Abstract
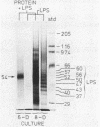
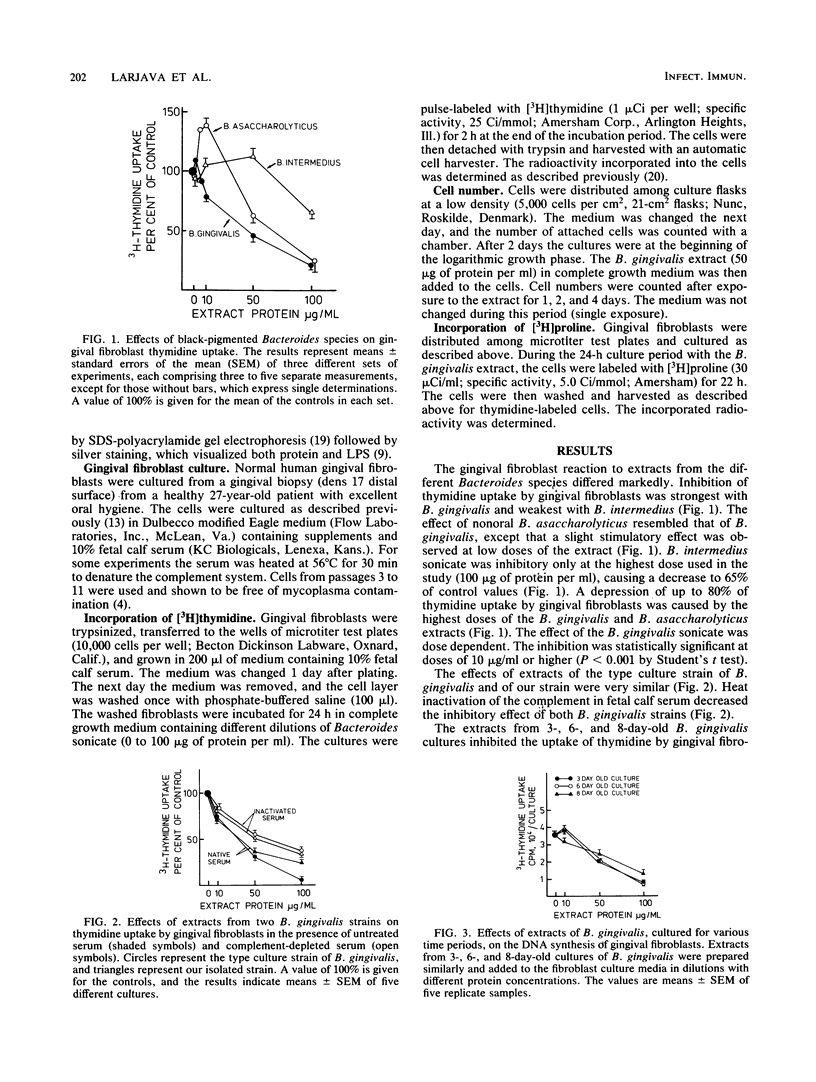
Human gingival fibroblasts were exposed in culture to cell extracts of different black-pigmented Bacteroides species, and their growth was monitored by determining thymidine uptake and counting cells. Of the Bacteroides species tested (B. gingivalis, B. asaccharolyticus, and B. intermedius), B. gingivalis gave the extract with the strongest inhibitory effect on fibroblast thymidine uptake. Linear inhibition reaching 80% of the control level was obtained with a dose of 100 micrograms of B. gingivalis extract protein per ml. The effect of B. asaccharolyticus resembled that of B. gingivalis, but even at the highest dose tested B. intermedius had only a slight inhibitory effect. When fibroblasts were counted after 2- and 4-day exposures to B. gingivalis extracts, a clear depression in the number of fibroblasts was found. The effects of extracts obtained from early and late growth phases of B. gingivalis cultures were similar. A fraction of B. gingivalis consisting essentially of lipopolysaccharides (LPSs) was obtained by degrading the extract proteins with proteinase K. Silver staining of polyacrylamide gels revealed a LPS pattern with a molecular mass ranging from 37 to 60 kilodaltons. This LPS-rich fraction caused inhibition of thymidine uptake by gingival fibroblasts similar to that caused by the native extract alone. Thus, the inhibition of gingival fibroblast growth by B. gingivalis appeared to be LPS mediated. This inhibitory effect of B. gingivalis on oral fibroblast growth may be a virulence factor of this bacterium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allenspach-Petrzilka G. E., Guggenheim B. Bacterial invasion of the periodontium; an important factor in the pathogenesis of periodontitis? J Clin Periodontol. 1983 Nov;10(6):609–617. doi: 10.1111/j.1600-051x.1983.tb01299.x. [DOI] [PubMed] [Google Scholar]
- Allenspach-Petrzilka G. E., Guggenheim B. Bacteroides melaninogenicus ss. intermedius invasion of rat gingival tissue. J Periodontal Res. 1982 Sep;17(5):456–459. doi: 10.1111/j.1600-0765.1982.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Höfling J. F., Sundqvist G. K. Degradation of albumin, haemopexin, haptoglobin and transferrin, by black-pigmented Bacteroides species. J Med Microbiol. 1984 Aug;18(1):39–46. doi: 10.1099/00222615-18-1-39. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Duguid R., Al-Makadsi F., Cowley G. C. Cytotoxic material extracted from human dental plaque and oral streptococci. Arch Oral Biol. 1980;25(5):349–352. doi: 10.1016/0003-9969(80)90045-x. [DOI] [PubMed] [Google Scholar]
- Duguid R. Inhibition of [3H]-thymidine uptake in human gingival fibroblasts by extracts from human dental plaque, oral bacteria of the Streptococcus and Actinomyces species. Arch Oral Biol. 1985;30(1):89–91. doi: 10.1016/0003-9969(85)90030-5. [DOI] [PubMed] [Google Scholar]
- Genco R. J., Slots J. Host responses in periodontal diseases. J Dent Res. 1984 Mar;63(3):441–451. doi: 10.1177/00220345840630031601. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino Y., Hopps R. M. The bone-resorbing activities in tissue culture of lipopolysaccharides from the bacteria Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Capnocytophaga ochracea isolated from human mouths. Arch Oral Biol. 1984;29(1):59–63. doi: 10.1016/0003-9969(84)90043-8. [DOI] [PubMed] [Google Scholar]
- Kamen P. R. The effects of bacterial sonicates on human keratinizing stratified squamous epithelium in vitro. J Periodontal Res. 1981 May;16(3):323–330. doi: 10.1111/j.1600-0765.1981.tb00981.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larjava H., Jalkanen M., Penttinen R., Paunio K. Enhanced synthesis of hyaluronic acid by human gingival fibroblasts exposed to human dental bacterial extract. J Periodontal Res. 1983 Jan;18(1):31–39. doi: 10.1111/j.1600-0765.1983.tb00332.x. [DOI] [PubMed] [Google Scholar]
- Manti F., Kornman K., Goldschneider I. Effects of an immunomodulating agent on peripheral blood lymphocytes and subgingival microflora in ligature-induced periodontitis. Infect Immun. 1984 Jul;45(1):172–179. doi: 10.1128/iai.45.1.172-179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair B. C., Mayberry W. R., Dziak R., Chen P. B., Levine M. J., Hausmann E. Biological effects of a purified lipopolysaccharide from Bacteroides gingivalis. J Periodontal Res. 1983 Jan;18(1):40–49. doi: 10.1111/j.1600-0765.1983.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Naito Y., Okuda K., Kato T., Takazoe I. Monoclonal antibodies against surface antigens of Bacteroides gingivalis. Infect Immun. 1985 Oct;50(1):231–235. doi: 10.1128/iai.50.1.231-235.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnemaa T., Doherty N. S. Effect of serum and liver extracts from hypercholesterolemic rats on the synthesis of collagen by isolated aortas and cultured aortic smooth muscle cells. Atherosclerosis. 1977 Mar;26(3):261–272. doi: 10.1016/0021-9150(77)90079-x. [DOI] [PubMed] [Google Scholar]
- Saglie F. R., Carranza F. A., Jr, Newman M. G., Cheng L., Lewin K. J. Identification of tissue-invading bacteria in human periodontal disease. J Periodontal Res. 1982 Sep;17(5):452–455. doi: 10.1111/j.1600-0765.1982.tb02024.x. [DOI] [PubMed] [Google Scholar]
- Saglie F. R., Carranza F. A., Jr, Newman M. G. The presence of bacteria within the oral epithelium in periodontal disease. I. A scanning and transmission electron microscopic study. J Periodontol. 1985 Oct;56(10):618–624. doi: 10.1902/jop.1985.56.10.618. [DOI] [PubMed] [Google Scholar]
- Saglie R., Newman M. G., Carranza F. A., Jr, Pattison G. L. Bacterial invasion of gingiva in advanced periodontitis in humans. J Periodontol. 1982 Apr;53(4):217–222. doi: 10.1902/jop.1982.53.4.217. [DOI] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Gatewood C., Hammond B. F. Cytotoxicity of the bacterium Actinobacillus actinomycetemcomitans extracts in human gingival fibroblasts. Arch Oral Biol. 1983;28(11):981–987. doi: 10.1016/0003-9969(83)90051-1. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Sela M. N., Shapira J., Hammond B. F. Detection of a fibroblast proliferation inhibitory factor from Capnocytophaga sputigena. Infect Immun. 1980 Jan;27(1):271–275. doi: 10.1128/iai.27.1.271-275.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew J. G., Marshall D. R., Burmeister J. A., Ranney R. R. Relationship between gingival crevicular fluid and serum antibody titers in young adults with generalized and localized periodontitis. Infect Immun. 1985 Sep;49(3):487–493. doi: 10.1128/iai.49.3.487-493.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touw J. J., van Kampen G. P., van Steenbergen T. J., Veldhuijzen J. P., de Graaff J. The effect of culture filtrates of oral strains of black-pigmented Bacteroides on the matrix production of chick embryo cartilage cells in vitro. J Periodontal Res. 1982 Jul;17(4):351–357. doi: 10.1111/j.1600-0765.1982.tb01164.x. [DOI] [PubMed] [Google Scholar]
- Touw J. J., van Steenbergen T. J., De Graaff J. Butyrate: a cytotoxin for Vero cells produced by Bacteroides gingivalis and Bacteroides asaccharolyticus. Antonie Van Leeuwenhoek. 1982;48(4):315–325. doi: 10.1007/BF00418285. [DOI] [PubMed] [Google Scholar]
- Williams G. D., Holt S. C. Characteristics of the outer membrane of selected oral Bacteroides species. Can J Microbiol. 1985 Mar;31(3):238–250. doi: 10.1139/m85-046. [DOI] [PubMed] [Google Scholar]
- Wilson M. E. Effects of bacterial endotoxins on neutrophil function. Rev Infect Dis. 1985 May-Jun;7(3):404–418. doi: 10.1093/clinids/7.3.404. [DOI] [PubMed] [Google Scholar]
- van Kampen G. P., van Steenbergen T. J., Schipper C. A., de Graaff J., Veldhuijzen J. P. Proteoglycan production by chick embryonic chondrocytes is inhibited by culture filtrate of Bacteroides gingivalis. J Periodontal Res. 1984 Sep;19(5):483–488. doi: 10.1111/j.1600-0765.1984.tb01303.x. [DOI] [PubMed] [Google Scholar]
- van Steenbergen T. J., dan Ouden M. D., Touw J. J., de Graaff J. Cytotoxic activity of Bacteroides gingivalis and Bacteroides asaccharolyticus. J Med Microbiol. 1982 May;15(2):253–258. doi: 10.1099/00222615-15-2-253. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff A. J., Carlee A. W., de Graaff J. Bacteroides endodontalis and other black-pigmented Bacteroides species in odontogenic abscesses. Infect Immun. 1985 Sep;49(3):494–497. doi: 10.1128/iai.49.3.494-497.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]