Abstract
Background
Different mechanisms of diabetic-induced NO dysfunction have been proposed and central to most of them are significant changes in eNOS function as the rate-limiting step in NO bioavailability. eNOS exists in both monomeric and dimeric conformations, with the dimeric form catalyzing the synthesis of nitric oxide, while the monomeric form catalyzes the synthesis of superoxide (O2-). Diabetic-induced shifts to decrease the dimer:monomer ratio is thought to contribute to the degradation of nitric oxide (NO) bioavailability. Exercise has long been useful in the management of diabetes. Although exercise-induced increases expression of eNOS has been reported, it is unclear if exercise may alter the functional coupling of eNOS.
Methods
To investigate this question, Goto-Kakizaki rats (a model of type II diabetes) were randomly assigned to a 9-week running program (train) or sedentary (sed) groups.
Results
Exercise training significantly (p < .05) increased plantaris muscle cytochrome oxidase, significantly improved glycosylated hemoglobin (sed: 7.33 ± 0.56%; train: 6.1 ± 0.18%), ad improved insulin sensitivity. Exercise increased both total eNOS expression and the dimer:monomer ratio in the left ventricle LV (sed: 11.7 ± 3.2%; train: 41.4 ± 4.7%). Functional analysis of eNOS indicated that exercise induced significant increases in nitric oxide (+28%) production and concomitant decreases in eNOS-dependent superoxide (-12%) production. This effect was observed in the absence of tetrahydrobiopterin (BH4), but not in the presence of exogenous BH4. Exercise training also significantly decreased NADPH-dependent O2- activity.
Conclusion
Exercise-induced increased eNOS dimerization resulted in an increased coupling of the enzyme to facilitate production of NO at the expense of ROS generation. This shift that could serve to decrease diabetic-related oxidative stress, which should serve to lessen diabetic-related complications.
Background
In the management of diabetes there is considerable evidence to demonstrate the benefits of exercise including improved glycemic control, an increased quality of life, and a reduction of cardiovascular risk factors. Exercise with and without dietary changes resulted in a significant reduction in glycosylated hemoglobin (HbA1c), increased insulin sensitivity, improved blood lipid levels, and lowered blood pressure [1,2]. Even low intensity forms of exercise such as walking will benefit NIDDM patients [1].
Exercise induces angiogenesis and altered vasculature reactivity in different vascular beds [3,4]. Exercise increases the sensitivity to endothelium-dependent relaxation by acetylcholine, but not the endothelium-independent response to sodium nitroprusside [3]. Chronic exercise increases NO production as early as one week after the start of training [4]. These changes are thought to be the result of increased eNOS protein [5,6]. Training effects may be limited to the vasculature of the working muscles; no effect was observed in mesenteric arterioles, suggesting that exercise-induced increases in stress may have be the responsible mechanism [7]. Several groups have reported that shear stress induces increases in eNOS expression [8,9]. However, studies in both diabetic patients and in diabetic animals have yielded different results; that vascular beds not participating in the response to exercise demonstrate significant improvements, suggesting that mechanisms other than localized stimuli are important [10,11].
Nitric oxide (NO) signaling regulates vascular tone, inhibits components of the atherogenic process, and influences myocardial energy consumption [12,13]. NO synthesis is governed by nitric oxide synthase (NOS). Three isoforms of NOS have been identified which are the products of three separate genes; endothelial NOS (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS). These isoforms share about 50–60% sequence identity and all use L-arginine, O2, and NADPH to catalyze the synthesis of NADP, citrulline, and NO as well as superoxide. Structural domain studies of the NOS molecule have identified separate oxygenase and reductase domains [14]. Dimerization is a requirement for catalytic activity of eNOS, although the truly active form is a complex that includes calmodulin, FAD, tetrahydrobiopterin (BH4), and iron protoporphyrin IX (haem) [14]. The dimeric form catalyzes the rate-limiting step in the synthesis of nitric oxide, while the monomeric form catalyzes the synthesis of O2-, a highly reactive oxidant species (ROS) [15]. The products catalyzed by eNOS are subject to complex regulation that we are just now beginning to understand. NO is an autocrine factor that regulates myocardial functioning via multiple mechanisms [16]. More recently Zhang et.al demonstrated that exercise training was associated with increased myocardial eNOS levels and enhanced myocardial contractility [17].
Different mechanisms of diabetic-induced NO dysfunction have been proposed and central to most of them are significant changes in eNOS function as the rate-limiting step in NO bioavailability. Several studies have reported decreased eNOS activity/protein levels in diabetic patients or animal models of diabetes [18-20]. The composition of the eNOS complex is critical for the relative formation of NO or superoxide formation. The mechanisms responsible for eNOS dysfunction remain unclear, however, a decrease in the dimer to monomer eNOS ratio within the myocardium of diabetic animals has been reported [15]. Although exercise-induced increases in eNOS expression have been documented, it is unclear if exercise may also alter the functional coupling of eNOS. To investigate this question, Goto-Kakizaki rats, a model of NIDDM, were exercise trained, to test if chronic exercise could improve eNOS function and enhance NO bioavailability.
Methods
Training Protocol
Twenty male GK rats were randomly assigned to exercise training (train) or sedentary (sed) groups. Rats were run on a motor driven treadmill set at a ten-degree incline. Animals were initially run at approximately 50% VO2max and the animals were run for up to 60 minutes and 5 days/week for 9 weeks. While training, animals were closely monitored to ensure animal safety and training compliance. Experimental protocols had institutional approval and animals were maintained in accordance with APS's Guiding Principles in the Care and Use of Animals and the Guide for the Care and Use of Laboratory Animals.
Glucose Tolerance Test
Following an overnight fast, animals were injected with Nembutal (40 mg/kg i.p.). To perform the glucose tolerance test, sterile glucose (1.0 g glucose/kg i.p.) was injected into the abdominal cavity and tail vein blood sampled at selected intervals. Insulin was determined from plasma samples by ELISA (Crystal Chem, Downers Grove, IL). Following the glucose tolerance test, animals were given additional Nembutal (100 mg/kg i.p.) and sacrificed for tissue harvest.
Low Temperature (LT) Electrophoresis and Western Blot Analysis
Tissues were stored at -80°C until used. LV samples were homogenized in ice-cold buffer (20 mM HEPES pH7.5, 50 mM NaCl, 1% SDS, 1× protease inhibitor (Sigma-Aldrich, P-8340). Protein concentration was determined by the Bradford method (BioRad reagent). LT electrophoresis is an in vitro test to determine the ratio of dimerized and monomerized forms of eNOS, with an increase in this ratio reflective increased coupling of the eNOS enzyme. For LT electrophoresis, samples were mixed with Lamelli buffer (without β-mercaptoethanol except where noted), before samples were loaded onto a 7.5% SDS-PAGE and electrophoresis was performed at 4°C. For total eNOS determinations, samples were mixed with Lamelli buffer, heated to 95°C for 5 min, and loaded onto a 7.5% SDS-PAGE and electrophoresis was perform at room temperature. The gels were blotted onto Hybond-P (Amersham Biosciences, Piscataway NJ) by a semi-dry transfer protocol. Western analysis was performed as described previously [21]. Antibodies used included eNOS (BD Transduction, San Jose, CA) and alpha-MHC derived from the BA-G5 cell line (ATCC, Manassas, VA). Antibody binding was visualized using the Amersham ECL Plus kit. Band density was quantified using AlphaEaseFC software (AlphaInnotech, San Leando CA). The dimer:monomer ratio calculated from band densities: % Dimerized eNOS = {dimer density/(dimer density + monomer density)}*100. Values presented are mean ± SEM.
eNOS Activity
eNOS enzyme activity was determined by fluorescence spectroscopy method, using 2,3-diaminonaphthalene (DAN), and modified from that described by Misko et.al. [22]. In brief, LV was homogenized in ice-cold buffer (20 mM HEPES-pH 7.4, 0.1 mM EDTA, 1 mM glutathione, 10 μM BH4, 1× proteinase inhibitor. The homogenate was centrifuged at 1000 × g for 10 minutes at 4°C, and the supernatant retained for protein determination. To measure activity; 100 μg protein was incubated in reaction buffer (30 μM arginine, 1 μM FAD, 0.5 mM NADPH, 50 nM calmodulin). The reaction mixture was incubated for 3 hours at 37°C before the addition of acidified DAN. Fluorescence was determined using a Kontron SFM 25 spectrofluorometer (excitation 365 nm; emission 450 nm). eNOS specific activity was the difference in fluorescence between CaCl2 and calcium-free EGTA buffered solutions. To determine this, separate reactions were performed in the presence of 1 mM CaCl2 or 0.5 mM EGTA and the difference taken as eNOS specific activity. To examine the role of BH4 the reactions were also performed in the presence or absence of 7.5 μM BH4. Nitrite formation was calculated from standards using sodium nitrite. Using 750 nM 7-nitroindazole (a nNOS specific inhibitor) preliminary experiments indicated that nNOS did not contribute to left ventricular calcium-dependent NOS activity (data not shown). Values presented are mean ± SEM of arbitrary fluorescent units.
Superoxide Activity
Superoxide activity was determined by two methods. In brief, LV was homogenized in ice-cold buffer (20 mM HEPES-pH 7.4, 0.1 mM EDTA, 1 mM glutathione, 10 μM BH4, 1× proteinase inhibitor. Protein concentration was determined by the Bradford method. NADPH oxidase activity, using 5 μM lucigenin, was determined as we have previously described [23]. NOX-dependent activity was determined by the addition of 300 μM apocynin. eNOS dependent superoxide activity was determined using dihydroethidium (DHE), as described by Zhao et.al. [24] To measure eNOS-driven superoxide activity, separate solutions containing either 10 μM BH4 or vehicle were added to reaction buffer (30 μM arginine, 1 μM FAD, 0.5 mM NADPH, 1 mM CaCl2, 50 nM calmodulin). 200 μg protein was incubated at 37°C and the development of fluorescence measured using an Infinite M200 plate reader (Tecan, Research Triangle Park, NC) (excitation; 480 nm, emission; 573 nm). Values presented are mean ± SEM of normalized arbitrary optical density (OD) units.
Cytochrome Oxidase Assay
Cytochrome oxidase activity was determined as previously described [21]. The rate of cytochrome C oxidation was followed at 550 nm (ε = 21.0 mM-1 cm-1), using an Ultrospec 3100 spectrophometer (Amersham Biosciences, Piscataway NJ).
RNA Analysis
Total RNA from left ventricle was isolated using a FASTRNA ProGreen Kit (Q-Biogene, Irvine CA). Quantification of mRNA levels was done by QRT-PCR by real-time fluorescent using a Stratagene MX3000p as described previously [21]. The following genes were studied: αMHC (f;5'-CTACAAGCGCCAGGCTGAGG-3' ; r; 5'-GTGGGATAGCAACAGCGAGGC-3'), NADPH Oxidase 1 (f; 5'-TCCTCACTGGCTGGGATAGC-3' ; r; 5'-TTGAGTACCGCCGACAGCAT-3'), NADPH Oxidase 2 (f; 5'-TTGAGTGGTTCGCAGACCT-3' ; r; 5'-GTTGGGCCGTCCATACAG-3'), p47 (f; 5'-ATCCCAACTACGCAGGTGAA-3'; r;5'-TATCTCCTCCCCAGCCTTCT -3'), GTP cyclohydrolase (f; 5'-AAGGGTCCATATTGGTTATCTTCCT-3' ; r; 5'-ACACCTCGCATGACCATACA-3'), eNOS (f; 5'-GGCATACAGAACCCAGGATGG-3'; r; 5'-GCAGGCTGCAGTCCTTTGAT-3'), β-actin (f; 5'-GCGGTGACCATAGCCCTCTTT-3'; r; 5'-TGCCACTCCCAAAGTAAAGGGTCA-3'). The data was normalized by Δ2Ct method using β-actin, and the fidelity of the reactions was verified by melting point analysis. Data presented are the mean ± SEM with respect to sedentary control values. β-actin expression was compared to hypoxanthine phosphoribosyltransferase (HPRT) another "housekeeping" gene and no differences were observed.
Statistical analysis
Statistical analyses were performed using NCSS Software (NCSS, Kaysville UT). Where appropriate, student t-test or ANOVA was utilized; post-hoc analysis was done using a Fisher's LSD analysis. Values presented are mean ± SEM, and statistical significance was set at p < .05.
Results
Plantaris muscle cytochrome oxidase enzyme activity, a marker of aerobic metabolism and peripheral training adaptations was significantly increased following 9 weeks of training in the diabetic rats compared to sedentary controls (Table 1). Similarly, training significantly increased left ventricular weight (sedentary: 0.72 ± 0.02, train; 0.80 ± 0.01 g), while body weights were unaltered (Table 1). We have previously reported that high intensity exercise will increase left ventricular α-MHC [21]. In the present study using a lower intensity of exercise training, we did not observe a change in left ventricular α-MHC protein (sedentary; 100 ± 6.2%, Train; 100 ± 5.4%) or α-MHC-mRNA levels. Increases in skeletal muscle cytochrome oxidase activity and left ventricular weights are both indicative of training adaptations to endurance exercise.
Table 1.
Morphometric and Blood Parameter Data
| Body mass | LV/BW | Plantaris Cytochrome Oxidase | Fasting Glucose | Fasting insulin | 120 Insulin | ISI | HbA1c | |
| (g) | nmole/min/mg | (mg%) | (ng/ml) | (ng/ml) | (%) | |||
| Sedentary | 384 | 1.86 | 0.31 | 205.3 | 2.57 | 4.56 | 16.6 | 7.36 |
| ± 7 | ± 0.05 | ± 0.02 | ± 9.5 | ± 0.27 | ± 0.66 | ± 1.60 | ± 0.56 | |
| Exercise Trained | 374 | 2.15 | 0.39 | 173.6 | 1.46 | 3.77 | 29.3 | 6.16 |
| ± 5 | ± 0.04 | ± 0.05 | ± 9.5 | ± 0.17 | ± 0.59 | ± 4.5 | ± 0.16 | |
| ns | p < .05 | p < .05 | p < .05 | p < .05 | ns | p < .05 | p < .05 | |
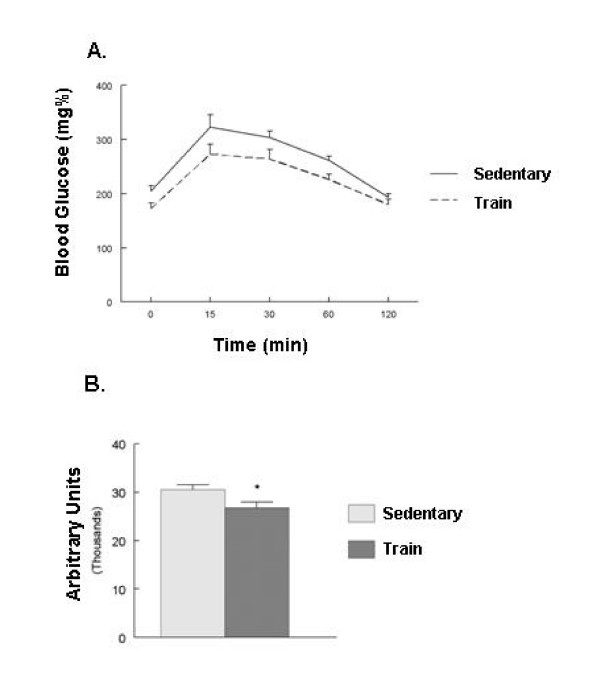
The exercise trained GK rats had a significant decrease in fasting blood glucose and consistent with this, a significant decrease in HbA1c (Table 1). Exercise has been useful in the management of diabetes in obese models of NIDDM, in part due to improved handling of blood glucose levels. To determine if training altered the sensitivity to glucose, a glucose tolerance test was performed. A significant decrease in the area under the curve analysis (AUC) in the trained animals was observed compared to sedentary animals (Figure 1). Plasma insulin levels were determined prior to the start of the glucose tolerance test and after 120 minutes. Fasting levels were significantly lower in the trained animals compared to sedentary animals (Table 1). Calculation of an insulin sensitivity index as described by Matsuda and DeFronzo, determined that exercise training significantly increased insulin sensitivity (Table 1) [25].
Figure 1.
Exercise improves glucose tolerance in the GK rats. Sedentary and exercise trained GK rats were anesthetized and then injected with 1.0 g/kg glucose as described in Methods. A. Tail blood glucose wad determined at select intervals. B. Area under the curve analysis was performed using the NCSS software. Values are mean ± SEM of 10–14 animals; * p < .05 compared to control.
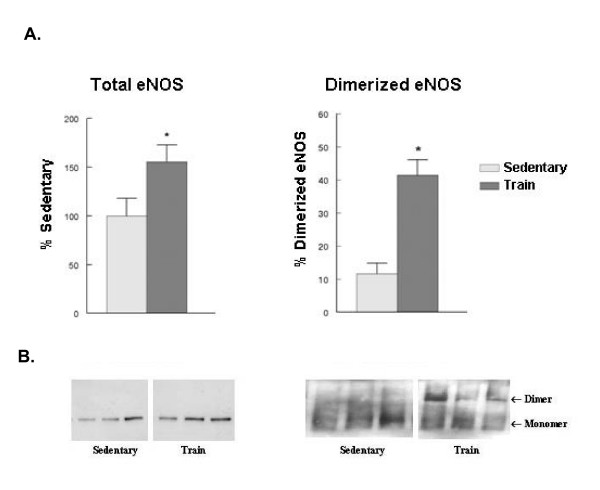
Exercise training has been reported to increase eNOS protein in different tissues [5,6]. Consistent with those findings, we observed a significant increase in left ventricular total eNOS (Figure 2). Dimerization of eNOS is required for the synthesis of NO and nitric oxide, and a decrease in the dimer:monomer eNOS ratio has been reported in diabetic myocardium [15]. When low temperature electrophoresis was performed under nonreducing conditions, we found that the ratio of dimer:monomer eNOS was also significantly increased by exercise training (Figure 2). When the samples were treated with 0.2% β-mercaptoethanol and low temperature electrophoresis was performed, the ratio of dimer:monomer eNOS was compressed but still significantly increased (sed: 17.8 ± 1.4%; train: 28.5 ± 4.1%). In most studies changes in eNOS have only been observed in those tissues undergoing exercise-induced increases in blood flow. In the present study total kidney eNOS levels tended to be increased, but a high level of variance blunted the statistical evaluation (sed; 100 ± 11.1%; train: 151.5 ± 22.3%: p = 0.06). However, a significant increase in the dimer:monomer ratio was observed in the eNOS protein from kidney (sed: 12.0 ± 1.7%; train: 20.7 ± 2.9%). Thus exercise training increased dimerization of eNOS in both tissues.
Figure 2.
Exercise training increased eNOS protein levels and eNOS dimerization. Total protein from the left ventricle was prepared and electrophoresis under normal or low temperature conditions were carried out as described in Methods. A. Quantitative analysis of eNOS expression. Values are mean ± SEM of 6–10 animals per group; * p < .05 compared to control. B. Representative western blots of eNOS protein.
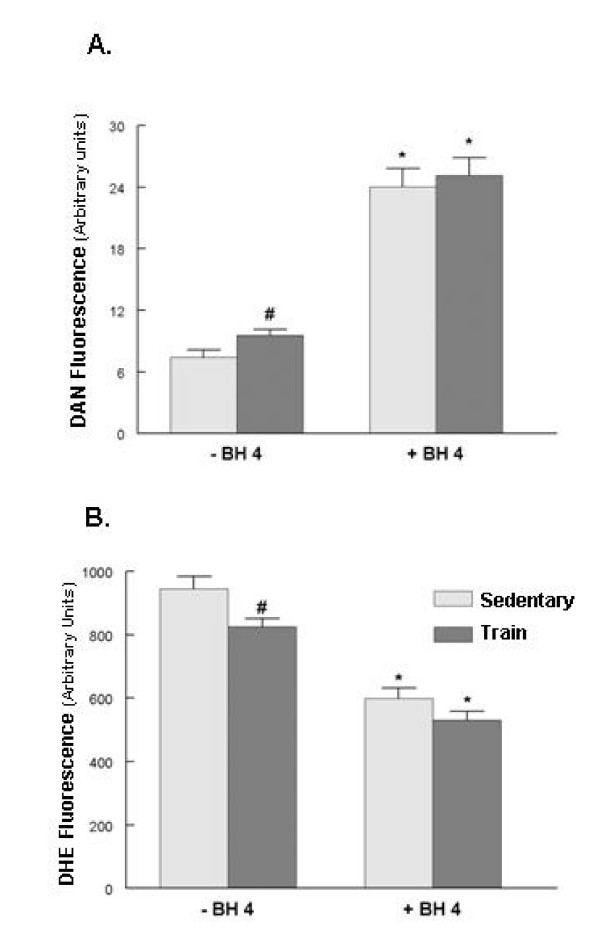
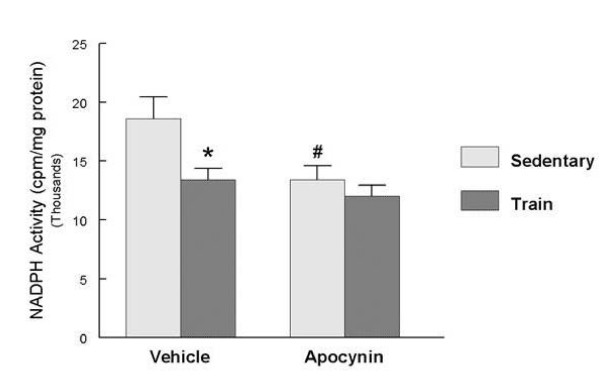
Increased dimerization of the eNOS protein should shift its enzymatic activity towards improved NO generation, at the expense of superoxide production. To examine this concept, two in vitro determinations of eNOS function were made. In the absence of exogenous BH4, exercise training significantly increased eNOS activity compared to sedentary controls (Figure 3A). The addition of exogenous BH4 to the reaction buffer significantly increased eNOS activity in both groups, but canceled the differences between the groups. Determination of eNOS-driven superoxide generation found that in the absence of added BH4, exercise training resulted in significantly decreased superoxide production compared to the sedentary group (Figure 3B). The addition of BH4 to the reaction media decreased eNOS-driven superoxide generation in both groups and no difference between sedentary and exercise trained groups were observed. Exercise training also significantly decreased ventricular NADPH oxidase activity (Figure 4). In the presence of the NADPH oxidase inhibitor apocynin, NADPH oxidase activity was significantly decreased only in the sedentary group and the differences between sedentary and trained groups were eliminated.
Figure 3.
Exercise training enhances eNOS function in the absence of BH4. A. eNOS activity was measured using in the absence or present of BH4. B. eNOS-dependent superoxide formation was measured as described in Methods. Values are mean ± SEM of 10 animals per group; # p < .05 compared to respective control, * p < .05 compared to respective -BH4 assay group.
Figure 4.
Exercise training decreased LV superoxide generation. LV homogenates were prepared as described in Methods to determine superoxide generation. Activity was assay in a Krebs-HEPES buffer using 5 μM lucigenin, 200 μM NADPH and in the absence or presence of 300 μM apocynin. Values are mean ± SEM cpm/mg protein of 10 animals per group; # p < .05 compared to respective control, * p < .05 compared to respective -BH4 assay group.
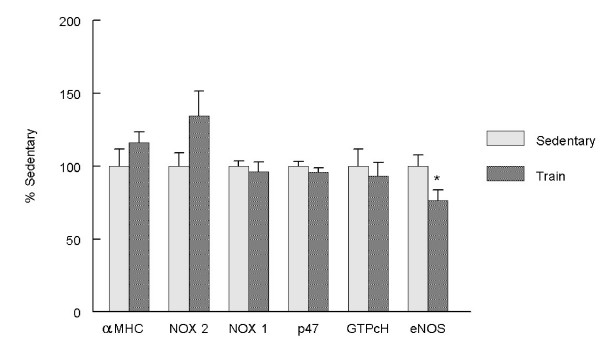
The apparent shift in NADPH oxidase activity suggests that this enzyme complex is either less active or that exercise decreased expression of the constitutive subunits. However, when expression of three subunits (NOX1, NOX2, and p47) was examined no significant changes in the relative mRNA levels were observed between the sedentary and exercised trained groups. Similarly, expression of GTP cyclohydrolase, the rate limiting step in BH4 synthesis, was unaltered by exercise training. In contrast to increased levels of eNOS protein, exercise training did cause a significant decrease in eNOS-mRNA levels (Figure 5).
Figure 5.
Exercise training decreased LV eNOS-mRNA levels. Total LV RNA was isolated and quantification of mRNA levels was done by QRT-PCR as described in Methods. Data was normalized by the Δ2Ct method using β-actin, and the fidelity of the reactions was verified by melting point analysis. Values presented are % control mean ± SEM of 10 animals per group; * p < .05 compared control.
Discussion
Even low intensity forms of exercise such as walking have been shown to benefit NIDDM levels, and patients [1]. Exercise lowers HbA1c, increases insulin sensitivity, improves blood lipid levels, and lowers blood pressure in diabetic individuals [1,2]. Exercise-induced increases in tissue eNOS expression have been reported and our results in the heart are consistent with those findings. The major finding of this study was that exercise training increased eNOS function, in part by an increase in dimerization, as one mechanism for increasing NO bioavailability in the diabetic heart.
GK rats have elevated fasting glucose (150–250 mg%), are hyperinsulinemic at a young age, have impaired response to glucose, and increased HbA1c [26]. As a model of type 2 diabetes, the GK rats do not have the confounding factors of hyperlipidemia or hypercholesterolemia observed in obese-diabetic rats. The etiology of the GK rats is unknown, but it has been suggested that impaired pancreatic mitochondrial function may partially explain the depressed insulin release [27]. GK rats display symptoms associated with diabetic complications, including reduced nerve conduction velocity indicative of peripheral neuropathy [28]. Progressive renal involvement in GK rats presents in a manner similar to NIDDM in humans; that includes thickening of the glomerular basement membrane [29]. Similar to others, we have also found endothelium-dependent microvascular dysfunction in the GK rats (data not shown) [26,30,31]. GK animals also display increases in oxidative stress markers and susceptibility to lipid peroxidation in the hearts of older (12–18 months) but not younger (3–6 months) animals [32].
Exercise-induced increases in vascular eNOS protein have been known for sometime [5,6,33]. More recently, it has been shown that exercise will also alter myocardial eNOS protein and phosphorylation status [17]. Zhang et.al exercise trained rats by a 10 week swimming program [17]. They demonstrated that exercise increased myocardial NOx production, eNOS protein levels, and increased the sensitivity to insulin-stimulated phosphorylation of eNOS. The shift in eNOS phosphorylation (ser1179) status was mediated through the Akt signaling pathway and resulted in enhanced myocardial contractility [17]. Shifts in phosphorylation status do not fully explain our findings of increased eNOS dimerization. eNOS has 5 phosphorylation sites; serines 116, 617, 635, 1179 and threonine 497 (bovine coordinates). Whereas phosphorylation of the serines 617, 635 and 1179 will activate eNOS, the Thr497 phosphorylation appears to serve as an intrinsic switch to enhance coupling of eNOS in favor of NO production at the expense of superoxide [34]. To date no one has reported exercise-induced alterations in eNOS-Thr497 phosphorylation status. Fulton et al demonstrated that phosphorylation of ser1179 was not required for correct intracellular localization [35]. eNOS differs from iNOS and nNOS in that the former contains the consensus sequences for both myristoylation and palmitoylation. Myristoylation is required for initial targeting of eNOS to the cell membrane, while palmitoylation may stabilize the eNOS membrane association [36,37]. To date no one has reported exercise-induced changes in myristoylation or palmitoylation. However, the extent of site specific phosphorylation is modified by subcellular localization and hence stimulus specific activation that is dependent upon localization would be a useful avenue for future work [38].
Exercise improved the eNOS coupling state; a shift that could serve to decrease diabetic-related oxidative stress and lessen diabetic-related complications. Two in vitro determinations of eNOS function were made. Both measures were influenced by the addition of exogenous BH4 to the reaction. eNOS coupling was greater in the left ventricle from the exercise trained group only in the absence of exogenous BH4. This suggests that BH4 may have been more tightly bound to eNOS in the trained group or eNOS had an increased sensitivity to residual BH4, producing a shift in eNOS functional state.
BH4 is both an anti-oxidant and an essential cofactor of eNOS and other members of the monooxygenase family [39]. In both healthy individuals and diabetics, a single oral glucose challenge can reduce NO-specific vasodilatation, an action that was blocked by supplementation with tetrahydrobiopterin (BH4) [40-42]. Although the exact mechanism of its actions are not clear, it is thought that BH4 stabilizes the dimer conformation and facilitates L-arginine binding as the first step in its conversion to citrulline [43,44]. Shifts in BH4 concentration directly influence the rate of NO or superoxide anion O2- production [39,45,46]. Both IDDM and NIDDM animal models have reported decreased concentrations of BH4 in the vasculature [47,48]. Differential effects of BH4 have also been observed with exercise in aged humans. Eskura et.al demonstrated that BH4 supplementation improved vasodilatation in sedentary but not exercise trained elderly humans, suggesting that chronic exercise improved BH4 bio-availability in that population [49]. We have demonstrated that exercise training improved eNOS function when BH4 may have been limiting. This suggests that chronic exercise enhanced eNOS function, in part through altered BH4 bioavailability.
We observed that exercise training reduced apocynin-sensitive chemiluminescence, indicative of a decrease in NAD(P)H oxidase activity in the left ventricle. Apocynin is thought to interfere with p47 activation of NAD(P)H oxidase, but more recently has also been suggested to act as an antioxidant [50,51]. If this latter mechanism was operational, we should have observed some decreases in both sedentary and training groups. However no differences were observed in the exercise trained group suggesting that chronic exercise modified the NAD(P)H oxidase complex activity. Plasma levels of angiotensin II (Ang II) are elevated in diabetics with poor glucose control and in untreated diabetic animal models [52-55]. Conversely, normalization of Ang II levels can be achieved with improved glucose handling or ACE inhibition [52,56]. Chronically elevated Ang II levels are associated with increased expression of NAD(P)H oxidase components and oxidative stress [56]. We did not observe changes in NOX 1, NOX 2, or p47-mRNA levels suggesting that altered expression was not influenced by training and that a shift in the activation of NADPH oxidase may have had a greater role in the observed improvements. ROS promotes oxidation of BH4 to dihydropterin and one consequence of decreased NAD(P)H oxidase activity may be to improve BH4 bioavailability [57].
Control of eNOS protein levels is a complex operation that is mediated on several levels including eNOS transcription, mRNA stability, and post-translational modifications. We have made the paradoxical observation that exercise training of diabetic animals lead to an increase in eNOS protein, but also a decrease in eNOS-mRNA. This outcome is the reverse observed by others who found that in comparison to controls, diabetes decreased eNOS protein but increased eNOS-mRNA [18,58]. Similarly, in cultured endothelial cells, elevated glucose levels increased eNOS-mRNA [59]. This paradox may be explained in part by diabetic-induced decreases in eNOS function which activates compensatory mechanisms. Drummond et.al. demonstrated that exogenous H2O2 increased eNOS transcription and eNOS-mRNA stability [60]. Our observation that exercise training decreased both eNOS-driven and NADPH oxidase derived ROS should serve to lessen this driving force for eNOS transcription. Second, hyperglycemia acts as a NO scavenger and the exercise-induced improvements in glycemic control should improve NO bioavailability [61]. Third, increased NO bioavailability is known to serve as a negative feedback mechanism to decrease eNOS transcription via a cGMP-mediated pathway [62,63]. Thus the decrease in eNOS-mRNA may have been the result of the decreased driving forces for eNOS transcription.
Summary
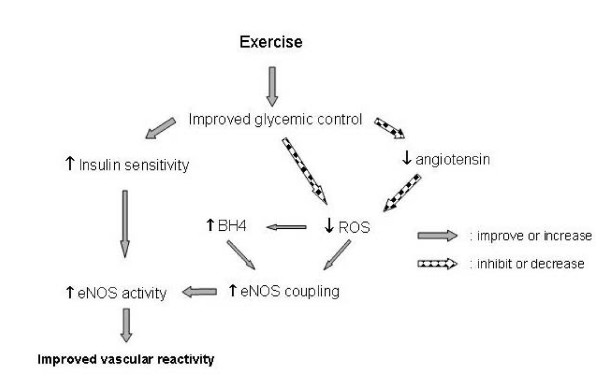
Regulation of eNOS function is showing itself to be an increasingly complex event that diabetes clearly disrupts. Exercise-induced increases in eNOS expression have been demonstrated in several reports as well as exercise-induced shifts of eNOS phosphorylation status. The major findings of this study are that exercise at least partially reversed diabetic-induced eNOS dysfunction through increased dimerization leading to increased NO generation at the expense of superoxide production. As shown in Figure 6, it is likely that this was achieved through several distinct mechanisms that converge on the management of cellular ROS and BH4 metabolism.
Figure 6.
Exercise induced alterations in the diabetic myocardium leading to improvements in eNOS function.  ; decrease in level or inhibition of function,
; decrease in level or inhibition of function,  ; increase in level or improvement of function. Improvements derived from increased insulin sensitivity are from the findings of Zhang et.al. [17].
; increase in level or improvement of function. Improvements derived from increased insulin sensitivity are from the findings of Zhang et.al. [17].
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JG and SH trained animals, performed data collection, assisted in data analysis and help write the manuscript, X.Z. and S.M. performed data collection and assisted in data analysis, P.K. and M.W. assisted in assay development, J.E. designed and coordinated the study and wrote the manuscript. All authors have read and approved the manuscript.
Acknowledgments
Acknowledgements
Supported in part by NIH PO1HL43023 and the New York Medical College Research Endowment Fund.
Contributor Information
James Grijalva, Email: JAMES_GRIJALVA@nymc.edu.
Steven Hicks, Email: hickss@upstate.edu.
Xiangmin Zhao, Email: XIANGMIN_ZHAO@nymc.edu.
Sushma Medikayala, Email: Sushma_Medikayala@nymc.edu.
Pawel M Kaminski, Email: PAWEL_KAMINSKI@nymc.edu.
Michael S Wolin, Email: MIKE_WOLIN@nymc.edu.
John G Edwards, Email: j_edwards@nymc.edu.
References
- Walker K, Jones J, Piers L, O'Dea K, Putt R. Effects of regular walking on cardiovascular risk factors and body composition in normoglycemic women and women with type 2 diabetes. Diabetes Care. 1999;22:555–561. doi: 10.2337/diacare.22.4.555. [DOI] [PubMed] [Google Scholar]
- Boule NG, Kenny GP, Haddad E, Wells GA, Sigal RJ. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia. 2003 doi: 10.1007/s00125-003-1160-2. [DOI] [PubMed] [Google Scholar]
- Laughlin MH. Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exerc. 1995;27:1135–44. [PubMed] [Google Scholar]
- Delp MD. Effects of exercise training on endothelium-dependent peripheral vascular responsiveness. Med Sci Sports Exerc. 1995;27:1152–7. [PubMed] [Google Scholar]
- Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994;74:349–53. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Rush JW, Turk JR, Price EM, Laughlin MH. Short-term exercise training increases ACh-induced relaxation and eNOS protein in porcine pulmonary arteries. J Appl Physiol. 2001;90:1102–10. doi: 10.1063/1.1383260. [DOI] [PubMed] [Google Scholar]
- Sun D, Huang A, Koller A, Kaley G. Adaptation of flow-induced dilation of arterioles to daily exercise. Microvasc Res. 1998;56:54–61. doi: 10.1006/mvre.1998.2083. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Xhang Z, Diamond SL. Shear Stress Induction of the Endothelial Nitric Oxide Synthase Gene is Calcium-Dependent But Not Calcium-Activated. Jour of Cell Phys. 1997;171:205–211. doi: 10.1002/(SICI)1097-4652(199705)171:2<205::AID-JCP11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ziegler T, Silacci P, Harrison V, Hayoz D. Nitric oxide synthase expression in endothelial cells exposed to mechanical forces. Hypertension. 1998;32:351–355. doi: 10.1161/01.hyp.32.2.351. [DOI] [PubMed] [Google Scholar]
- Minami A, Ishimura N, Harada N, Sakamoto S, Niwa Y, Nakaya Y. Exercise training improves acetylcholine-induced endothelium-dependent hyperpolarization in type 2 diabetic rats, Otsuka Long-Evans Tokushima fatty rats. Atherosclerosis. 2002;162:85–92. doi: 10.1016/S0021-9150(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Hamdy O, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, et al. Lifestyle modification improves endothelial function in obese subjects with the insulin resistance syndrome. Diabetes Care. 2003;26:2119–25. doi: 10.2337/diacare.26.7.2119. [DOI] [PubMed] [Google Scholar]
- Radomski MW, Palmer RM, Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987;92:639–46. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchia FA, Osorio JC, Chandler MP, Xu X, Panchal AR, Lopaschuk GD, et al. Reduced synthesis of NO causes marked alterations in myocardial substrate metabolism in conscious dogs. Am J Physiol Endocrinol Metab. 2002;282:E197–206. doi: 10.1152/ajpendo.2002.282.1.E197. [DOI] [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–26. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balligand JL, Cannon PJ. Nitric oxide synthases and cardiac muscle. Autocrine and paracrine influences. Arterioscler Thromb Vasc Biol. 1997;17:1846–58. doi: 10.1161/01.atv.17.10.1846. [DOI] [PubMed] [Google Scholar]
- Zhang QJ, Li QX, Zhang HF, Zhang KR, Guo WY, Wang HC, et al. Swim training sensitizes myocardial response to insulin: role of Akt-dependent eNOS activation. Cardiovasc Res. 2007;75:369–80. doi: 10.1016/j.cardiores.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Zhao G, Zhang X, Smith CJ, Xu X, Ochoa M, Greenhouse D, et al. Reduced coronary NO production in conscious dogs after the development of alloxan-induced diabetes. Am J Physiol. 1999;277:H268–78. doi: 10.1152/ajpheart.1999.277.1.H268. [DOI] [PubMed] [Google Scholar]
- Rabini RA, Staffolani R, Fumelli P, Mutus B, Curatola G, Mazzanti L. Decreased nitric oxide synthase activity in platelets from IDDM and NIDDM patients. Diabetologia. 1998;41:101–4. doi: 10.1007/s001250050873. [DOI] [PubMed] [Google Scholar]
- Martina V, Bruno GA, Trucco F, Zumpano E, Tagliabue M, Di Bisceglie C, et al. Platelet cNOS activity is reduced in patients with IDDM and NIDDM. Thromb Haemost. 1998;79:520–2. [PubMed] [Google Scholar]
- Rafalski K, Abdourahman A, Edwards JG. Early adaptations to training: upregulation of alpha-myosin heavy chain gene expression. Med Sci Sports Exerc. 2007;39:75–82. doi: 10.1249/01.mss.0000240324.08406.3d. [DOI] [PubMed] [Google Scholar]
- Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993;214:11–6. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, et al. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2003;23:418–24. doi: 10.1161/01.ATV.0000061735.85377.40. [DOI] [PubMed] [Google Scholar]
- Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–68. doi: 10.1016/S0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- Witte K, Jacke K, Stahrenberg R, Arlt G, Reitenbach I, Schilling L, et al. Dysfunction of soluble guanylyl cyclase in aorta and kidney of Goto-Kakizaki rats: influence of age and diabetic state. Nitric Oxide. 2002;6:85–95. doi: 10.1006/niox.2001.0363. [DOI] [PubMed] [Google Scholar]
- Oliveira PJ, Rolo AP, Seica R, Sardao V, Monteiro P, Goncalves L, et al. Impact of diabetes on induction of the mitochondrial permeability transition. Rev Port Cardiol. 2002;21:759–66. [PubMed] [Google Scholar]
- Murakawa Y, Zhang W, Pierson CR, Brismar T, Ostenson CG, Efendic S, et al. Impaired glucose tolerance and insulinopenia in the GK-rat causes peripheral neuropathy. Diabetes Metab Res Rev. 2002;18:473–83. doi: 10.1002/dmrr.326. [DOI] [PubMed] [Google Scholar]
- Phillips AO, Baboolal K, Riley S, Grone H, Janssen U, Steadman R, et al. Association of prolonged hyperglycemia with glomerular hypertrophy and renal basement membrane thickening in the Goto Kakizaki model of non-insulin-dependent diabetes mellitus. Am J Kidney Dis. 2001;37:400–10. doi: 10.1053/ajkd.2001.21322. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Matsumoto T, Ooishi K, Kamata K. Differential expression of alpha2D-adrenoceptor and eNOS in aortas from early and later stages of diabetes in Goto-Kakizaki rats. Am J Physiol Heart Circ Physiol. 2004;287:H135–48. doi: 10.1152/ajpheart.01074.2003. [DOI] [PubMed] [Google Scholar]
- Cheng ZJ, Vaskonen T, Tikkanen I, Nurminen K, Ruskoaho H, Vapaatalo H, et al. Endothelial dysfunction and salt-sensitive hypertension in spontaneously diabetic Goto-Kakizaki rats. Hypertension. 2001;37:433–9. doi: 10.1161/01.hyp.37.2.433. [DOI] [PubMed] [Google Scholar]
- Santos DL, Palmeira CM, Seica R, Dias J, Mesquita J, Moreno AJ, et al. Diabetes and mitochondrial oxidative stress: a study using heart mitochondria from the diabetic Goto-Kakizaki rat. Mol Cell Biochem. 2003;246:163–70. doi: 10.1023/A:1023475022025. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Muller JM, Laughlin MH, Price M. Induction of nitric oxide synthase mRNA in coronary resistance arteries isolated from exercise-trained pigs. Am J Physiol. 1997;273:H2575–9. doi: 10.1152/ajpheart.1997.273.6.H2575. [DOI] [PubMed] [Google Scholar]
- Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA, Jr, et al. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J Biol Chem. 2003;278:44719–26. doi: 10.1074/jbc.M302836200. [DOI] [PubMed] [Google Scholar]
- Fulton D, Fontana J, Sowa G, Gratton JP, Lin M, Li KX, et al. Localization of endothelial nitric-oxide synthase phosphorylated on serine 1179 and nitric oxide in Golgi and plasma membrane defines the existence of two pools of active enzyme. J Biol Chem. 2002;277:4277–84. doi: 10.1074/jbc.M106302200. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Busconi L, Michel T. Agonist-modulated palmitoylation of endothelial nitric oxide synthase. J Biol Chem. 1995;270:995–8. doi: 10.1074/jbc.270.46.27403. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, et al. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–22. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Kou R, Lin AJ, Golan DE, Michel T. Subcellular targeting and agonist-induced site-specific phosphorylation of endothelial nitric-oxide synthase. J Biol Chem. 2002;277:39554–60. doi: 10.1074/jbc.M207299200. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T. Regulation of nitric oxide production by tetrahydrobiopterin. Circulation. 1995;91:248–50. doi: 10.1161/01.cir.91.1.248. [DOI] [PubMed] [Google Scholar]
- Ihlemann N, Rask-Madsen C, Perner A, Dominguez H, Hermann T, Kober L, et al. Tetrahydrobiopterin restores endothelial dysfunction induced by an oral glucose challenge in healthy subjects. Am J Physiol Heart Circ Physiol. 2003;285:H875–82. doi: 10.1152/ajpheart.00008.2003. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000;43:1435–8. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- Bagi Z, Koller A. Lack of nitric oxide mediation of flow-dependent arteriolar dilation in type I diabetes is restored by sepiapterin. J Vasc Res. 2003;40:47–57. doi: 10.1159/000068938. [DOI] [PubMed] [Google Scholar]
- Cai S, Alp NJ, McDonald D, Smith I, Kay J, Canevari L, et al. GTP cyclohydrolase I gene transfer augments intracellular tetrahydrobiopterin in human endothelial cells: effects on nitric oxide synthase activity, protein levels and dimerisation. Cardiovasc Res. 2002;55:838–49. doi: 10.1016/S0008-6363(02)00460-1. [DOI] [PubMed] [Google Scholar]
- Tiefenbacher CP. Tetrahydrobiopterin: a critical cofactor for eNOS and a strategy in the treatment of endothelial dysfunction? Am J Physiol Heart Circ Physiol. 2001;280:H2484–8. doi: 10.1152/ajpheart.2001.280.6.H2484. [DOI] [PubMed] [Google Scholar]
- Mayer B, Werner ER. In search of a function for tetrahydrobiopterin in the biosynthesis of nitric oxide. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:453–63. doi: 10.1007/BF00171035. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, et al. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220–5. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meininger CJ, Marinos RS, Hatakeyama K, Martinez-Zaguilan R, Rojas JD, Kelly KA, et al. Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydrobiopterin deficiency. Biochem J. 2000;349:353–6. doi: 10.1042/0264-6021:3490353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar MS, Wahid S, Mustafa S, Al-Saleh E, Dhaunsi GS, Al-Mulla F. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur J Pharmacol. 2005;511:53–64. doi: 10.1016/j.ejphar.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–65. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JM, Hart BA, Ip Vai Ching TR, van Dijk H, Labadie RP. Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radic Biol Med. 1990;8:251–8. doi: 10.1016/0891-5849(90)90070-Y. [DOI] [PubMed] [Google Scholar]
- Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, et al. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–7. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- Ferriss JB, O'Hare JA, Kelleher CC, Sullivan PA, Cole MM, Ross HF, et al. Diabetic control and the renin-angiotensin system, catecholamines, and blood pressure. Hypertension. 1985;7:II58–63. doi: 10.1161/01.hyp.7.6_pt_2.ii58. [DOI] [PubMed] [Google Scholar]
- Lip PL, Chatterjee S, Caine GJ, Hope-Ross M, Gibson J, Blann AD, et al. Plasma vascular endothelial growth factor, angiopoietin-2, and soluble angiopoietin receptor tie-2 in diabetic retinopathy: effects of laser photocoagulation and angiotensin receptor blockade. Br J Ophthalmol. 2004;88:1543–6. doi: 10.1136/bjo.2004.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K, Kobayashi T, Hayashi Y, Matsumoto T, Kamata K. Enalapril improves impairment of SERCA-derived relaxation and enhancement of tyrosine nitration in diabetic rat aorta. Eur J Pharmacol. 2007;556:121–8. doi: 10.1016/j.ejphar.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Hollenberg NK, Stevanovic R, Agarwal A, Lansang MC, Price DA, Laffel LM, et al. Plasma aldosterone concentration in the patient with diabetes mellitus. Kidney Int. 2004;65:1435–9. doi: 10.1111/j.1523-1755.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Akimoto K, Gross SS, Hattori S, Kasai K. Angiotensin-II-induced oxidative stress elicits hypoadiponectinaemia in rats. Diabetologia. 2005;48:1066–74. doi: 10.1007/s00125-005-1766-7. [DOI] [PubMed] [Google Scholar]
- Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, et al. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–8. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- Cai S, Khoo J, Mussa S, Alp NJ, Channon KM. Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia. 2005;48:1933–40. doi: 10.1007/s00125-005-1857-5. [DOI] [PubMed] [Google Scholar]
- Ding QF, Hayashi T, Packiasamy AR, Miyazaki A, Fukatsu A, Shiraishi H, et al. The effect of high glucose on NO and O2- through endothelial GTPCH1 and NADPH oxidase. Life Sci. 2004;75:3185–94. doi: 10.1016/j.lfs.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res. 2000;86:347–54. doi: 10.1161/01.res.86.3.347. [DOI] [PubMed] [Google Scholar]
- Brodsky SV, Yamamoto T, Tada T, Kim B, Chen J, Kajiya F, et al. Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. Am J Physiol Renal Physiol. 2002;282:F1140–9. doi: 10.1152/ajprenal.00329.2001. [DOI] [PubMed] [Google Scholar]
- Grumbach IM, Chen W, Mertens SA, Harrison DG. A negative feedback mechanism involving nitric oxide and nuclear factor kappa-B modulates endothelial nitric oxide synthase transcription. J Mol Cell Cardiol. 2005;39:595–603. doi: 10.1016/j.yjmcc.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Wang XQ. cGMP-mediated negative-feedback regulation of endothelial nitric oxide synthase expression by nitric oxide. Hypertension. 1999;34:1237–41. doi: 10.1161/01.hyp.34.6.1237. [DOI] [PubMed] [Google Scholar]