Abstract
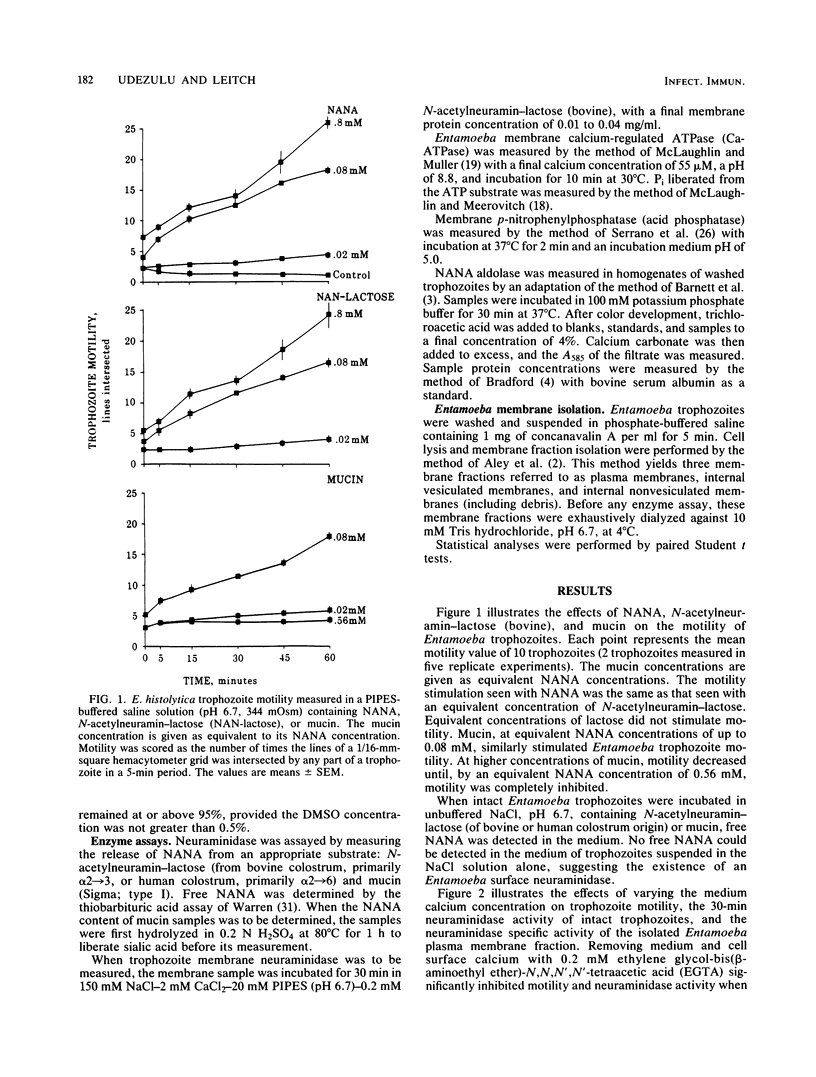
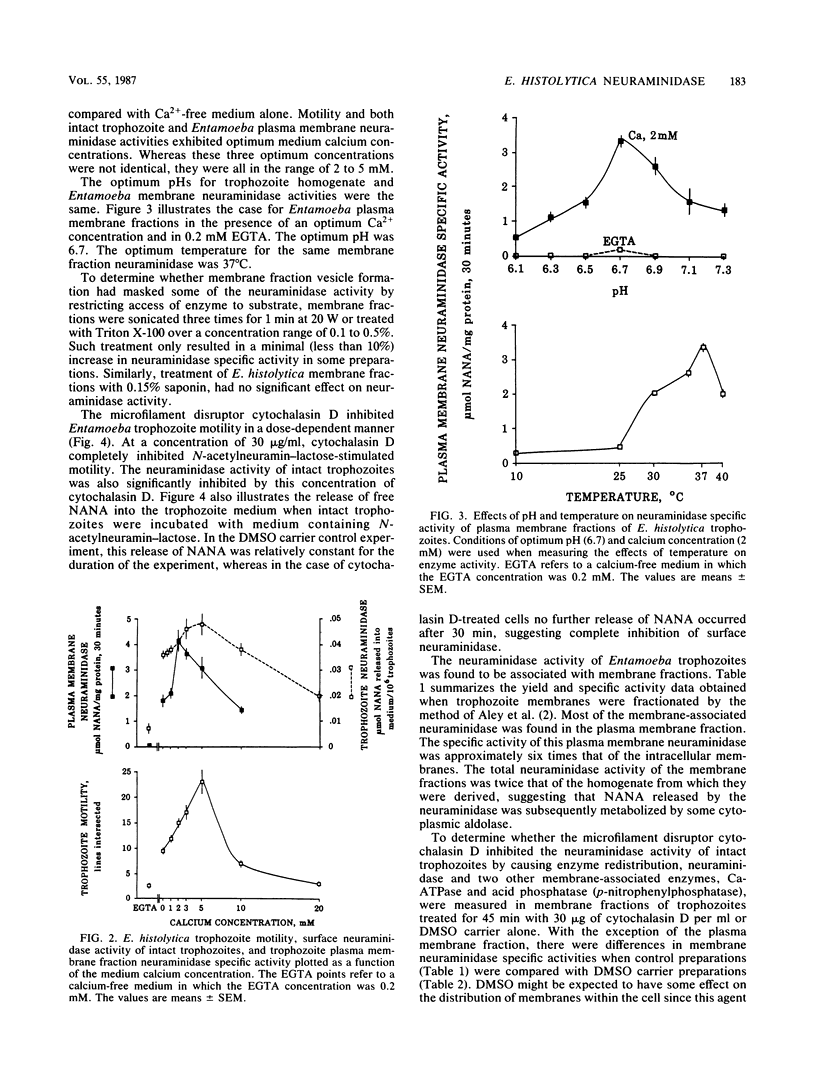
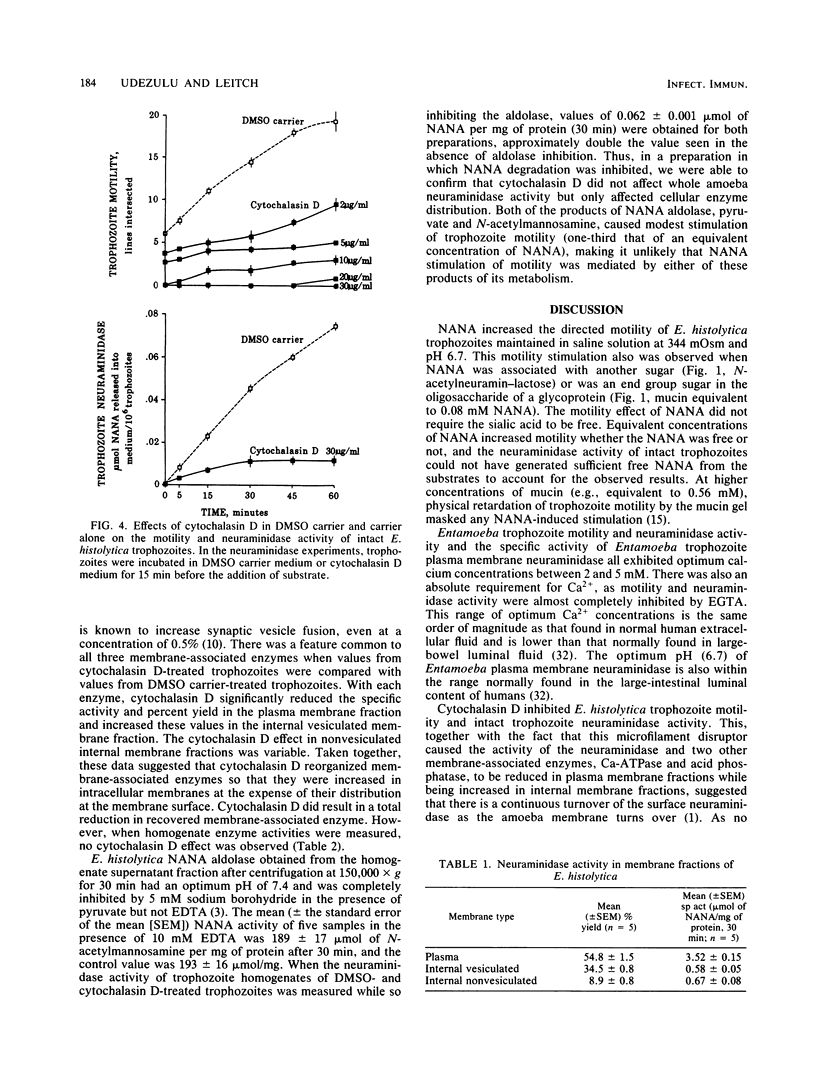
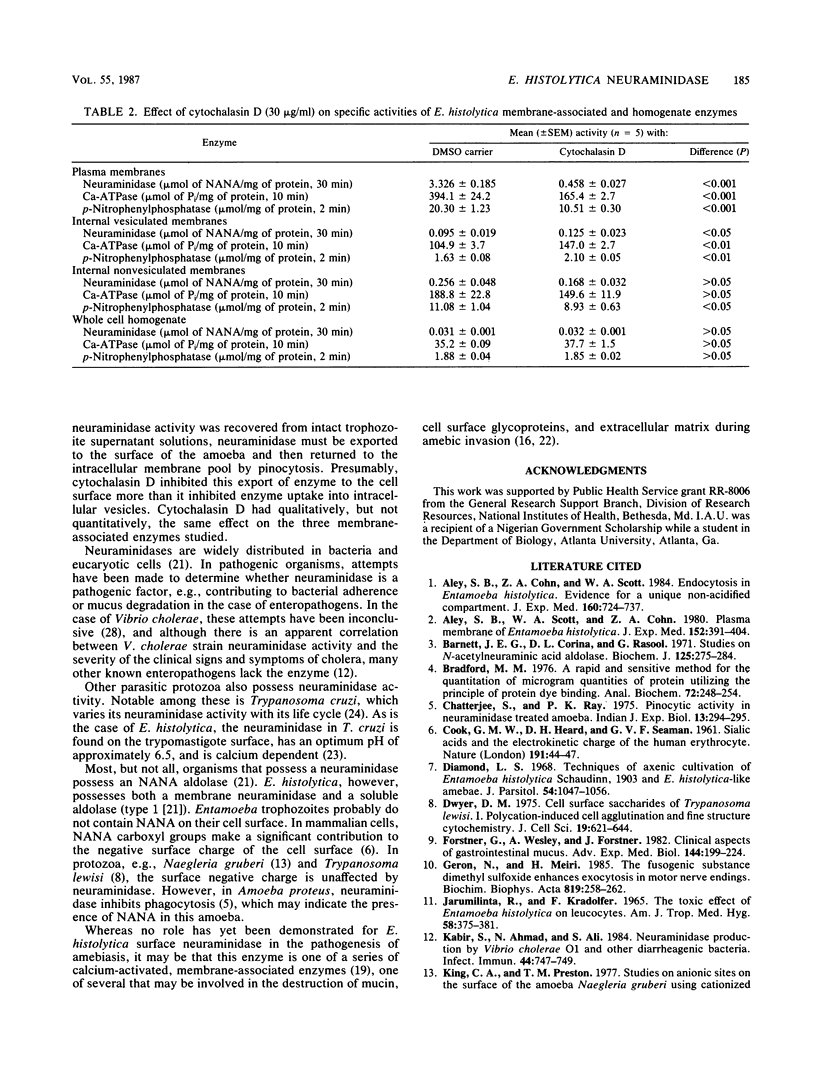
Trophozoites of the parasitic amoeba Entamoeba histolytica HM-1:IMSS possess a surface neuraminidase capable of liberating N-acetylneuraminic acid (NANA) from N-acetylneuramin-lactose (alpha 2----3 or alpha 2----6) or mucin in their medium. The neuraminidase was found to be membrane associated, with more than 50% of the yield being recovered in the plasma membrane fraction. The neuraminidase specific activity of the plasma membrane fraction was six times that of internal membrane fraction enzyme. The optimum pH and temperature for this enzyme were 6.7 and 37 degrees C, respectively. Neuraminidase activity was inhibited by ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid, and the optimum Ca2+ concentration was 2 mM. The microfilament disruptor cytochalasin D (30 micrograms/ml) inhibited motility and neuraminidase activity of intact Entamoeba trophozoites. The cytochalasin D-induced loss of surface neuraminidase activity was explained in part by a redistribution of enzyme with a loss of plasma membrane enzyme and an increase in intracellular membrane enzyme. A qualitatively similar cytochalasin D effect was observed with two other membrane-associated enzymes, calcium-regulated ATPase and acid phosphatase. Membrane-associated enzyme was minimally affected by Triton X-100 and saponin. An N-acetylneuraminic acid aldolase, optimum pH, 7.4, was found in trophozoite homogenate supernatant fractions. NANA and NANA-containing compounds stimulated trophozoite-directed motility. This motility stimulation by NANA-containing compounds did not apparently require prior release of free NANA by the trophozoite surface neuraminidase. Entamoeba neuraminidase is one of a series of enzymes that may modify the mucus blanket and target cell surface and thereby play a role in the pathogenesis of amebiasis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aley S. B., Cohn Z. A., Scott W. A. Endocytosis in Entamoeba histolytica. Evidence for a unique non-acidified compartment. J Exp Med. 1984 Sep 1;160(3):724–737. doi: 10.1084/jem.160.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley S. B., Scott W. A., Cohn Z. A. Plasma membrane of Entamoeba histolytica. J Exp Med. 1980 Aug 1;152(2):391–404. doi: 10.1084/jem.152.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett J. E., Corina D. L., Rasool G. Studies on N-acetylneuraminic acid aldolase. Biochem J. 1971 Nov;125(1):275–284. doi: 10.1042/bj1250275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- COOK G. M., HEARD D. H., SEAMAN G. V. Sialic acids and the electrokinetic charge of the human erythrocyte. Nature. 1961 Jul 1;191:44–47. doi: 10.1038/191044a0. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Ray P. K. Pinocytic activity in neuraminidase treated ameba. Indian J Exp Biol. 1975 May;13(3):294–295. [PubMed] [Google Scholar]
- Diamond L. S. Techniques of axenic cultivation of Entamoeba histolytica Schaudinn, 1903 and E. histolytica-like amebae. J Parasitol. 1968 Oct;54(5):1047–1056. [PubMed] [Google Scholar]
- Dwyer D. M. Cell surface saccharides of Trypanosoma lewisi. I. Polycation-induced cell agglutination and fine-structure cytochemistry. J Cell Sci. 1975 Dec;19(3):621–644. doi: 10.1242/jcs.19.3.621. [DOI] [PubMed] [Google Scholar]
- Forstner G., Wesley A., Forstner J. Clinical aspects of gastrointestinal mucus. Adv Exp Med Biol. 1982;144:199–224. doi: 10.1007/978-1-4615-9254-9_32. [DOI] [PubMed] [Google Scholar]
- Geron N., Meiri H. The fusogenic substance dimethyl sulfoxide enhances exocytosis in motor nerve endings. Biochim Biophys Acta. 1985 Oct 10;819(2):258–262. doi: 10.1016/0005-2736(85)90181-6. [DOI] [PubMed] [Google Scholar]
- JARUMILINTA R., KRADOLFER F. THE TOXIC EFFECT OF ENTAMOEBA HISTOLYTICA ON LEUCOCYTES. Ann Trop Med Parasitol. 1964 Sep;58:375–381. doi: 10.1080/00034983.1964.11686259. [DOI] [PubMed] [Google Scholar]
- Kabir S., Ahmad N., Ali S. Neuraminidase production by Vibrio cholerae O1 and other diarrheagenic bacteria. Infect Immun. 1984 Jun;44(3):747–749. doi: 10.1128/iai.44.3.747-749.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. A., Preston T. M. Studies of anionic sites on the cell surface of the amoeba Naegleria gruberi using cationized ferritin. J Cell Sci. 1977 Dec;28:133–149. doi: 10.1242/jcs.28.1.133. [DOI] [PubMed] [Google Scholar]
- Kobiler D., Mirelman D. Lectin activity in Entamoeba histolytica trophozoites. Infect Immun. 1980 Jul;29(1):221–225. doi: 10.1128/iai.29.1.221-225.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch G. J., Dickey A. D., Udezulu I. A., Bailey G. B. Entamoeba histolytica trophozoites in the lumen and mucus blanket of rat colons studied in vivo. Infect Immun. 1985 Jan;47(1):68–73. doi: 10.1128/iai.47.1.68-73.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J., Meerovitch E. A simple and sensitive modification of the Chen procedure for orthophosphate determination in the presence of Triton X-100. Anal Biochem. 1976 Feb;70(2):643–644. doi: 10.1016/0003-2697(76)90495-4. [DOI] [PubMed] [Google Scholar]
- McLaughlin J., Müller M. A calcium regulated adenosine triphosphatase in Entamoeba histolytica. Mol Biochem Parasitol. 1981 Oct;3(6):369–379. doi: 10.1016/0166-6851(81)90037-2. [DOI] [PubMed] [Google Scholar]
- Mora-Galindo J., Martínez-Palomo A., González-Robles A. Interacción entre Entamoeba histolytica y epitelio cecal del cobayo. Estudio cuantitativo. Arch Invest Med (Mex) 1982;13 (Suppl 3):233–243. [PubMed] [Google Scholar]
- Muńoz M. L., Calderón J., Rojkind M. The collagenase of Entamoeba histolytica. J Exp Med. 1982 Jan 1;155(1):42–51. doi: 10.1084/jem.155.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. E. A developmentally regulated neuraminidase activity in Trypanosoma cruzi. Science. 1983 Mar 25;219(4591):1444–1446. doi: 10.1126/science.6338592. [DOI] [PubMed] [Google Scholar]
- Pereira M. E. A rapid and sensitive assay for neuraminidase using peanut lectin hemagglutination: application to Vibrio cholera and Trypanosoma cruzi. J Immunol Methods. 1983 Sep 30;63(1):25–34. doi: 10.1016/0022-1759(83)90206-5. [DOI] [PubMed] [Google Scholar]
- Ravdin J. I., Murphy C. F., Salata R. A., Guerrant R. L., Hewlett E. L. N-Acetyl-D-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. I. Partial purification and relation to amoebic virulence in vitro. J Infect Dis. 1985 May;151(5):804–815. doi: 10.1093/infdis/151.5.804. [DOI] [PubMed] [Google Scholar]
- Serrano R., Deas J. E., Warren L. G. Entamoeba histolytica: membrane fractions. Exp Parasitol. 1977 Apr;41(2):370–384. doi: 10.1016/0014-4894(77)90109-6. [DOI] [PubMed] [Google Scholar]
- Silberberg A., Meyer F. A. Structure and function of mucus. Adv Exp Med Biol. 1982;144:53–74. doi: 10.1007/978-1-4615-9254-9_6. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Phillips B. P. Electron microscope studies of experimental Entamoeba histolytica infection in the guinea pig. I. Penetration of the intestinal epithelium by trophozoites. Am J Trop Med Hyg. 1975 Jan;24(1):34–48. doi: 10.4269/ajtmh.1975.24.34. [DOI] [PubMed] [Google Scholar]
- Trissl D., Martínez-Palomo A., de la Torre M., de la Hoz R., Pérez de Suárez E. Surface properties of Entamoeba: increased rates of human erythrocyte phagocytosis in pathogenic strains. J Exp Med. 1978 Nov 1;148(5):1137–1143. doi: 10.1084/jem.148.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WRONG O., METCALFE-GIBSON A., MORRISON R. B., NG S. T., HOWARD A. V. IN VIVO DIALYSIS OF FAECES AS A METHOD OF STOOL ANALYSIS. I. TECHNIQUE AND RESULTS IN NORMAL SUBJECTS. Clin Sci. 1965 Apr;28:357–375. [PubMed] [Google Scholar]


