Abstract
Steroidogenesis is now recognized as a global phenomenon in the brain, but how it is regulated and its relationship to circulating steroids of peripheral origin have remained more elusive issues. Neurosteroids, steroids synthesized de novo in nervous tissue, have a large range of actions in the brain, but it is only recently that the role of neuroprogesterone in the regulation of arguably the quintessential steroid dependent neural activity, regulation of the reproduction has been appreciated. Circuits involved in controlling the LH surge and sexual behaviors were thought to be influenced by estradiol and progesterone synthesized in the ovary and perhaps the adrenal. It is now apparent that estradiol of ovarian origin regulates the synthesis of neuroprogesterone, and it is the locally produced neuroprogesterone that is involved in the initiation of the LH surge and subsequent ovulation. In this model, estradiol induces the transcription of progesterone receptors while stimulating synthesis of neuroprogesterone. Although the complete signaling cascade has not been elucidated, many of the features have been characterized. The synthesis of neuroprogesterone occurs primarily in astrocytes and requires the interaction of membrane associated estrogen receptor-α with metabotropic glutamate receptor-1a. This G protein-coupled receptor activates a phospholipase C that in turn increases inositol trisphosphate (IP3) levels mediating the release of intracellular stores of Ca2+ via an IP3 receptor gated Ca+2 channel. The large increase in free cytoplasmic Ca2+ ([Ca2+]i) stimulates the synthesis of progesterone, which can then diffuse out of the astrocyte and activate estradiol-induced progesterone receptors in local neurons to trigger the neural cascade to produce the LH surge. Thus, it is a cooperative action of astrocytes and neurons that is needed for estrogen positive feedback and stimulation of the LH surge.
Keywords: neurosteroids, estrogen positive feedback, neurotransmission, estrogen receptor, mGluR, progesterone, progesterone receptor
Introduction
The brain has always been considered a target for sex steroid hormones produced by peripheral steroidogenic organs, the gonads and the adrenal glands, but it is now well accepted that the brain synthesizes neurosteroids de novo, and converts circulating steroids to neuroactive steroids (Corpechot et al., 1981; Guennoun et al., 1995; Jung-Testas et al., 1989; Jung-Testas et al., 1991; Kohchi et al., 1998; Micevych et al., 2007; Robel, 1995; Sanne and Krueger, 1995; Sinchak et al., 2003; Zwain and Yen, 1999). Regardless of their origin, steroids affect brain function through actions at their cognate receptors, or by affecting receptors whose primary transmitter is not a steroid (e.g., GABA receptors).
As with a number of different signaling molecules, the site of their synthesis has been used to classify them as hormones or neurotransmitters. Similarly, sex steroids of peripheral origin are hormones. They are released into the general circulation to act on distal target sites that have the appropriate receptors, which includes nervous tissue. Neurosteroids are neurotransmitters: they are made in the brain, their synthesis and levels are regulated and they influence neuronal activity by modulating intracellular signaling pathways, channels and transcription.
This awareness of neurosteroid function led Kawato et al. (Kawato et al., 2003) to classify these compounds fourth generation (4-G) neurotransmitters. In this schema, first generation transmitters are the small molecular weight messengers (e.g., acetylcholine. glutamate and GABA). Second generation neurotransmitters are catecholamines (e.g., dopamine, serotonin), and third generation neurotransmitters are large family of neuropeptides (e.g., neuropeptide Y (NPY), cholecystokinin (CCK), β-endorphin). Although Kiwato et al., (Kawato et al., 2003) suggest that neurosteroids are the 4-G transmitters, this class of neurotransmitter should include not only neurosteroids (e.g., progesterone, estrogen), but also gaseous transmitters (e.g, nitric oxide, carbon monoxide) and endocannabinoids. The 4-G transmitters employ a volumetric mode of transmission affecting a region of the brain rather than the more classical point to point neurotransmission of the first generation neurotransmitters. Moreover, 4-G neurotransmitters are unique, they are regulated at the level of synthesis unlike other classes of transmitters which are stored and whose release is tightly controlled. Once 4-G neurotransmitters are synthesized - they are rapidly released to affect surrounding cells.
One of the more intriguing questions has been the relationship of peripheral steroids to neurosteroids. Free steroids (i.e., steroids not bound to carrier proteins) are capable of diffusing across the blood-brain-barrier to bind both membrane-associated steroid receptors and intracellular receptors. Thus, levels of a particular steroid in the brain are a composite of steroids from the periphery, converted peripheral steroids, and neurosteroids. Additionally, hormonal steroids also regulate the site-specific synthesis of neurosteroid levels (Maguire and Mody, 2007; Micevych et al., 2003) and their cognate receptors (Chappell and Levine, 2000; MacLusky and McEwen, 1978; Soma et al., 2005) that affect neurosteroid levels and function. Such peripheral sex steroid-neurosteroid interactions are the subject of this review, especially as it relates to neuroprogesterone synthesis.
Model of estrogen positive feedback
In a cycling rat, steroidogenesis in ovarian follicles is stimulated by gonadotropins released from the pituitary gland. As the cycle advances the levels of circulating estradiol increase until they peak on the afternoon of proestrus. This spike of estradiol signals the process of estrogen positive feedback that stimulates the surge release of gonadotropin releasing hormone that triggers the of surge release of LH from the pituitary. In the ovary, LH induces ovulation and the luteinization of the ruptured follicles that secrete progesterone. The mechanism of estrogen positive feedback has remained elusive, but several components are known. First, high levels of estradiol are absolutely essential (Brom and Schwartz, 1968; Ferin et al., 1969; Labhsetwar, 1970). Second, estradiol induced progesterone receptors (PR) in the hypothalamus are needed (Chappell et al., 2000; DePaolo, 1988; Hibbert et al., 1996; Mahesh and Brann, 1992; Sanchez-Criado et al., 1994; Snyder et al., 1984). Third, preovulatory progesterone is also essential for the gonadotropin surge (DePaolo, 1988; Hibbert et al., 1996; Mahesh et al., 1992; Micevych et al., 2003; Remohi et al., 1988). Most models of the LH surge use both estradiol and progesterone priming because ovariectomized rats treated with only estradiol show a physiological, but blunted LH surge, but treatment with progesterone increases the magnitude and duration of the surge (DePaolo and Barraclough, 1979).
The source of the preovulatory progesterone has been the source of some controversy. Both the ovary and the adrenal have been proposed as sources of preovulatory progesterone (Buckingham et al., 1978; Mahesh and Brann, 1998; Putnam et al., 1991; Shaikh and Shaikh, 1975), but estradiol primed ovariectomized and adrenalectomized (ovx/adx) rats continue to produce an LH surge (Mann et al., 1975; Micevych et al., 2003). Antagonizing PR prevents the estradiol-induced LH surge (DePaolo, 1988; Hibbert et al., 1996; Mahesh et al., 1992; Sanchez-Criado et al., 1994; Snyder et al., 1984). In ovx/adx rats, the estradiol-stimulated LH surge is disrupted if 3β-HSD (3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase), which converts pregnenolone to progesterone, is blocked (Micevych et al., 2003). Moreover, hypothalamic neuroprogesterone concentrations were positively correlated with surge levels of plasma LH (r2 = 0.77). These results are highly suggestive that neurosteroids may be involved in estrogen positive feedback regulating the LH surge.
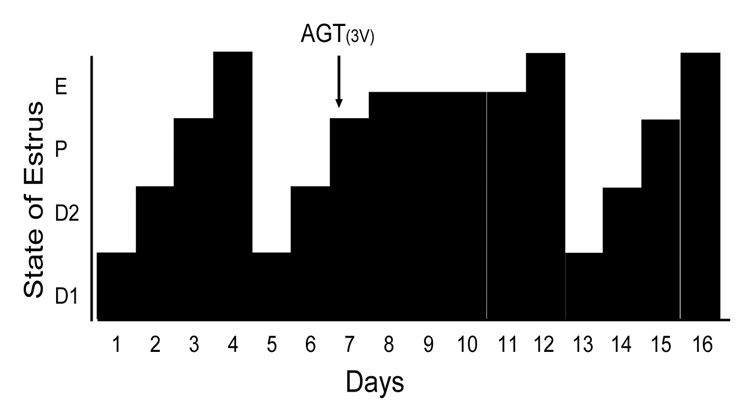
To further explore the relationship between hypothalamic neurosteroid synthesis and the regulation of ovulation, we blocked the first step in steroidogenesis, the conversion of cholesterol to pregnenolone. On the morning of proestrus in gonadally intact cycling rats, aminoglutethemide (AGT), the P450scc (cytochrome P450side-chain cleavage) enzyme inhibitor, was infused into the third ventricle to inhibit steroidogenesis in the hypothalamus. Following AGT treatment, rats had a vaginal cytology of predominately nucleated cells (proestrus pattern), and a mixture of nucleated and cornified cells (a cytology midway between proestrus and estrus), indicating that an LH surge had not occurred (Fig 1). In rats treated with AGT, ovaries had increased numbers of antral follicles but lacked corpora lutea compared with the vehicle treated rats. AGT rats also had atrophied uterine walls and uterine cavities filled with fluid indicating estradiol stimulation, but no LH or progesterone stimulation. The increased number of developing follicles in the ovaries, and the swollen uterus were similar to those described in neuronal ERα knockout mice that could not have an LH surge (Wintermantel et al., 2006). Four to six days after AGT treatment, rats eventually developed a cornified vaginal cytology (estrous pattern), associated with an LH surge, and rats resumed a regular 4 day estrous pattern. To verify that AGT had not leaked out of the brain and blocked peripheral steroidogenesis, estradiol levels were measured. Plasma levels of estradiol were comparable between control and the AGT infused groups (28.4 ± 12.3 vs. 17.7 ± 5.0 pg/ml; n = 11) confirming that peripheral steroidogenesis had not been disrupted by third ventricular AGT treatment. On the other hand, AGT treated rats had significantly reduced circulating progesterone levels (27.9 ± 7.6 vs. 10.4 ± 4.4 ng/ml; p < 0.05). In the hypothalamus neuroprogesterone levels were also significantly reduced by AGT treatment (49.8 ± 16.7 vs. 18.4 ± 12.2 pg/mg; p<0.05). These results indicate that AGT inhibited neurosteroidogenesis in the hypothalamus preventing the LH surge, and underscoring the importance of neuroprogesterone in initiating the LH surge even in an animal with ovaries and adrenals.
Figure 1.
Blocking neuroprogesterone synthesis alters the pattern of estrous cycles in gonadally intact rats. The four day rat estrous cycle is diagrammatically presented. Each day of the cycle is indicated on the ordinate: D1 = diestrous day 1, D2 = diestrous day 2, P = proestrus and E=estrus. Neuroprogesterone synthesis was blocked by infusion of a P450scc inhibitor, aminoglutethimide (AGT), into the third ventricle (3V) via an implanted cannula. All animals treated with DMSO (5%, vehicle n = 14; data not shown) had normal 4 day estrous cycles as determined by vaginal cytology. In contrast, 11/14 AGT treated rats (0800 hrs on proestrus, indicated by the arrow) had disrupted estrous cycles with delayed onset of estrous as determined by vaginal cytology.
Regulation of Neurosteroidogenesis
Steroids are derived from cholesterol. There are two sources of cholesterol for steroidogenesis: the lipoproteins in the circulation and from de novo synthesis in the individual cells (Freeman, 1987). However, circulating cholesterol cannot cross the blood-brain-barrier, and cholesterol is produced de novo in the brain (reviewed in (Bjorkhem and Meaney, 2004) Almost all brain cholesterol is unesterified, and comprises a structural component of myelin sheaths (oligodendrocytes) and the plasma membranes (astrocytes and neurons) (Snipes and Suter, 1997). The first enzymatic step of steroidogenesis is the removed of the cholesterol side chain is by P450scc located along the inner mitochondrial membrane. In the smooth endoplasmic reticulum, pregnenolone is converted to progesterone by 3β-HSD (for reviews see (Mensah-Nyagan et al., 1999; Payne and Hales, 2004).
The overall rate-limiting step of steroidogenesis is the transport of cholesterol from the outer to the inner mitochondrial membrane effected by steroid acute regulatory protein (StAR), peripheral-type benzodiazepine receptor (PBR) and its endogenous ligand, diazepam binding inhibitor (DBI; (Granot et al., 2002; Kallen et al., 1998; King et al., 2002; Stocco, 2001; Stocco and Clark, 1996) for reviews see (Niswender, 2002; Papadopoulos, 1993)). In the nervous system, StAR, PBR and DBI have been localized in astrocytes (Karri et al., 2007; Lamacz et al., 1996; Young, 1994). In peripheral steroidogenic tissues, steroid production is regulated by gonadotropins released from the anterior pituitary that activate G protein-coupled receptor (GPCR) that induce the phosphorylation of protein kinases (Alila et al., 1990; Rao et al., 1987; Shalem et al., 1988; Shemesh et al., 1984) and lead to activation of enzyme activity and transcription (Momoi et al., 1992; Waterman, 1994). Phosphorylation of StAR and PBR are part of a rapid phase of steroidogenesis that does not involve gene transcription (Arakane et al., 1997; Jo et al., 2005; Papadopoulos et al., 1997a; Papadopoulos et al., 1997b; Stocco et al., 2005).
In the brain, cell-types have preferential steroid products (Zwain et al., 1999). The most steroidogenic cell is the astrocyte, and its primary steroid product is neuroprogesterone. Oligodendrocytes preferentially synthesize pregnenolone, and neurons aromatize circulating androgens to estrogens. (Guennoun et al., 1995; Jung-Testas et al., 1989; Jung-Testas et al., 1991; Kohchi et al., 1998; Micevych et al., 2007; Robel, 1995; Sanne et al., 1995; Sinchak et al., 2003; Zwain et al., 1999),
Estradiol facilitates neuroprogesterone synthesis
To examine mechanism by which estradiol increases neuroprogesterone synthesis, we began with an in vivo preparation. In ovx/adx rats, systemic estradiol significantly increased hypothalamic 3β-HSD mRNA levels and 3β-HSD enzyme activity (Soma et al., 2005). The increase in the progesterone synthesizing enzyme, 3β-HSD, and its activity occur several hours before the expected LH surge, and suggest that these events were physiologically relevant to estrogen positive feedback of the LH surge. Moreover the results support the AGT studies that indicated elevated levels of hypothalamic neuroprogesterone initiate the LH surge. In contrast, estradiol treatment had no effect on mRNA levels of P450scc or StAR (Soma et al., 2005), suggesting that in vivo estradiol-induced neuroprogesterone synthesis has a transcriptional component, a hallmark of the long-term phase of neurosteroidogenesis.
The in vivo model does not allow for the examination of estradiol cell signaling that facilitates neuroprogesterone synthesis, therefore, we turned to enriched hypothalamic astrocyte cultures as a valuable tool for understanding the mechanism of estradiol regulation of neurosteroidogenesis. Post-pubertal hypothalamic astrocytes cultured from female rats expressed StAR, P450scc and 3β-HSD mRNAs and synthesized neuroprogesterone (Micevych et al., 2007). Treating astrocyte cultures with estradiol induced the synthesis of neuroprogesterone (Micevych et al., 2007; Sinchak et al., 2003). This action was mediated through ERs. Both intracellular and membrane associated ERα and ERβ were localized in astrocytes suggesting two possible mechanisms for estradiol regulation of neuroprogesterone synthesis: increasing levels of proteins involved in neurosteroidogenesis (intracellular, long-term regulation), or increasing the activity of such proteins (membrane, rapid regulation) (Chaban et al., 2004). Relative qRT-PCR measurements indicated that estradiol did not increase P450scc, 3β-HSD, or StAR mRNA levels, suggesting that in vitro, estradiol induces neuroprogesterone synthesis via a rapid, membrane initiated mechanism.
Further support for this mode of estradiol action was provided by a series of experiments that demonstrated a rapid estradiol-initiated increased in free cytoplasmic Ca2+ ([Ca2+]i) in astrocytes. The ability of the membrane impermeable estradiol coupled at the 6-carbon to bovine serum albumin (E-6-BSA) to mimic free estradiol suggested that these rapid estradiol actions were mediated through membrane associated ERs (Chaban et al., 2004). To test whether ERα or ERβ mediated this membrane effect, selective ER agonists were used. Stimulation of astrocytes with propylpyrazoletriol (PPT), the ERα selective agonist, mimicked the estradiol-induced [Ca2+]i flux, whereas diaprylpropionitrile (DPN), the ERβ selective agonist, was ineffective. These results strongly suggest that the membrane ER involved in increasing [Ca2+]i flux is ERα. Finally, using a Ca2+-free media with BAPTA we demonstrated that the estradiol-induced [Ca2+]i flux was dependent on intracellular stores of Ca2+, and its release is dependent on stimulation of intracellular pathways associated with G protein signaling (Chaban et al., 2004; Micevych et al., 2007).
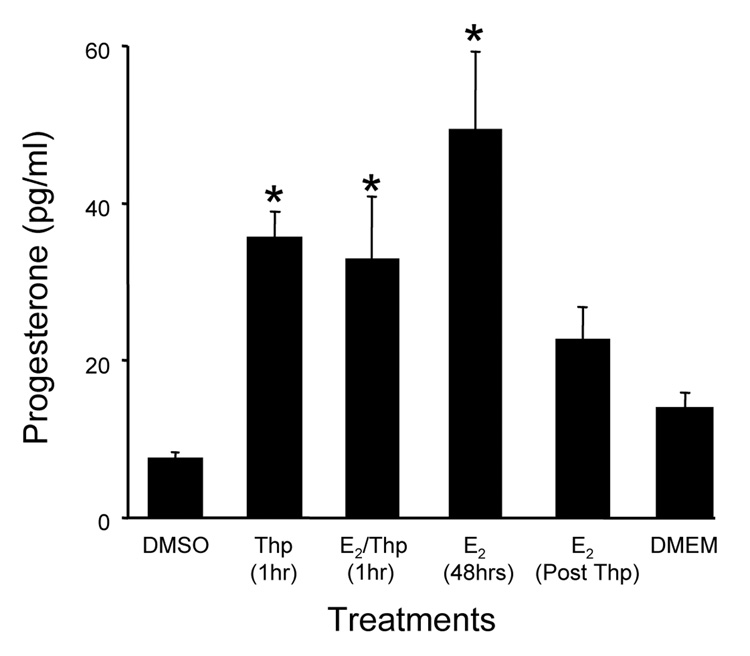
Thapsigargin, a drug that causes massive release of IP3-sensitive intracellular Ca2+ stores, was used to determine whether the estradiol-increased [Ca2+]i flux is associated with estradiol-induced neuroprogesterone synthesis. Treating cells with thapsigargin allowed us to examine the proximal intracellular signaling (Jackson et al., 1988), and references therein). Astrocytes treated with thapsigargin had significantly greater levels of neuroprogesterone, indicating increased synthesis. Combining thapsigargin and estradiol did not augment neuroprogesterone levels indicating that estradiol-stimulated neuroprogesterone synthesis depends on releasing intracellular Ca2+ stores (Fig 2). Thus, increased [Ca2+]i flux in astrocytes is a good marker for estradiol activation of neuroprogesterone synthesis (Micevych and Sinchak, 2008a).
Figure 2.
The rapid release of Ca2+ from internal stores by thapsigargin (Thp) induces neuroprogesterone synthesis in post-pubertal female hypothalamic astrocyte cultures. Astrocytes were treated with Thp or Thp (10−7 M) with 10−6 M estradiol (E2/Thp) for one hour. Media was collected and replaced with either estradiol-free DMEM/F12 (DMEM Post Thp) or 10−6 M estradiol (E2 48hrs Post Thp). The neuroprogesterone concentration was significantly higher following treatment with E2 (48 hrs), or Thp or Thp+E2 (1 hr). Following an hour of Thp, treatment with either DMEM or E2 for 48 hours did not statistically increase the concentration of neuroprogesterone. Data are mean ± SEM (n = 4). * Represent values significantly different (p < 0.05) compared to the control media, DMEM + DMSO (DMSO) (Micevych et al., 2007).
Estradiol signaling in astrocytes is mediated by metabotropic glutamate receptors
How is membrane ERα coupled to [Ca2+]i flux? Blocking PLC and IP3 receptor prevented the estradiol-induced [Ca2+]i flux indicating that G protein activated intracellular signaling pathway is involved (Chaban et al., 2004). PLC is coupled to GPCR via a Gq, suggesting several possibilities: i) the membrane ERα is itself a GPCR (Hammes and Levin, 2007; Razandi et al., 1999), ii) membrane ERα interacts with another protein that activates G proteins (Boulware et al., 2005; Dewing et al., 2007), or iii) the estradiol binding membrane protein is a GPCR novel receptor (GPR30 (Filardo et al., 2000; Filardo et al., 2002); STX-binding membrane protein (Qiu et al., 2003); and ER-X (Toran-Allerand et al., 2002; Toran-Allerand et al., 1999)). In astrocytes the best evidence is that membrane-initiated estradiol signaling requires a classical ER: both ERα and ERβ are located in the membrane, estradiol-induced [Ca2+]i flux and neuroprogesterone synthesis is blocked by ICI 182,780, E-6-BSA mimic estradiol effects, and only ERα selective, but not Erβ selective agonists stimulate a [Ca2+]i flux in hypothalamic astrocytes. We hypothesized that membrane ER was coupled to intracellular cascades through a mGluR based on results from studies in neurons, In neurons, ER has been shown to directly interact with the mGluR to initiate intracellular signaling (Boulware et al., 2005; Dewing et al., 2007).
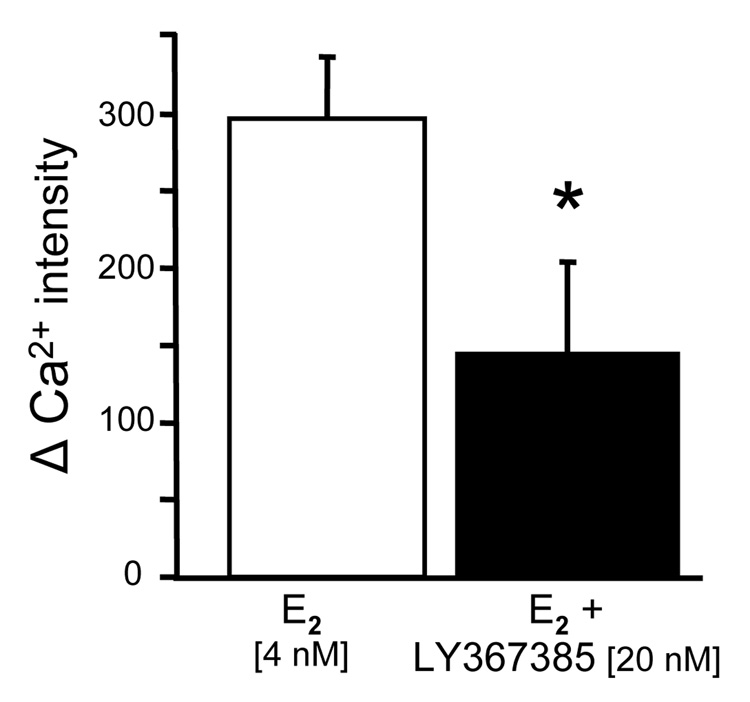
The metabotropic glutamate receptors (mGluRs) are a family of GPCR that are divided into three groups. Group I mGLuR1a are coupled to Gq and activate PLC forming diacyl glycerol (DAG) and IP3 in neurons and astrocytes (Boulware et al., 2005; Zur Nieden and Deitmer, 2006). Interactions between ER and mGluR have been demonstrated to mediate rapid estradiol actions in hypothalamic circuits that induce sexual receptivity, and mediate anti-nociceptive estradiol actions in dorsal root ganglion (DRG) neurons (Chaban et al., 2007; Chaban et al., 2003). Since astrocytes like neurons express both the mGluR1a and ERs, we tested whether these proteins could interact. Using co-immunoprecipitation, we identified such an interaction between ERα and mGluR1a in astrocyte membrane fractions (Kuo et al., 2008). To demonstrate that the ERα/mGluR1a interaction is part of estradiol signaling in astrocytes, mGluR1a was antagonized by LY367,385 in the presence of estradiol, and the [Ca2+]i flux was blocked (Fig 3). Conversely, we stimulated a [Ca2+]i flux with the agonist of mGluR1a, DHPG. Together these results indicate that membrane–initiated estradiol signaling is dependent on an ERα/mGluR1a interaction, and suggests that while ERα is not a GPCR, it can stimulate G protein signaling pathways though the mGluR1a. Interestingly, joint application of estradiol and DHPG produced and augmented Ca2+ response that was significantly greater than that produced with either agent alone. This effect was not observed in neurons and may suggest a pathway through which neural activity that involves the release of glutamate can potentiate the response to estradiol.
Figure 3.
Effect of mGluR1a antagonist, LY 367385, on the estradiol-induced [Ca2+]i flux in post-pubertal hypothalamic astrocytes. LY367385 inhibited the estradiol (E2) stimulated release of Ca2+ from internal stores. Astrocytes were stimulated with E2 and then washed for 7 min. prior to the second E2 stimulation, LY367385 was added. * Significantly different compared with E2 stimulation alone (p<0.05, Student’s t-test).
Menopause
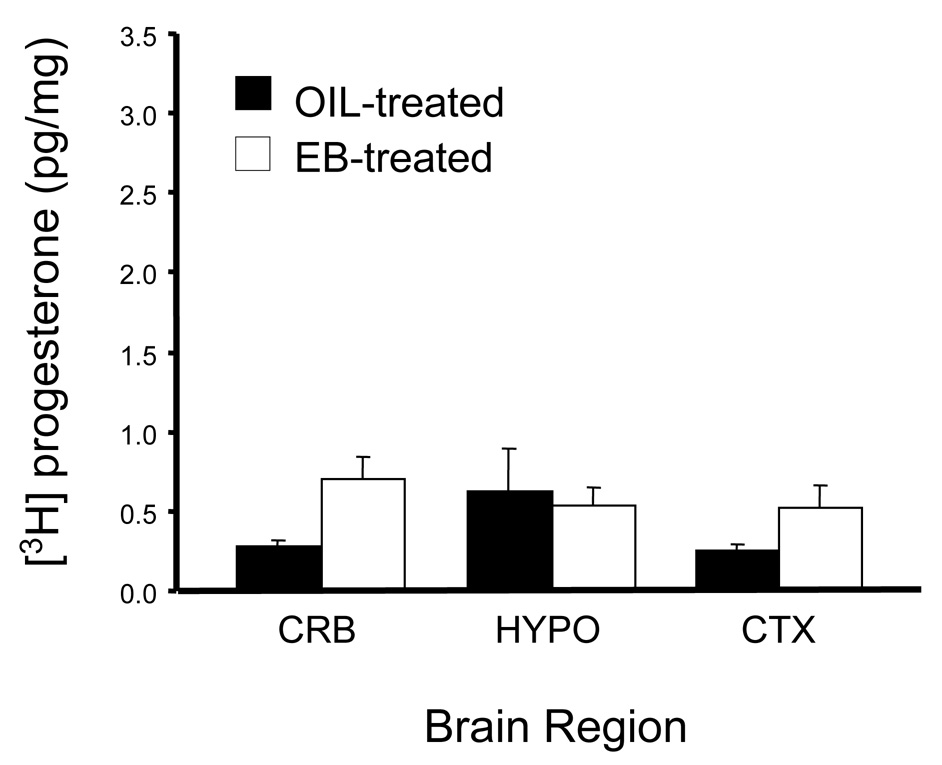
A consequence of the loss of neuroprogesterone synthesis may be reproductive senescence in which estrogen positive feedback is attenuated and then lost (Micevych et al., 2008b). One of the first signs of reproductive aging is a reduction in the magnitude of the LH surge (Cooper et al., 1980; Nass et al., 1984; Wise, 1982), which is followed by irregular estrous cycles suggesting impaired estrogen positive feedback. Eventually, the rat becomes acyclic, exhibiting a cornified vaginal cytology - a persistent or constant estrous state (CE). Rats in CE have moderately elevated circulating estradiol (Lu et al., 1979), but low progesterone with no LH surges (Lu et al., 1979; Matt et al., 1986). One idea is that such reproductive senescence is associated with a loss of progesterone synthesis since estradiol + progesterone induce an LH surge within the first 30 days of CE, but estradiol alone, unlike in young rats, does not. Moreover, the levels of estradiol-induce progesterone receptor mRNA remain relatively high during the CE (Mills et al., 2002). Females in CE for 90 days or longer completely loose their response to progesterone and their progesterone receptor mRNA is down-regulated. CE rats were examined to test the idea that neuroprogesterone synthesis might be compromised, Circulating progesterone levels in ovx/adx CE rats were similar to levels in young, ovx/adx females (Corpechot et al., 1993; Lu et al., 1979; Nass et al., 1984). In distinction to young rats (Micevych et al., 2003)), CE females do not respond to estradiol with an increase of hypothalamic (or cerebral or cerebellar) neuroprogesterone progesterone levels (2 way ANOVA (2,23), F = 2.237, p = 0.15; Fig 4) or serum levels (t-test df = 6, t = 1.34, p = 0.23). These results suggest that the loss of estrogen positive feedback and the LH surge in CE rats is due to a loss in the estradiol-induced hypothalamic neuroprogesterone.
Figure 4.
Estradiol did not induce neuroprogesterone synthesis in reproductively senescent, 9 month old Long-Evans female rats exhibiting continuous cornified vaginal smears) for 30 days (indicating they were in constant estrous (CE)). CE rats were ovx/adx and subsequently subcutaneously injected with estradiol (50 µg EB) or the safflower oil vehicle. Brain tissue was examined for neuroprogesterone 45 hr after EB. Unlike young animals, estradiol treatment had no effect on hypothalamic, progesterone levels (2 way ANOVA (2,23), F = 2.237, p = 0.15) or serum levels (t-test df = 6, t = 1.34, p = 0.23 (Fig 4).Values are means ± SEM of at least 9 animals per group. Abbreviations: CRB: cerebellum; HYPO: hypothalamus; CTX: cortex.
Summary
In addition to its role as a hormone, progesterone made in the nervous system, neuroprogesterone, may be one of a family of 4-G neurotransmitters that are regulated at the level of their synthesis. They diffuse and activate cognate receptors to influence neuronal activity and function. Neuroprogesterone and its metabolites also have the ability to activate different receptors expanding their role as neuroactive compounds. Neuroprogesterone has been implicated in a variety of tropic and regulatory functions including: normal brain development, myelination and maintenance of neuronal survival. We have discussed the role of neuroprogesterone as a regulator of the central event in reproduction – ovulation. Previously, it had been assumed that the source of progesterone that regulates reproductive circuits in the brain were the ovaries and adrenal glands. While it is undeniable that peripheral steroidogenic organs produce the bulk of circulating progesterone, the importance of brain steroidogenesis is increasingly being appreciated. In our laboratories, we have been interested in understanding the interactions of circulating estradiol and neuroprogesterone with respect to estrogen positive feedback of the LH surge. Our working hypothesis is that estradiol of ovarian origin binds to an intracellular receptor to initiate the expression of progesterone receptors in neurons. As levels of estradiol peak on the morning of proestrus, estradiol binds to membrane ERs initiating an interaction with mGluR1a increasing [Ca2+]i flux and stimulating the synthesis of neuroprogesterone in astrocytes. Thus, estradiol acts at both “presynaptic” and “postsynaptic” sites to increase ligand concentration and its cognate receptor. Thus, it is neuroprogesterone acting at the newly expressed PR that activates a neuronal circuit leading to a surge release of GnRH, which in turn stimulates the surge release of LH and ovulation. In the reproductively senescent, CE rat a lack of estrogen positive feedback and LH surge has been correlated with the inability of estradiol to stimulate neuroprogesterone synthesis in the hypothalamus, suggesting that at least one part of reproductive aging is the loss of estradiol-induced neuroprogesterone synthesis. Although there are still many details to elucidate in this schema, it does provide an important heuristic model to understand estrogen positive feedback and the physiology of neurosteroids in the central control of reproduction.
ACKNOWLEDGEMENTS
We appreciate the contributions of our collaborators Drs Dewing, Chaban, Kuo and Bondar. The research was supported by NIH grant HD042635.
Grants: The research was supported by NIH grant HD042635.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alila HW, Davis JS, Dowd JP, Corradino RA, Hansel W. Differential effects of calcium on progesterone production in small and large bovine luteal cells. J. Steroid Biochem. 1990;36:687–693. doi: 10.1016/0022-4731(90)90189-y. [DOI] [PubMed] [Google Scholar]
- Arakane F, King SR, Du Y, Kallen CB, Walsh LP, Watari H, Stocco DM, Strauss JF., 3rd Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J. Biol. Chem. 1997;272:32656–32662. doi: 10.1074/jbc.272.51.32656. [DOI] [PubMed] [Google Scholar]
- Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J. Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brom GM, Schwartz NB. Acute changes in the estrous cycle following ovariectomy in the golden hamster. Neuroendocrinol. 1968;3:366–377. doi: 10.1159/000121725. [DOI] [PubMed] [Google Scholar]
- Buckingham JC, Deohler KD, Wilson CA. Activity of the pituitary-adrenocortical system and thyroid gland during the oestrous cycle of the rat. Journal of Endocrinol. 1978;78:359–366. doi: 10.1677/joe.0.0780359. [DOI] [PubMed] [Google Scholar]
- Chaban V, Li J, McDonald J, Rapkin A, Micevych P. Estradiol attenuates ATP-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat DRG neurons. Society for Neuronscience. Neuroscience Meeting Planner. 2007 doi: 10.1002/jnr.22718. Program No. 519.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinol. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neurosci. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinol. 2000;141:1477–1485. doi: 10.1210/endo.141.4.7428. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Conn PM, Walker RF. Characterization of the LH surge in middle-aged female rats. Biol. Reprod. 1980;23:611–615. doi: 10.1095/biolreprod23.3.611. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc. Natl. Acad. Sci. USA. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpechot C, Young J, Calvel M, Wehrey C, Veltz JN, Touyer G, Mouren M, Prasad VV, Banner C, Sjovall J. Neurosteroids: 3 alpha-hydroxy-5 alpha-pregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinol. 1993;133:1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- DePaolo LV. Attenuation of preovulatory gonadotrophin surges by epostane: a new inhibitor of 3 beta-hydroxysteroid dehydrogenase. J. Endocrinol. 1988;118:59–68. doi: 10.1677/joe.0.1180059. [DOI] [PubMed] [Google Scholar]
- DePaolo LV, Barraclough CA. Dose dependent effects of progesterone on the facilitation and inhibition of spontaneous gonadotropin surges in estrogen treated ovariectomized rats. Biol. Reprod. 1979;21:1015–1023. doi: 10.1095/biolreprod21.4.1015. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J. Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferin M, Tempone A, Zimmering PE, Van de Wiele RL. Effect of antibodies to 17beta-estradiol and progesterone on the estrous cycle of the rat. Endocrinol. 1969;85:1070–1078. doi: 10.1210/endo-85-6-1070. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR, Jr., Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol. Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Freeman DA. Cyclic AMP mediated modification of cholesterol traffic in Leydig tumor cells. J. Biol. Chem. 1987;262:13061–13068. [PubMed] [Google Scholar]
- Granot Z, Silverman E, Friedlander R, Melamed-Book N, Eimerl S, Timberg R, Hales KH, Hales DB, Stocco DM, Orly J. The life cycle of the steroidogenic acute regulatory (StAR) protein: from transcription through proteolysis. Endocrine Research. 2002;28:375–386. doi: 10.1081/erc-120016812. [DOI] [PubMed] [Google Scholar]
- Guennoun R, Fiddes RJ, Gouezou M, Lombes M, Baulieu EE. A key enzyme in the biosynthesis of neurosteroids, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase (3 beta-HSD), is expressed in rat brain. Brain Res. Mol. Brain Res. 1995;30:287–300. doi: 10.1016/0169-328x(95)00016-l. [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr. Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- Hibbert ML, Stouffer RL, Wolf DP, Zelinski-Wooten MB. Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc. Natl. Acad. Sci. USA. 1996;93:1897–1901. doi: 10.1073/pnas.93.5.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson TR, Patterson SI, Thastrup O, Hanley MR. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem. J. 1988;253:81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y, King SR, Khan SA, Stocco DM. Involvement of protein kinase C and cyclic adenosine 3',5'-monophosphate-dependent kinase in steroidogenic acute regulatory protein expression and steroid biosynthesis in Leydig cells. Biol. Reprod. 2005;73:244–255. doi: 10.1095/biolreprod.104.037721. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Hu ZY, Baulieu EE, Robel P. Neurosteroids: biosynthesis of pregnenolone and progesterone in primary cultures of rat glial cells. Endocrinol. 1989;125:2083–2091. doi: 10.1210/endo-125-4-2083. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Renoir JM, Gasc JM, Baulieu EE. Estrogen-inducible progesterone receptor in primary cultures of rat glial cells. Exp. Cell Res. 1991;193:12–19. doi: 10.1016/0014-4827(91)90532-y. [DOI] [PubMed] [Google Scholar]
- Kallen CB, Arakane F, Christenson LK, Watari H, Devoto L, Strauss JF., 3rd Unveiling the mechanism of action and regulation of the steroidogenic acute regulatory protein. Molecular and cellular endocrinology. 1998;145:39–45. doi: 10.1016/s0303-7207(98)00167-1. [DOI] [PubMed] [Google Scholar]
- Karri S, Dertien JS, Stocco DM, Syapin PJ. Steroidogenic acute regulatory protein expression and pregnenolone synthesis in rat astrocyte cultures. J. Neuroendocrinol. 2007;19:860–869. doi: 10.1111/j.1365-2826.2007.01600.x. [DOI] [PubMed] [Google Scholar]
- Kawato S, Yamada M, Kimoto T. Brain neurosteroids are 4th generation neuromessengers in the brain: cell biophysical analysis of steroid signal transduction. Adv. Biophys. 2003;37:1–48. doi: 10.1016/s0065-227x(03)80002-3. [DOI] [PubMed] [Google Scholar]
- King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K, Walsh LP, Parker KL, Stocco DM, Smith RG, Lamb DJ. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. J. Neurosci. 2002;22:10613–10620. doi: 10.1523/JNEUROSCI.22-24-10613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohchi C, Ukena K, Tsutsui K. Age- and region-specific expressions of the messenger RNAs encoding for steroidogenic enzymes p450scc, P450c17 and 3beta-HSD in the postnatal rat brain. Brain Res. 1998;801:233–238. doi: 10.1016/s0006-8993(98)00585-x. [DOI] [PubMed] [Google Scholar]
- Kuo J, Hariri O, Bondar G, Ogi J, Shung MS, Micevych P. Membrane estradiol receptors interact with metabotropic glutamate receptors in hypothalamic astrocytes. Reproduct. Sci. 2008 15.#36. [Google Scholar]
- Labhsetwar AP. Role of estrogens in ovulation: A study using the estrogen-antagonist, I.C.I. 46,474. Endocrinol. 1970;87:542–551. doi: 10.1210/endo-87-3-542. [DOI] [PubMed] [Google Scholar]
- Lamacz M, Tonon MC, Smih-Rouet F, Patte C, Gasque P, Fontaine M, Vaudry H. The endogenous benzodiazepine receptor ligand ODN increases cytosolic calcium in cultured rat astrocytes. Brain Res. Mol. Brain Res. 1996;37:290–296. doi: 10.1016/0169-328x(95)00330-u. [DOI] [PubMed] [Google Scholar]
- Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol. Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, McEwen BS. Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature. 1978;274:276–278. doi: 10.1038/274276a0. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J. Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahesh VB, Brann DW. Interaction between ovarian and adrenal steroids in the regulation of gonadotropin secretion. J. Steroid Biochem. Mol. Biol. 1992;41:495–513. doi: 10.1016/0960-0760(92)90375-s. [DOI] [PubMed] [Google Scholar]
- Mahesh VB, Brann DW. Neuroendocrine mechanisms underlying the control of gonadotropin secretion by steroids. Steroids. 1998;63:252–256. doi: 10.1016/s0039-128x(98)00031-2. [DOI] [PubMed] [Google Scholar]
- Mann DR, Korowitz CD, Barraclough CA. Adrenal gland involvement in synchronizing the preovulatory release of LH in rats. Proc. Soc. Exp. Biol. Med. 1975;150:115–120. doi: 10.3181/00379727-150-38985. [DOI] [PubMed] [Google Scholar]
- Matt DW, Lee J, Sarver PL, Judd HL, Lu JK. Chronological changes in fertility, fecundity and steroid hormone secretion during consecutive pregnancies in aging rats. Biol. Reprod. 1986;34:478–487. doi: 10.1095/biolreprod34.3.478. [DOI] [PubMed] [Google Scholar]
- Mensah-Nyagan AG, Do-Rego JL, Beaujean D, Luu-The V, Pelletier G, Vaudry H. Neurosteroids: expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol. Rev. 1999;51:63–81. [PubMed] [Google Scholar]
- Micevych P, Sinchak K. Mini-reviews: Synthesis and function of hypothalamic neuroprogesterone in reproduction. Endocrinol. 2008a doi: 10.1210/en.2008-0011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinol. 2003;78:29–35. doi: 10.1159/000071703. [DOI] [PubMed] [Google Scholar]
- Micevych P, Soma KK, Sinchak K. Neuroprogesterone: Key to estrogen positive feedback? Brain Res. Rev. 2008b;57:470–480. doi: 10.1016/j.brainresrev.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Chaban V, Ogi J, Lakhter A, Lu JKH, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinol. 2007;148:782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- Mills RH, Romeo HE, Lu JK, Micevych PE. Site-specific decrease of progesterone receptor mRNA expression in the hypothalamus of middle-aged persistently estrus rats. Brain Res. 2002;955:200–206. doi: 10.1016/s0006-8993(02)03440-6. [DOI] [PubMed] [Google Scholar]
- Momoi K, Waterman MR, Simpson ER, Zanger UM. 3',5'-cyclic adenosine monophosphate-dependent transcription of the CYP11A (cholesterol side chain cleavage cytochrome P450) gene involves a DNA response element containing a putative binding site for transcription factor Sp1. Mol. Endocrinol. 1992;6:1682–1690. doi: 10.1210/mend.6.10.1333053. [DOI] [PubMed] [Google Scholar]
- Nass TE, Lapolt PS, Judd HL, Lu JK. Alterations in ovarian steroid and gonadotrophin secretion preceding the cessation of regular oestrous cycles in ageing female rats. J. Endocrinol. 1984;100:43–50. doi: 10.1677/joe.0.1000043. [DOI] [PubMed] [Google Scholar]
- Niswender GD. Molecular control of luteal secretion of progesterone. Reproduction. 2002;123:333–339. doi: 10.1530/rep.0.1230333. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V. Peripheral-type benzodiazepine/diazepam binding inhibitor receptor: biological role in steroidogenic cell function. Endocr. Rev. 1993;14:222–240. doi: 10.1210/edrv-14-2-222. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, Hardwick M, Li H, Vidic B, Brown AS, Reversa JL, Bernassau JM, Drieu K. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997a;62:21–28. doi: 10.1016/s0039-128x(96)00154-7. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Amri H, Li H, Boujrad N, Vidic B, Garnier M. Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J. Biol. Chem. 1997b;272:32129–32135. doi: 10.1074/jbc.272.51.32129. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocrine Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Putnam CD, Brann DW, Mahesh VB. Acute activation of the adrenocorticotropin-adrenal axis: effect on gonadotropin and prolactin secretion in the female rat. Endocrinol. 1991;128:2558–2566. doi: 10.1210/endo-128-5-2558. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J. Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MC, Palfrey HC, Nash NT, Greisman A, Jayatilak PG, Gibori G. Effects of estradiol on calcium-specific protein phosphorylation in the rat corpus luteum. Endocrinol. 1987;120:1010–1018. doi: 10.1210/endo-120-3-1010. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene G, Levin E. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol. Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Remohi J, Balmaceda JP, Rojas FJ, Asch RH. The role of preovulatory progesterone in the midcycle gonadotrophin surge, ovulation and subsequent luteal phase: studies with RU486 in rhesus monkeys. Hum. Reprod. 1988;3:431–435. doi: 10.1093/oxfordjournals.humrep.a136722. [DOI] [PubMed] [Google Scholar]
- Robel P, Baulieu EE. Neurosteroids and neuroactive steroids. In: Micevych PE, Hammer RP, editors. Neurobiological Effects of Sex Steroid Hormones. Cambridge: Cambridge University Press; 1995. pp. 281–296. [Google Scholar]
- Sanchez-Criado JE, Hernandez G, Bellido C, Gonzalez D, Tebar M, Diaz-Cruz MA, Alonso R. Periovulatory LHRH, LH and FSH secretion in cyclic rats treated with RU486: effects of exogenous LHRH and LHRH antagonist on LH and FSH secretion at early oestrus. J. Endocrinol. 1994;141:7–14. doi: 10.1677/joe.0.1410007. [DOI] [PubMed] [Google Scholar]
- Sanne JL, Krueger KE. Expression of cytochrome P450 side-chain cleavage enzyme and 3 beta- hydroxysteroid dehydrogenase in the rat central nervous system: a study by polymerase chain reaction and in situ hybridization. J. Neurochem. 1995;65:528–536. doi: 10.1046/j.1471-4159.1995.65020528.x. [DOI] [PubMed] [Google Scholar]
- Shaikh AA, Shaikh SA. Adrenal and ovarian steroid secretion. Endocrinol. 1975;96:37–44. doi: 10.1210/endo-96-1-37. [DOI] [PubMed] [Google Scholar]
- Shalem Z, Izhar M, Shore LS, Shemesh M, Hansel W, Strauss JF., 3rd Control of bovine placental progestin synthesis: calcium dependent steroidogenesis is modulated at the site of the cholesterol side chain cleavage enzyme. J. Steroid Biochem. 1988;31:835–838. doi: 10.1016/0022-4731(88)90293-2. [DOI] [PubMed] [Google Scholar]
- Shemesh M, Hansel W, Strauss JF., 3rd Calcium-dependent, cyclic nucleotide-independent steroidogenesis in the bovine placenta. Proc. Natl. Acad. Sci. USA. 1984;81:6403–6407. doi: 10.1073/pnas.81.20.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK, Micevych P. Estrogen induces de novo progesterone synthesis in astrocytes. Dev. Neurosci. 2003;25:343–348. doi: 10.1159/000073511. [DOI] [PubMed] [Google Scholar]
- Snipes GJ, Suter U. Cholesterol and myelin. Subcell. Biochem. 1997;28:173–204. doi: 10.1007/978-1-4615-5901-6_7. [DOI] [PubMed] [Google Scholar]
- Snyder BW, Beecham GD, Schane HP. Inhibition of ovulation in rats with epostane, an inhibitor of 3beta-hydroxysteroid dehydrogenase. Proc. Soc. Exp. Biol. Med. 1984;176:238–242. doi: 10.3181/00379727-176-41865. [DOI] [PubMed] [Google Scholar]
- Soma KK, Sinchak K, Lakhter A, Schlinger BA, Micevych PE. Neurosteroids and female reproduction: estrogen increases 3beta-HSD mRNA and activity in rat hypothalamus. Endocrinol. 2005;146:4386–4390. doi: 10.1210/en.2005-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Ann. Review Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ. Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochem. Pharmacol. 1996;51:197–205. doi: 10.1016/0006-2952(95)02093-4. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol. Endocrinol. 2005;19:2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr., Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J. Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Singh M, Setalo G., Jr. Novel mechanisms of estrogen action in the brain: new players in an old story. Front. Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- Waterman MR. Biochemical diversity of cAMP-dependent transcription of steroid hydroxylase genes in the adrenal cortex. J. Biol. Chem. 1994;269:27783–27786. [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM. Alterations in proestrous LH, FSH, and prolactin surges in middle-aged rats. Proc. Soc. Exp. Biol. Med. 1982;169:348–354. doi: 10.3181/00379727-169-41356. [DOI] [PubMed] [Google Scholar]
- Young JK. Immunoreactivity for diazepam binding inhibitor in Gomori-positive astrocytes. Regul. Pept. 1994;50:159–165. doi: 10.1016/0167-0115(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Zur Nieden R, Deitmer JW. The role of metabotropic glutamate receptors for the generation of calcium oscillations in rat hippocampal astrocytes in situ. Cereb. Cortex. 2006;16:676–687. doi: 10.1093/cercor/bhj013. [DOI] [PubMed] [Google Scholar]
- Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinol. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]