabbit anti-goat polyclonal antibodies-FITC conjugated antibodies (DakoCytomation) diluted in the binding buffer and used at 1:1000 dilution. Samples were thereafter incubated for 60 min at RT and analyzed by flow cytometry.
Western blotting
P. gingivalis (0.75 − 6 × 105 cells) harvested from six-day old agar plates were incubated with NHS (5%) for 60 min at 37°C with shaking in a final volume of 50 µl. Serum proteins (0.15 µl NHS/well) were separated by gel electrophoresis under non-reducing conditions using 5% gel and transferred to PVDF membrane using semi-dry blotting system. The membranes were blocked with 50 mM Tris-HCl, 150 mM NaCl, 2 mM CaCl2, 0.1% Tween 20 and 3% fish gelatin, pH 8.0. C4BP was detected using the monoclonal MK104 antibody followed by goat-anti mouse antibody conjugated to HRP and 3,3′-diaminobenzidine tetrahydrochloride colorimetric substrate (Sigma-Aldrich). The amount of C4BP was quantified after digital scanning using ImageGauge software (Fujifilm).
Measurement of enzymatic activity of gingipains
The assay was performed as previously described (32). Briefly, purified HRgpA (ranging from 0.2 to 4 µg/ml) or the P.gingivalis strain J4261 collected from 9-day old plates and washed twice with GVB++ (2.5×105 to 5×106 cells), were added to the wells of microtiter plate containing 100 µl of reaction buffer (200 mM Tris-HCl, 150 mM NaCl, 5 mM CaCl2, 0.02% NaN3, pH 7.6 containing freshly added 20 mM L-cysteine-HCl). Total volume was adjusted to 200 µl for each sample and left to incubate for 10 minutes at 37°C. Thereafter, 20 µl of L-BAPNA (Nα-Benzoyl-L-arginine p-nitroanilide hydrochloride) was added to the wells yielding the final substrate concentration of 1mM and hydrolysis of the substrate was measured spectrophotometically at 405 nm every 18 s for 5 min.
Statistical analysis
Student’s t-test was used to calculate p values in order to estimate if the observed differences were statistically significant.
Results
C4BP binds to all tested strains of P. gingivalis
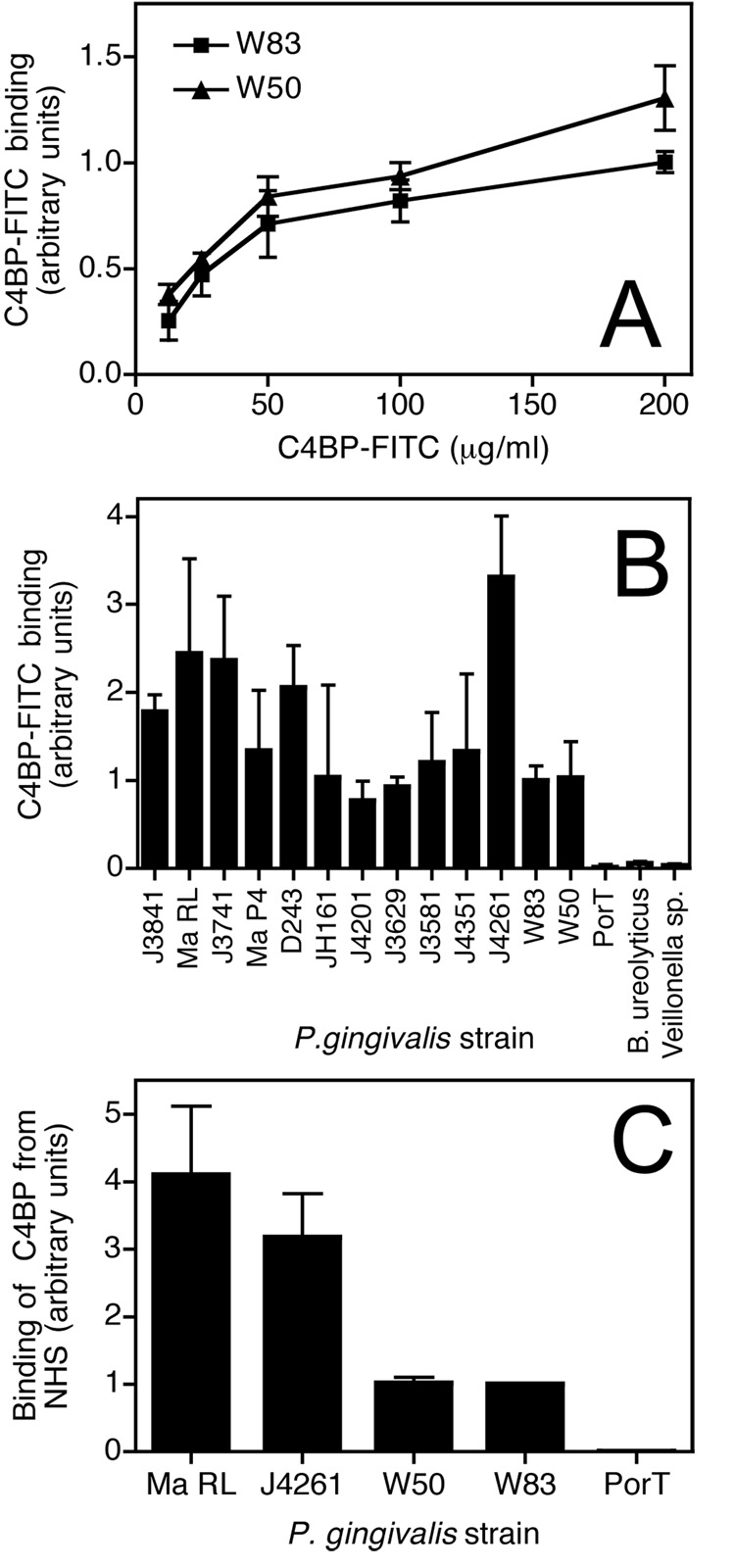
We started our investigation by testing whether the potent complement inhibitor C4BP can be captured by P. gingivalis. Using flow cytometric analysis, two widely used laboratory strains, W50 and W83, were found to bind plasma derived FITC-labelled C4BP. The binding of C4BP was concentration dependent and saturable (Fig. 1A). Importantly, we found that all clinical isolates of P. gingivalis tested in this study bound C4BP to varying extent (Fig. 1B). The majority of the clinical strains bound more C4BP than the two laboratory strains analyzed. Notably, the ability to bind C4BP was specific for P. gingivalis, since two other anaerobic bacteria species (Bacteroides ureolyticus and Veillonella sp.) cultured in the same conditions, i.e. solid medium, did not bind C4BP (Fig. 1B). Interestingly, a P. gingivalis mutant lacking PorT, an integral outer membrane protein involved in the secretion of gingipains (Nguyen et al. – manuscript in preparation; (30)) entirely lost the ability to bind C4BP in comparison with the parental strain W83. Importantly, P. gingivalis were able to bind C4BP from NHS as shown in Fig 1C. The level of C4BP captured from NHS corresponded well with the binding experiment using purified C4BP (Fig. 1B) as the clinical strains J4261 and Ma RL both displayed the strongest binding.
Fig. 1. Binding of C4BP-FITC to clinical isolates and laboratory strains of P. gingivalis.
Bacteria were grown for 6 days on TSB agar and suspended in the binding buffer at 6×105 cells/ml. A) Wild type strains W83 and W50 were incubated with indicated concentrations of C4BP-FITC for 1h at RT, washed and analyzed by flow cytometry. B) C4BP-FITC (50 µg/ml) was incubated with a number of strains of P. gingivalis for 30 minutes at RT, the cells washed and analyzed by flow cytometry. C) The bacteria were incubated with 5% NHS diluted in GVB++ followed by detection of bound C4BP with mAb 104 and secondary FITC-labelled anti-mouse antibody. Binding to every strain was analyzed three times in duplicates. Shown are mean values +/− standard deviation (SD).
C4BP binds to hemagglutinin/adhesin domain of HRgpA
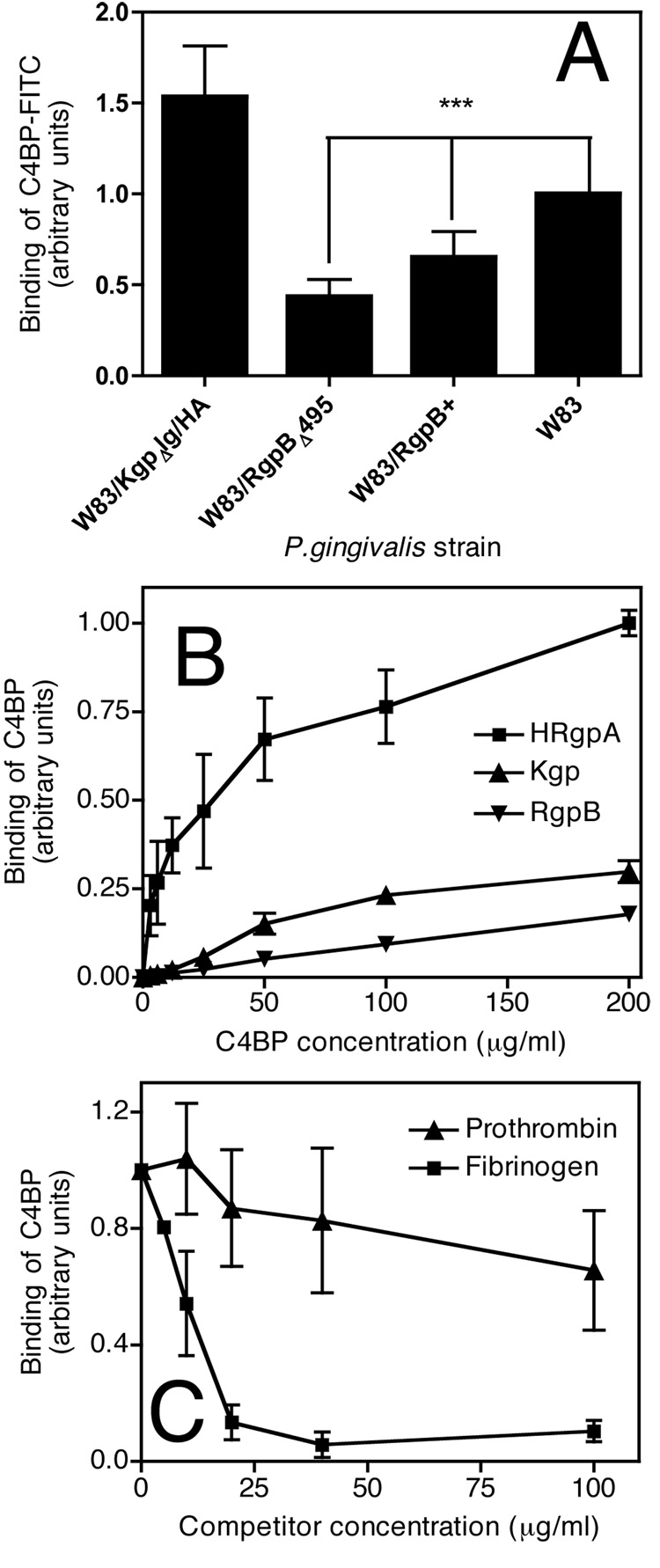
Our next goal was to identify the C4BP ligand on the surface of P. gingivalis. To this end we used P. gingivalis W83 mutants lacking various gingipains as described in Table I. We found that binding of C4BP was significantly decreased for both strains lacking HRgpA (Fig. 2A). Notably, the single rgpA gene mutant strain of P. gingivalis (W83/RgpB+) showed significantly higher binding in comparison with the double rgpA and rgpB genes deficient strain (W83/RgpBΔ495). This observation may indicate that RgpB is also partially involved, directly or indirectly, through processing of other surface proteins (33) that may be involved in binding of C4BP. The mutant lacking only Kgp (W83/KgpΔIg/HA) showed a slight increase in binding of C4BP compared to the parental W83 strain (Fig. 2A).
Fig. 2. C4BP binds mainly HRgpA.
A) Wild type strain W83 and its mutants lacking gingipains grown as described in Fig. 1 were incubated with (50 µg/ml) C4BP-FITC for 30 minutes at RT, washed and analyzed by flow cytometry. Binding of C4BP to W83 in each experiment was set as 1. Statistical significance of differences between tested stains was estimated with Student’s t-test, *** p<0.001. B) Purified HRgpA, RgpB and Kgp were immobilized on microtiter plates and incubated with indicated concentrations of C4BP, binding of which was detected with specific monoclonal antibodies. In each experiment, the binding of highest concentration of C4BP to HRgpA was set as 1 and all values were normalized C) C4BP (15 µg/ml) was incubated with HRgpA immobilized on microtiter plates in the presence of indicated concentrations of fibrinogen and prothrombin and the binding of C4BP was detected with a monoclonal antibody. Binding at every condition in A–C was analyzed three times in doublets. Shown are mean values +/− SD.
In order to test whether purified gingipains would also interact with C4BP, gingipains were immobilized on microtiter plates and the binding of purified C4BP was determined with specific antibodies. C4BP bound avidly to HRgpA (Fig. 2B) and to a much lower extent to Kgp and RgpB (Fig. 2B). Taken together, our results show that HRgpA serves as a ligand for C4BP.
In order to further identify the binding site for C4BP on HRgpA, we performed a competition assay with fibrinogen that interacts with the hemagglutinin/adhesion domains of gingipains. HRgpA was immobilized on microtiter plates and incubated in a solution containing 25 µg/ml of C4BP and increasing concentrations of fibrinogen or prothrombin that was included as a negative control. The binding of C4BP was detected using specific antibodies. We found that fibrinogen strongly competed with C4BP for binding to HRgpA while prothrombin had no significant effect (Fig. 2C). These findings imply that C4BP binds mainly to the hemagglutinin/adhesin domain of HRgpA.
Binding sites for P. gingivalis on C4BP are localized to CCP1 and CCP6-7 of the α-chains
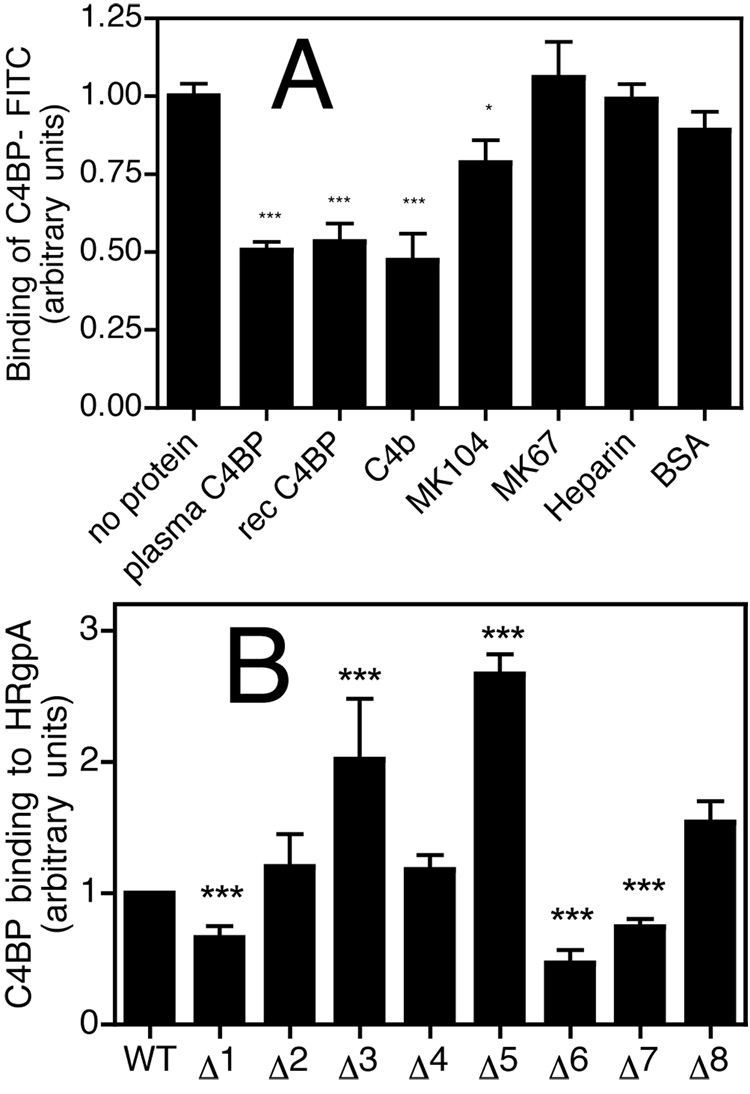
To further determine details of the interaction between C4BP and its bacterial ligand, and to elucidate which subunit of C4BP is responsible for binding to P. gingivalis, we incubated bacteria with FITC-labelled C4BP in the presence of various competitors. We found that the binding of C4BP-FITC was inhibited to the same degree by C4BP purified from plasma (composed of 7 α-chains and one β-chain with bound protein S) as by recombinant C4BP (containing 6 α-chains), implying that binding is localized to α-chains (Fig. 3A). In addition, the C4BP-P. gingivalis interaction could be inhibited by C4b and mAb104 but not mAb67 or albumin (BSA). C4b interacts with CCP1-3 of the α-chains (27), while mAb104 and mAb67 bind CCP1 and CCP4, respectively. Furthermore, binding of immobilized HRgpA to recombinant C4BP mutants lacking one CCP domain at the time showed that mutants missing CCP1, CCP6 and CCP7 have significantly decreased binding capacity to HRgpA (Fig. 3B). The mutants lacking CCP3 and CCP5 bound better than the wild type. Moreover, similar results were obtained with whole bacteria and mutated recombinant C4BP using flow cytometry analysis (data not shown). Taken together, there appears to be two binding sites for P. gingivalis on C4BP and they are localized to CCP1 and CCP6-7 of the α-chains.
Fig. 3. The binding site for P. gingivalis is localized to N-terminus of C4BP.
A) P. gingivalis strain W83 was incubated with 50 µg/ml C4BP-FITC in the presence of several competitors: plasma purified C4BP (20 µg/ml), recombinant C4BP (20 µg/ml), C4b (100 µg/ml), mAb 104 (100 µg/ml), mAb 67 (100 µg/ml), heparin (100 µg/ml) and BSA (100 µg/ml). After 1h incubation at RT, the bacteria were washed and analyzed by flow cytometry. B) Recombinant C4BP and its mutants lacking single CCP domains were incubated with HRgpA and the binding was detected with a polyclonal antibody. Binding at every condition was analyzed three times in duplicates. Shown are mean values +/− SD. Statistical significance of differences between experimental conditions without (set as 1) and with competitors was estimated with Student’s t test, *p<0.05, ** p<0.01, *** p<0.001.
Binding of C4BP is related to the age of P. gingivalis cultures
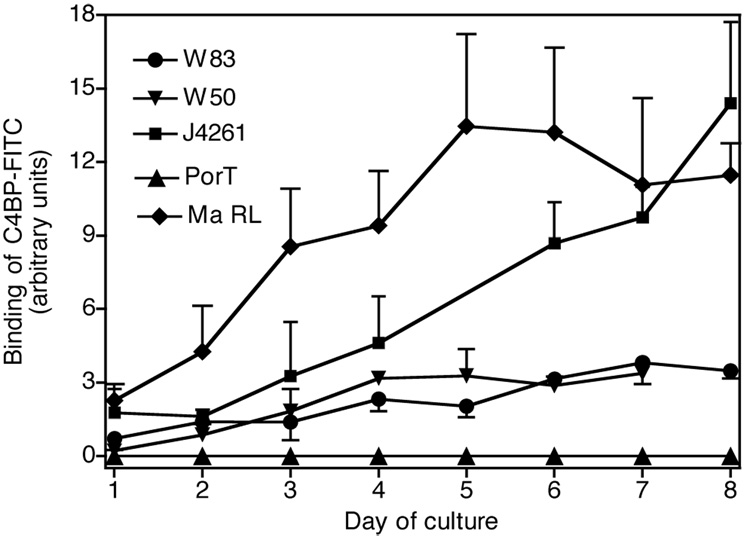
Next we tested whether binding of C4BP depends on stage of bacterial growth and maturity of colonies. Flow cytometry was used to assess binding of C4BP-FITC to strains W83, W50, J4261 and Ma RL cultured for 1–8 days on TSB agar plates (solid medium) and the binding of C4BP was observed to be strongly related to the age of P. gingivalis culture (Fig. 4). In agreement with our previous data, the clinical strains Ma RL and J4261 were the strongest binders of C4BP and the binding increased proportionally to the time of culture (Fig. 4). The PorT mutant showed no ability to bind C4BP irrespective of the cultivation time.
Fig. 4. Binding of C4BP depends on the age of culture.
Bacteria were grown for up to 8 days on TSB agar, harvested every day starting from day 1 after spreading on plates, washed twice and suspended in binding buffer at 6×105 cells/ml. Afterwards C4BP-FITC (50 µg/ml) was added for 30 minutes at RT, the cells were washed and analyzed by flow cytometry. Binding was investigated at least three times in duplicates for each time point.
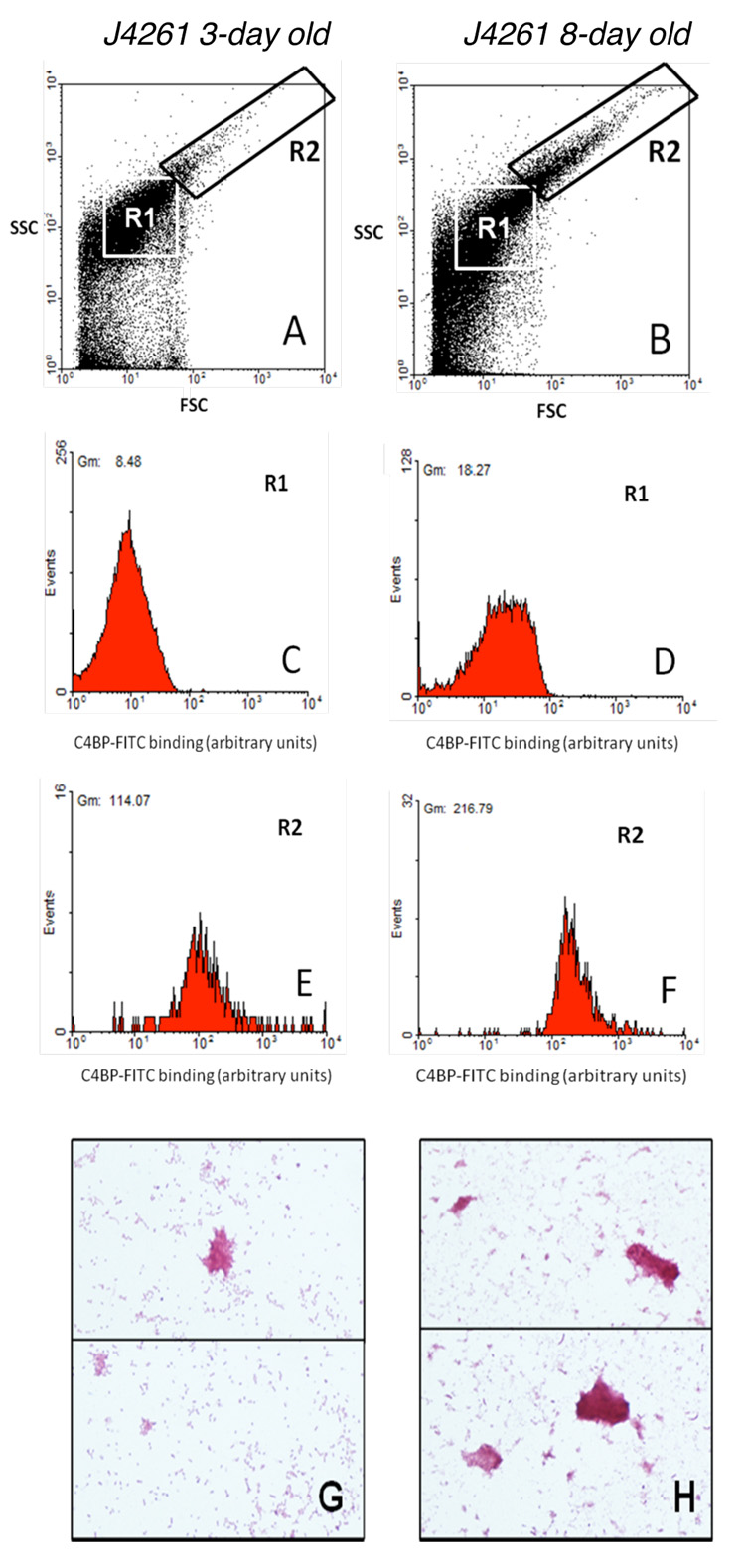
Furthermore, analysis of flow cytometry data indicated that a new subset of bacteria (strain J4261) was formed during culture aging (Fig 5B, gate R2). The bacterial cells in this new population appeared to be larger than single bacteria (Fig 5, gate R1) and was hypothesized to be aggregates of bacteria, which was confirmed microscopically after Gram staining (Fig. 5 G, H). We also found that dispersed bacterial cells of J4261 (gate R1) from 8-day old colonies bound C4BP to a greater extent than dispersed cells derived from 3-day old colonies (Fig. 5C, D). Importantly, the bacterial population in gate R2 that appeared in colonies after 8 days of culture bound very large amounts of C4BP. These aggregates bound more C4BP (Fig. 5F) than dispersed cells derived from 3 days (Fig. 5C) and 8 days (Fig. 5D) cultures. Similar data were obtained for the W83 strain although the aggregates were not as pronounced as in the case of the J4261 strain. On the contrary, binding of C4BP to PorT mutant was constantly at background levels irrespective of the cultivation time (not shown). Furthermore, the PorT mutant did not form any aggregates.
Fig. 5. Bacteria form aggregates during culture, which bind C4BP stronger than dispersed bacterial cells.
Wild type strain J4261 was harvested on a third and eight day of culture and incubated with 50 µg/ml C4BP-FITC. The binding was detected by flow cytometry. After 8 days of culture a subsets of bacteria forming aggregates appeared in gate R2 (B), which are not to be observed after 3 days of culture (A). These aggregates bound more C4BP (E, F) than single cells after both 3 (C) and 8 (D) days of culture. One representative flow cytometry graph of at least three independently performed is shown for each condition. The bacteria were also visualized using Gram staining after three (G) and eight (H) days of culture.
Binding of C4BP to P. gingivalis leads to decreased complement attack
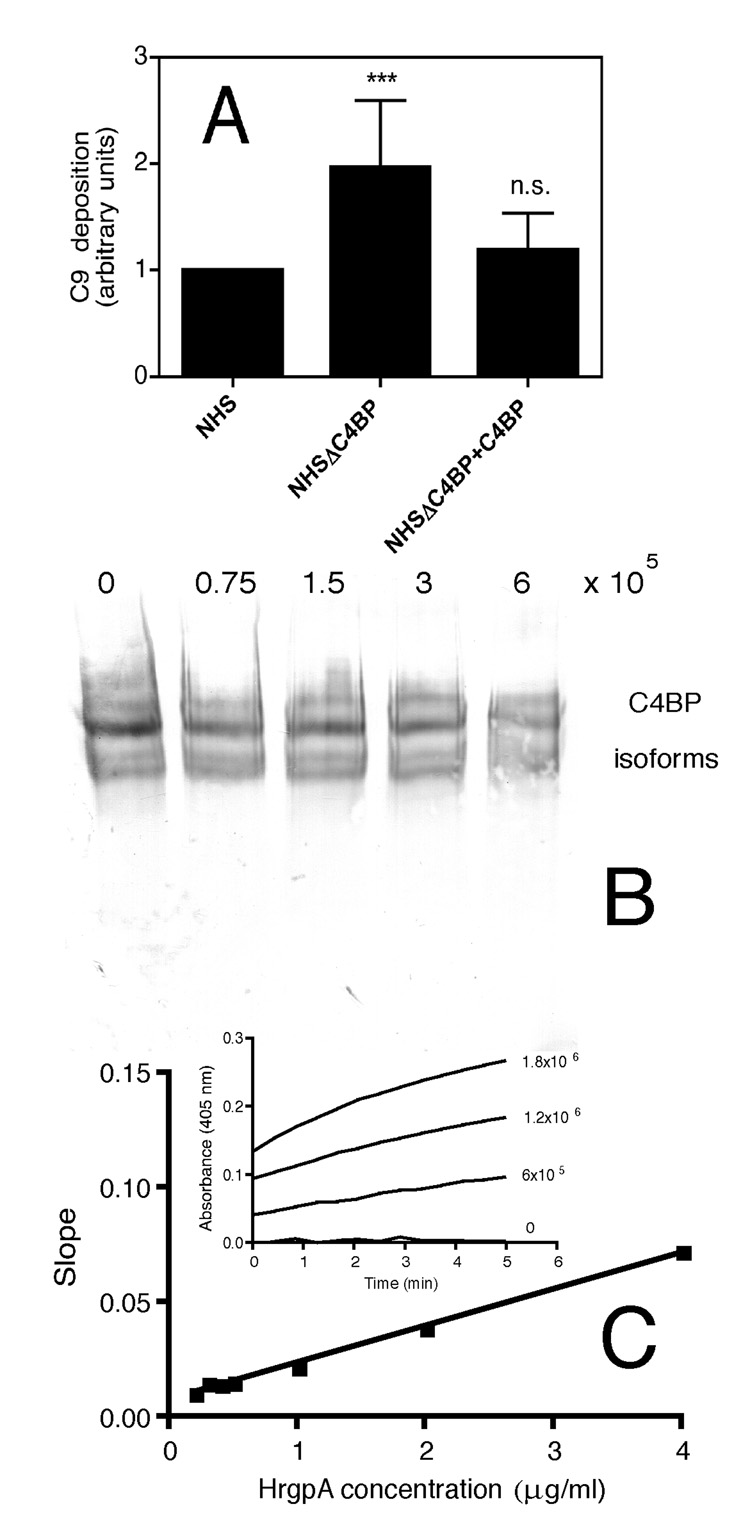
In order to investigate the functional consequence of the binding of C4BP, we compared the amount of deposited C9, the final component of the complement cascade, on the bacterial surface upon incubation with NHS as well as serum from which C4BP was depleted using affinity chromatography. We chose the J4261 strain for these experiments because it was one of the best binders of C4BP. When P. gingivalis J4261 strain was incubated with C4BP-depleted serum, twice as much C9 was deposited on the bacterial surface as compared to those incubated with NHS (Fig. 6A). Notably, upon adding back C4BP at a physiological concentration of 0.2 mg/ml to the C4BP-depleted serum, the amount of deposited C9 did not differ to the one in NHS (Fig. 6A). This implies that binding of C4BP provides an increased level of protection from complement attack.
Fig. 6. In the absence of C4BP P. gingivalis is more readily attacked by complement.
A) P. gingivalis strain J4261 was harvested after 9 days of culture. 6 × 105 cells were incubated in a total volume of 50 µl with 5% NHS in GVB++, 5% NHS lacking C4BP and 5% C4BP-depleted NHS reconstituted with C4BP, respectively. Thereafter, deposition of C9 on bacteria was investigated using polyclonal antibodies followed by flow cytometry analysis. Heat inactivated serum (56°C, 30 minutes) was used as negative control and the background value was subtracted from the responses obtained in the sera experimentation. The values are presented relative to the signal obtained with NHS. Statistical significance of differences between depleted and reconstituted sera and NHS was estimated with Student’s t test, *** p<0.001, n.s. – not significant B) NHS incubated with indicated amounts of the J4261 strain under the same conditions as in A was analyzed by western blotting under non-reducing conditions and C4BP detected with monoclonal antibodies. Under these conditions several isoforms of C4BP are separated C) Indicated concentrations of purified HRgpA were incubated with 1mM L-BAPNA and the kinetic reaction was followed by measurement of absorbance at 405 nm corresponding to the released product. The slopes of obtained linear curves were plotted against concentration of HRgpA. The strain J4261 at indicated concentrations was then incubated with L-BAPNA (insert).
Since gingipains are efficient proteinases we investigated if they would degrade C4BP in NHS in the conditions used for the C9 deposition assay. We found that at least 50% of C4BP remained intact at the end of the incubation period (Fig. 6B). C1q remained intact in the same samples while C3 was degraded with a similar efficiency as C4BP (not shown). In order to test if gingipains of the J4261 strain were active we used a kinetic assay. The standard curve was obtained for purified HRgpA (Fig. 6C) and the slopes of kinetic curves obtained for the J4261 strain allowed us to estimate that 6×105 bacteria used in the experiment presented in Fig 6A displayed an activity of arginine-specific gingipains corresponding to 67 +/− 13 ng of the purified enzyme.
Discussion
All successful human bacterial pathogens must develop strategies to circumvent the complement system. Complement-mediated killing is relevant for P. gingivalis since complement components are present in the gingival crevicular fluid at 70% of the serum concentration (34) and that P. gingivalis have been reported to activate both the classical and alternative pathways of complement (35). It has also been demonstrated in vivo that there is a high level of complement activation in the gingival crevicular fluid of patients with periodontitis (36, 37). Furthermore, specific antibody responses against P. gingivalis are of importance as there is a relationship between antibody titers, opsonic activity and accumulation of C3 (38) indicating the importance of the classical pathway in defense against this pathogen. However, P. gingivalis is able to override these host defense mechanisms as it is exceptionally resistant to bactericidal activity of human serum. One strategy of evasion depends on the production of large amounts of proteinases (gingipains), which are able to degrade several complement factors (39). Another strategy, as employed by many successful human bacterial pathogens, involves the capture of human complement inhibitors such as FH or C4BP in order to down-regulate complement attack. We could not detect interaction between FH and P. gingivalis (data not shown) but we did detect binding of C4BP. All tested clinical and laboratory strains of P. gingivalis bound C4BP. Notably, several clinical strains (J4261, Ma RL, J3741) bound more C4BP than the laboratory strains of P. gingivalis, whereas two other tested gram-negative anaerobic bacteria showed no binding. The fact that clinical isolates bound more C4BP than laboratory strains may suggest that there is a correlation between the ability to bind C4BP and strain virulence. We also observed that P. gingivalis form aggregates during culture and that these bind more C4BP than dispersed bacterial cells. However, even dispersed cells of P. gingivalis bound C4BP better after longer period of culture on agar plates. Taken together, it appeared that both aggregate formation (increased number of binding sites per particle) and accumulation of gingipains on the bacterial surface (mainly RgpA) resulted in an increase of C4BP binding to the bacteria.
Upon examining potential C4BP ligands on the bacterial surface we found that C4BP bound to gingipains, mainly to HRgpA. Accordingly, P. gingivalis mutants lacking RgpA showed significantly lower binding of C4BP. Significantly, purified RgpB showed only a weak binding of C4BP despite the fact that RgpB is practically identical to the catalytic domain of HRgpA. This observation suggests that a C4BP binding site may be located within the hemagglutinin/adhesin domain of RgpA (40). The hypothesis is further supported by the fact that interaction between C4BP and HRgpA was inhibited by fibrinogen that binds to the hemaglutinin domains of gingipains (9). The mutant lacking only Kgp showed a slight increase in binding of C4BP compared to the parental W83 strain. Since Kgp and HRgpA exist as a complex on the surface of the wild type strain (41), the absence of Kgp in the Kgp-null mutant may result in greater exposure of the C4BP binding site on the RgpA molecule resulting in higher binding in this mutant. Interestingly, deletion of both rgpA and rgpB genes did not entirely abolish the ability of the bacteria to bind C4BP whereas there was absolutely no interaction with the PorT mutant lacking not only gingipains but also other cell surface associated proteins carrying a specific C-terminal domain (42, 43). This suggests the presence of other surface ligand(s) in addition to HRgpA that may play a role in C4BP binding. One potential candidate for such a ligand is hemagglutinin A (HagA), in which some hemagglutinin/adhesin subdomains present in RgpA and Kgp are repeated several times (44) and exert the same hemagglutination, platelet aggregation, and hemoglobin binding activities as gingipains (45).
So far, all known binding sites for various pathogens including P. gingivalis as shown in this study are localized to the α-chains of C4BP, which is in agreement with the fact that the β-chain of C4BP is always occupied by protein S with which forms a high affinity, hydrophobic interaction (46). However, various domains of α-chains are utilized for interaction by pathogens. Neisseria gonorrhoeae (17) and Streptococcus pyogenes (19) bind the most N-terminal 70 amino acids i.e. CCP1. Bordetella pertussis (47) and Candida albicans (23) bind to somewhat larger area covering CCP1-2 with C. albicans also interacting with CCP6. Neisseria meningitidis (48) binds CCP2-3, while Moraxella catarrhalis (20) and Haemophilus influenzae (24) interact with CCP2 and CCP7 and Escherichia coli K1 with CCP3 (the main site) and CCP8 (21). In case of P. gingivalis we found two major interaction sites in CCP1 and CCP6-7. The interaction with CCP1 is further supported by the fact that the binding was inhibited by addition of mAb104 and C4b that both bind to this domain (27). Interestingly heparin, binding to CCP2-3 (27) and some positively charged amino acids on the interface between CCP1 and 2 (49) did not affect the binding supporting the hypothesis that P. gingivalis does not extend its binding into CCP2. Somewhat surprisingly, the binding of C4BP lacking CCP3 and CCP5 to HRgpA was increased in comparison to the wild type. Perhaps the binding site on CCP1 and CCP6-7 become more adjacent or oriented in a more preferred conformation, which yields better interaction. We have observed such effect for other ligands that have binding sites on both N- and C- terminus of the α-chains (manuscript in preparation). Most importantly, irrespectively of the binding domain for a particular pathogen C4BP always remains active when bound because of its polymeric nature. Even if several of its α-chains are engaged in interaction with pathogen, others are free to inhibit complement as we have shown numerous times previously (17, 20, 22–24, 48).
C4BP bound to the bacterial surface should inhibit complement activation by decreasing the level of C4 and C3 activation and subsequent downstream effects such as opsonisation with C3b, release of anaphylatoxins and formation of MAC. However, experiments proving that binding of C4BP to P. gingivalis impair their destruction by complement proved to be challenging. For example, we could not compare complement deposition on the wild-type and mutant strains lacking gingipains since these proteases by themselves are strong inhibitors of complement (39) and their proteolytic activity could not be dissociated from the ability to bind C4BP. Subsequently, comparison of complement deposition on clinical strains of P. gingivalis was found to be highly variable due to the differing initial amounts of C1 deposition leading to large differences in activation of complement, thus, precluding studies of the effect of C4BP binding. Finally, the PorT mutant lost entirely the ability to bind C1 but instead, acquired the capacity to intensively activate the alternative pathway. However, we did show that C4BP binding to bacteria has functional importance since bacteria challenged with C4BP-depleted serum exhibited a two-fold increase in C9 deposition on their surfaces in comparison to bacteria incubated with serum containing C4BP. Importantly, a large fraction of C4BP in NHS remained intact at the end of the incubation period with the bacteria harbouring active gingipains, indicating that they are relatively resistant to degradation by these proteases. Taken together, our data suggests that binding to C4BP is another strategy P. gingivalis could employ to enhance survival in the host and the fact that gingipains act as ligands for C4BP further emphasize the role of these cysteine proteases in bacterial virulence.
Table II.
Description of clinical P. gingivalis strains used in this study.
| Strain | % of cultivable flora | Patient | ||
|---|---|---|---|---|
| Diagnosis | Gender | Age (years) | ||
| J384-1* | 13 | Chronic periodontitis | Female | 43 |
| J374-1 | 40 | Aggressive periodontitis | Female | 24 |
| J420-1* | 8 | Aggressive periodontitis | Female | 23 |
| J362-9 | 53 | Chronic periodontitis | Male | 47 |
| J378-1 | 52 | Chronic periodontitis | Female | 55 |
| J358-1 | 4 | Chronic periodontitis | Female | 64 |
| J435-1* | 57 | Chronic periodontitis | Female | 46 |
| J426-1* | 48 | Chronic periodontitis | Female | 40 |
| Ma RL* | 36 | Chronic periodontitis | Male | 72 |
| Ma P4 | 52 | Chronic periodontitis | Male | 42 |
| D-2-4-3 | 40 | Chronic periodontitis | Male | 39 |
| JH16-1 | 4 | Aggressive periodontitis | Female | 28 |
Patients with cardiovascular disease
Footnotes
This work was supported by Swedish Foundation for Strategic Research (INGVAR), Swedish Medical Research Council, Foundations of Österlund, Kock, King Gustav V’s 80th Anniversary, Knut and Alice Wallenberg, Inga-Britt and Arne Lundberg and research grants from the University Hospital in Malmö (to AB) and grants from Ministry of Science and Higher Education (Warsaw, Poland) and the National Institutes of Health Grant DE 09761, USA (to JP). Mrs Margareta Pålsson is acknowledged for expert technical help.
Abbreviations: C3b, activated complement factor 3; C4b, activated complement factor 4; C4BP, C4b-binding protein; CCP, complement control protein (domain); FH, factor H; GVB++, barbiturate buffer with gelatin; mAb, monoclonal antibody; MAC, membrane attack complex; MBL, mannose-binding lectin; NHS, normal human serum; pAb, polyclonal antibody; TSB, tryptic soy broth
References
- 1.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 2.Socransky SS, Haffajee AD, Smith C, Dibart S. Relation of counts of microbial species to clinical status at the sampled site. J Clin Periodontol. 1991;18:766–775. doi: 10.1111/j.1600-051x.1991.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 3.Whitney C, Ant J, Moncla B, Johnson B, Page RC, Engel D. Serum immunoglobulin G antibody to Porphyromonas gingivalis in rapidly progressive periodontitis: titer, avidity, and subclass distribution. Infect Immun. 1992;60:2194–2200. doi: 10.1128/iai.60.6.2194-2200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffen AL, Becker MR, Lyons SR, Moeschberger ML, Leys EJ. Prevalence of Porphyromonas gingivalis and periodontal health status. J Clin Microbiol. 1998;36:3239–3242. doi: 10.1128/jcm.36.11.3239-3242.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holt SC, Ebersole J, Felton J, Brunsvold M, Kornman KS. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988;239:55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- 6.Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13 Suppl 4:3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 7.Holt SC, Bramanti TE. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2:177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- 8.Potempa J, Sroka A, Imamura T, Travis J. Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr Protein Pept Sci. 2003;4:397–407. doi: 10.2174/1389203033487036. [DOI] [PubMed] [Google Scholar]
- 9.Pike RN, Potempa J, McGraw W, Coetzer TH, Travis J. Characterization of the binding activities of proteinase-adhesin complexes from Porphyromonas gingivalis. J Bacteriol. 1996;178:2876–2882. doi: 10.1128/jb.178.10.2876-2882.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potempa J, Banbula A, Travis J. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol 2000. 2000;24:153–192. doi: 10.1034/j.1600-0757.2000.2240108.x. [DOI] [PubMed] [Google Scholar]
- 11.Walport MJ. Complement. First of two parts. N. Engl. J. Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 12.Fujita T, Gigli I, Nussenzweig V. Human C4-binding protein. II. Role in proteolysis of C4b by C3b-inactivator. J. Exp. Med. 1978;148:1044–1051. doi: 10.1084/jem.148.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blom AM, Kask L, Dahlbäck B. CCP1-4 of the C4b-binding protein a-chain are required for Factor I mediated cleavage of C3b. Mol. Immunol. 2003;39:547–556. doi: 10.1016/s0161-5890(02)00213-4. [DOI] [PubMed] [Google Scholar]
- 14.Dahlbäck B, Stenflo J. High molecular weight complex in human plasma between vitamin K-dependent protein S and complement component C4b-binding protein. Proc. Natl. Acad. Sci. USA. 1981;78:2512–2516. doi: 10.1073/pnas.78.4.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blom AM, Villoutreix BO, Dahlbäck B. Complement inhibitor C4b-binding protein - friend or foe in the innate immune system? Mol. Immunol. 2004;40:1333–1346. doi: 10.1016/j.molimm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Carlsson F, Berggård K, Stalhammar-Carlemalm M, Lindahl G. Evasion of Phagocytosis through Cooperation between Two Ligand-binding Regions in Streptococcus pyogenes M Protein. J Exp Med. 2003;198:1057–1068. doi: 10.1084/jem.20030543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ram SM, Cullinane M, Blom AM, Gulati S, McQuillen DP, Monks BG, O’ Connell C, Boden R, Elkins C, Pangburn MK, Dahlbäck B, Rice PA. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J. Exp. Med. 2001;193:281–296. doi: 10.1084/jem.193.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ngampasutadol J, Ram S, Blom AM, Jarva H, Jerse AE, Lien E, Goguen J, Gulati S, Rice PA. Human C4b-binding protein selectively interacts with Neisseria gonorrhoeae and results in species-specific infection. Proc Natl Acad Sci U S A. 2005;102:17142–17147. doi: 10.1073/pnas.0506471102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blom AM, Berggård K, Webb JH, Lindahl G, Villoutreix BO, Dahlbäck B. Human C4b-binding protein has overlapping but not identical binding sites for C4b and streptococcal M proteins. J. Immunol. 2000;164:5328–5336. doi: 10.4049/jimmunol.164.10.5328. [DOI] [PubMed] [Google Scholar]
- 20.Nordström T, Blom AM, Forsgren A, Riesbeck K. The emerging pathogen Moraxella catarrhalis interacts with complement inhibitor C4b-binding protein through ubiquitous surface proteins A1 and A2. J. Immunol. 2004;173:4598–4606. doi: 10.4049/jimmunol.173.7.4598. [DOI] [PubMed] [Google Scholar]
- 21.Prasadarao NV, Blom AM, Villoutreix BO, Linsangan LC. A novel interaction of outer membrane protein A with C4b-binding protein mediates serum resistance of Escherichia coli K1. J. Immunol. 2002;169:6352–6360. doi: 10.4049/jimmunol.169.11.6352. [DOI] [PubMed] [Google Scholar]
- 22.Meri T, Cutler SJ, Blom AM, Meri S, Jokiranta TS. Relapsing fever spirochetes Borrelia recurrentis and B. duttonii acquire complement regulators C4b-binding protein and factor H. Infect Immun. 2006;74:4157–4163. doi: 10.1128/IAI.00007-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meri T, Blom AM, Hartmann A, Lenk D, Meri S, Zipfel PF. The yeast and hyphal forms of Candida albicans bind complement regulator C4b-binding protein. Infect. Immun. 2004;11:6633–6641. doi: 10.1128/IAI.72.11.6633-6641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallstrom T, Jarva H, Riesbeck K, Blom AM. Interaction with C4b-binding protein contributes to nontypeable Haemophilus influenzae serum resistance. J Immunol. 2007;178:6359–6366. doi: 10.4049/jimmunol.178.10.6359. [DOI] [PubMed] [Google Scholar]
- 25.Dahlbäck B. Purification of human C4b-binding protein and formation of its complex with vitamin K-dependent protein S. Biochem. J. 1983;209:847–856. doi: 10.1042/bj2090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb JH, Blom AM, Dahlbäck B. Vitamin K-dependent protein S localizing complement regulator C4b-binding protein to the surface of apoptotic cells. J. Immunol. 2002;169:2580–2586. doi: 10.4049/jimmunol.169.5.2580. [DOI] [PubMed] [Google Scholar]
- 27.Blom AM, Kask L, Dahlbäck B. Structural requirements for the complement regulatory activities of C4BP. J. Biol. Chem. 2001;276:27136–27144. doi: 10.1074/jbc.M102445200. [DOI] [PubMed] [Google Scholar]
- 28.Pike R, McGraw W, Potempa J, Travis J. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Isolation, characterization, and evidence for the existence of complexes with hemagglutinins. J Biol Chem. 1994;269:406–411. [PubMed] [Google Scholar]
- 29.Potempa J, Mikolajczyk-Pawlinska J, Brassell D, Nelson D, Thogersen IB, Enghild JJ, Travis J. Comparative properties of two cysteine proteinases (gingipains R), the products of two related but individual genes of Porphyromonas gingivalis. J Biol Chem. 1998;273:21648–21657. doi: 10.1074/jbc.273.34.21648. [DOI] [PubMed] [Google Scholar]
- 30.Sato K, Sakai E, Veith PD, Shoji M, Kikuchi Y, Yukitake H, Ohara N, Naito M, Okamoto K, Reynolds EC, Nakayama K. Identification of a new membrane-associated protein that influences transport/maturation of gingipains and adhesins of Porphyromonas gingivalis. J Biol Chem. 2005;280:8668–8677. doi: 10.1074/jbc.M413544200. [DOI] [PubMed] [Google Scholar]
- 31.Kask L, Trouw L, Dahlbäck B, Blom AM. C4b-binding protein-protein S complex inhibits phagocytosis of apoptotic cells. J. Biol. Chem. 2004;279:23869–23873. doi: 10.1074/jbc.C400159200. [DOI] [PubMed] [Google Scholar]
- 32.Potempa J, Nguyen KA. Current protocols in protein science / editorial board John E. Coligan … [et al. 2007. Purification and characterization of gingipains. Chapter 21. Unit 21 20. [DOI] [PubMed] [Google Scholar]
- 33.Kadowaki T, Nakayama K, Yoshimura F, Okamoto K, Abe N, Yamamoto K. Arg-gingipain acts as a major processing enzyme for various cell surface proteins in Porphyromonas gingivalis. J Biol Chem. 1998;273:29072–29076. doi: 10.1074/jbc.273.44.29072. [DOI] [PubMed] [Google Scholar]
- 34.Schenkein HA, Genco RJ. Gingival fluid and serum in periodontal diseases. I. Quantitative study of immunoglobulins, complement components, and other plasma proteins. J Periodontol. 1977;48:772–777. doi: 10.1902/jop.1977.48.12.772. [DOI] [PubMed] [Google Scholar]
- 35.Okuda K, Kato T, Naito Y, Ono M, Kikuchi Y, Takazoe I. Susceptibility of Bacteroides gingivalis to bactericidal activity of human serum. J Dent Res. 1986;65:1024–1027. doi: 10.1177/00220345860650070601. [DOI] [PubMed] [Google Scholar]
- 36.Attström R, Laurel AB, Lahsson U, Sjöholm A. Complement factors in gingival crevice material from healthy and inflamed gingiva in humans. J Periodontal Res. 1975;10:19–27. doi: 10.1111/j.1600-0765.1975.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 37.Schenkein HA, Genco RJ. Gingival fluid and serum in periodontal diseases. II. Evidence for cleavage of complement components C3, C3 proactivator (factor B) and C4 in gingival fluid. J Periodontol. 1977;48:778–784. doi: 10.1902/jop.1977.48.12.778. [DOI] [PubMed] [Google Scholar]
- 38.Cutler CW, Arnold RR, Schenkein HA. Inhibition of C3 and IgG proteolysis enhances phagocytosis of Porphyromonas gingivalis. J Immunol. 1993;151:7016–7029. [PubMed] [Google Scholar]
- 39.Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J. Immunol. 2007;178:7242–7250. doi: 10.4049/jimmunol.178.11.7242. [DOI] [PubMed] [Google Scholar]
- 40.Curtis MA, Kuramitsu HK, Lantz M, Macrina FL, Nakayama K, Potempa J, Reynolds EC, Aduse-Opoku J. Molecular genetics and nomenclature of proteases of Porphyromonas gingivalis. J Periodontal Res. 1999;34:464–472. doi: 10.1111/j.1600-0765.1999.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 41.Takii R, Kadowaki T, Baba A, Tsukuba T, Yamamoto K. A functional virulence complex composed of gingipains, adhesins, and lipopolysaccharide shows high affinity to host cells and matrix proteins and escapes recognition by host immune systems. Infect Immun. 2005;73:883–893. doi: 10.1128/IAI.73.2.883-893.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seers CA, Slakeski N, Veith PD, Nikolof T, Chen YY, Dashper SG, Reynolds EC. The RgpB C-terminal domain has a role in attachment of RgpB to the outer membrane and belongs to a novel C-terminal-domain family found in Porphyromonas gingivalis. J Bacteriol. 2006;188:6376–6386. doi: 10.1128/JB.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen K, Travis J, Potempa J. Does the importance of the C-terminal residues in the maturation of RgpB from Porphyromonas gingivalis reveal a novel mechanism for protein export in a subgroup of Gram-negative bacteria? J. Bacteriol. 2007;189:833–843. doi: 10.1128/JB.01530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kozarov E, Whitlock J, Dong H, Carrasco E, Progulske-Fox A. The number of direct repeats in hagA is variable among Porphyromonas gingivalis strains. Infect Immun. 1998;66:4721–4725. doi: 10.1128/iai.66.10.4721-4725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi Y, Ratnayake DB, Okamoto K, Abe N, Yamamoto K, Nakayama K. Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis. Construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J Biol Chem. 1999;274:17955–17960. doi: 10.1074/jbc.274.25.17955. [DOI] [PubMed] [Google Scholar]
- 46.Blom AM, Covell DG, Wallqvist A, Dahlbäck B, Villoutreix BO. The C4b-binding protein-protein S interaction is hydrophobic in nature. Biochim. Biophys. Acta. 1998;1388:181–189. doi: 10.1016/s0167-4838(98)00178-2. [DOI] [PubMed] [Google Scholar]
- 47.Berggård K, Lindahl G, Dahlbäck B, Blom AM. Bordetella pertussis binds to human C4b-binding protein (C4BP) at a site similar to that used by the natural ligand C4b. Eur J Immunol. 2001;31:2771–2780. doi: 10.1002/1521-4141(200109)31:9<2771::aid-immu2771>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 48.Jarva H, Ram S, Vogel U, Blom AM, Meri S. Binding of the complement inhibitor C4bp to serogroup B Neisseria meningitidis. J Immunol. 2005;174:6299–6307. doi: 10.4049/jimmunol.174.10.6299. [DOI] [PubMed] [Google Scholar]
- 49.Blom AM, Webb J, Villoutreix BO, Dahlbäck B. A cluster of positively charged amino acids in the N-terminal modules of the C4BP a-chain is crucial for C4b binding and factor I cofactor function. J. Biol. Chem. 1999;274:19237–19245. doi: 10.1074/jbc.274.27.19237. [DOI] [PubMed] [Google Scholar]
- 50.Sztukowska M, Sroka A, Bugno M, Banbula A, Takahashi Y, Pike RN, Genco CA, Travis J, Potempa J. The C-terminal domains of the gingipain K polyprotein are necessary for assembly of the active enzyme and expression of associated activities. Mol Microbiol. 2004;54:1393–1408. doi: 10.1111/j.1365-2958.2004.04357.x. [DOI] [PubMed] [Google Scholar]
- 51.Aduse-Opoku J, Davies NN, Gallagher A, Hashim A, Evans HE, Rangarajan M, Slaney JM, Curtis MA. Generation of lys-gingipain protease activity in Porphyromonas gingivalis W50 is independent of Arg-gingipain protease activities. Microbiology. 2000;146:1933–1940. doi: 10.1099/00221287-146-8-1933. [DOI] [PubMed] [Google Scholar]