Abstract
Background
Extended wakefulness disrupts acquisition of short term memories in mammals. However, the underlying molecular mechanisms triggered by extended waking and restored by sleep are unknown. Moreover, the neuronal circuits that depend on sleep for optimal learning remain unidentified.
Results
Learning was evaluated using Aversive Phototaxic Suppression (APS). In this task, flies learn to avoid light that is paired with an aversive stimulus (quinine /humidity). We demonstrate extensive homology in sleep deprivation induced learning impairment between flies and humans. Both 6 h and 12 h of sleep deprivation are sufficient to impair learning in Canton-S (Cs) flies. Moreover, learning is impaired at the end of the normal waking-day in direct correlation with time spent awake. Mechanistic studies indicate that this task requires intact mushroom bodies (MBs) and requires the Dopamine D1-like receptor (dDA1). Importantly, sleep deprivation induced learning impairments could be rescued by targeted gene expression of the dDA1 receptor to the MBs.
Conclusion
These data provide direct evidence that extended wakefulness disrupts learning in Drosophila. These results demonstrate that it is possible to prevent the effects of sleep deprivation by targeting a single neuronal structure and identify cellular and molecular targets adversely affected by extended waking in a genetically tractable model organism.
A single night of wakefulness impairs cognitive ability on a variety of tasks including those measuring working memory, adaptive learning and problem solving [1]. Surprisingly, the relatively short durations of wakefulness that define our working day (10–16h) are sufficient to impair cognitive performance [2]. Indeed, dose response studies indicate that the extent of cognitive impairment is correlated with the accumulated time spent awake [3]. Thus waking is associated with biological processes that build up over time and interfere with cognitive performance.
Although, the underlying molecular mechanisms that are triggered by extended waking are unknown, several groups have begun utilizing neuroimaging strategies to identify networks that underlie the cognitive deficits associated with sleep loss [4]. Results from these studies indicate that during extended waking, reduced activation in particular cortical structures (e.g. prefrontal cortex) is associated with a decline in performance. That is, performance decrements following waking may not be due to global brain impairments but may reflect a molecular vulnerability in specific neuronal circuits. Thus, it may be possible to manipulate a single molecular pathway in specific cell groups to prevent cognitive impairments associated with waking. We demonstrate that the effects of extended waking could be prevented by activating the Dopamine D1 receptor in a specific circuit known to be involved in learning/memory [5, 6]. These data provide the first demonstration that the negative effects of extended waking can be reversed by modifying the properties of a single brain structure.
Results
Learning assay
Sleep deprivation induced learning impairments were evaluated using an assay that requires flies to inhibit a prepotent attraction towards light [7]. In this task, flies are placed in a T-maze and allowed to choose between a lighted and a dark chamber (Supplementary Figure 1). Filter paper is wetted with 10−1M quinine hydrochloride solution and placed into the lighted chamber such that the quinine and the humidity provide an aversive stimulus. The percentage of times the fly visits the dark vial is tabulated during 16 trials. Flies learn to select the dark alley more frequently over the course of the 16 trials [7]. Learning reaches a maximum during the last 4 trials of the test and does not improve with additional training [7]. Thus the performance index is calculated as the percentage of times the fly chooses the dark vial during the last 4 trials. The assay will be referred to as Aversive Phototaxic Suppression (APS).
Sleep deprivation disrupts learning
Flies, like humans, are awake during the day and consolidate their sleep during the night [8, 9]. Cs flies exhibit a sleep rebound following 3h, 6h and 12h of sleep deprivation (Figure 1A). We show that 6 h and 12 h of sleep deprivation disrupt learning (Figure 1B; Supplemental Figure 2). Low motivation is an unlikely explanation for the impairment because the time to complete the 16 trials (TCT) was not significantly different from controls (Supplementary Table 1). Similarly, after sleep deprivation male flies (n=17) maintained motivation to court virgin females, another prepotent response, and were not different from controls (n=18) (p=.17, data not shown). Sleep deprivation does not alter the Photosensitivity Index (PI-percentage of photopositive choices in the T-maze in 10 trials in the absence of quinine/humidity) nor the Quinine Sensitivity Index (QSI-time in seconds flies reside on the non-quinine side of a chamber) indicating that the learning impairment is due to sleep loss and not due to sleep deprivation induced alterations in sensory thresholds. Indeed, sleep deprivation does not alter photosensitivity when measured over a range of light intensities (supplementary Figure 3A) nor does it change performance using a fast phototaxis assay (supplementary Figure 3B). Since flies must climb upward to enter either chamber, we evaluated the effects of sleep deprivation on geotaxis and found it to be unaffected by sleep deprivation (supplementary Figure 3C). Importantly, flies that have been selected to prefer climbing downward with gravity (Lo) learn as well as flies that have been selected to prefer climbing upward against gravity (high5; supplementary Figure 3D, [10]) indicating that geotaxis is not required in this assay. Together these data indicate that the effects of extended waking are not due to changes in sensory thresholds.
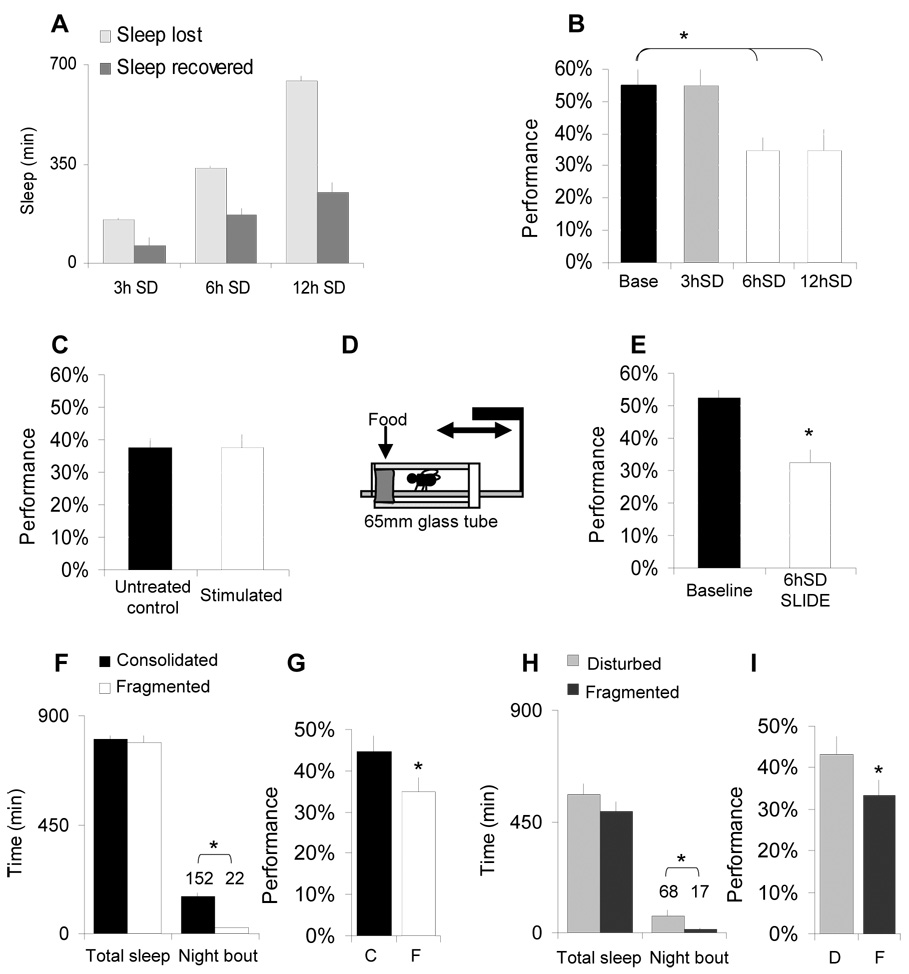
Figure 1. Sleep disruption impairs learning.
A, Sleep Deprivation (SD)successfully eliminated 100% of baseline sleep (light-grey) during to 3, 6 and 12h of SD using the Sleep Nullifying Apparatus (SNAP). Sleep recovered in 24h after 3, 6 and 12h of sleep deprivation (SD) is proportional to sleep lost (dark-grey). Flies were sleep deprived until tested. SD was started at ZT 18 and ZT 21 for 3 h and 6 h SD. B, Learning was not impaired following 3 h SD but was significantly lower after 6 or 12 h of SD. C, 6 h of stimulation in the SNAP between ZT0-ZT5:59 (‘stimulated’) does not impair performance compared to untreated circadian-matched controls (black). D–E, Performance is impaired following 6 h of SD using the Sleep Interrupting Device (SLIDE). F, Total sleep and average sleep bout duration for Cs flies with consolidated sleep (black) and their spontaneously sleep-fragmented siblings (white). G, Learning is impaired in spontaneously fragmented flies (F) compared to siblings with consolidated sleep bouts (C). Learning was evaluated between ZT0-ZT3; (n=15/group). H. Experimental fragmentation was induced for three consecutive days in otherwise sleep consolidated flies by activating the SNAP for 5 minutes every 30 minutes. Controls were sleep deprived for 4 hours (ZT11-ZT15) and thus received the same number of stimuli (1440) and similar total sleep loss while obtaining consolidated sleep bouts. I Experimental sleep fragmentation (F) impairs performance compared to flies that are disturbed (D) but with consolidated sleep. (*: p<0.05).
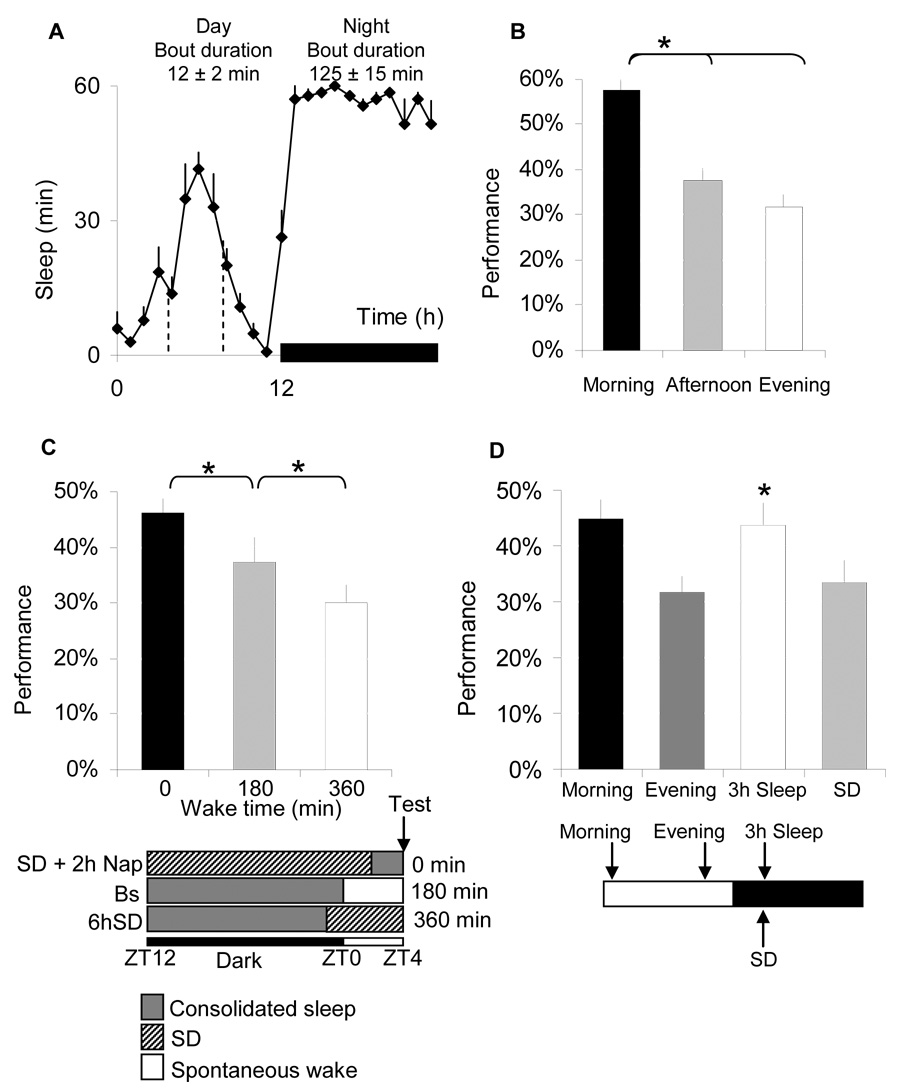
Figure 3. Performance is dependent on previous waking experience.
A, Sleep min/h for each hour of the 24-h day in female Cs flies maintained on a 12:12 LD schedule. The dark bar represents lights-out; inset number show average sleep bout during the day and night. B, Performance is impaired as waking accumulates across the biological day. Morning test (ZT0-3:59) Afternoon (ZT4-7:59) and Evening (ZT8-11:59). C, Learning was evaluated in three groups of flies at the same circadian time (ZT4-7:59). These flies differed in the amount of waking obtained since their last episode of consolidated sleep (defined as average sleep bout duration >30min). ‘0 minutes of waking’, (n=23) were sleep deprived from ZT12 until ZT2 and allowed to sleep unperturbed for 2 h (observed sleep time and sleep bout duration: 77 ± 6 min and 35 ± 8 min, respectively). ‘180 minutes of waking’, (n=17) were allowed 4 h of spontaneous sleep between ZT0-ZT3:59 (observed wake time, sleep time and sleep bout duration: 182 ± 17 min, 58 ± 17 min and 13.5 ± 1.65 min respectively). ‘360 min waking’, (n=17) were sleep deprived from ZT22 until ZT3:59 (observed sleep time =0 min). D, Learning was evaluated 3 h after lights-off in spontaneously sleeping flies compared to circadian-matched waking-controls that had been kept awake until ZT15 using the SNAP. Sleeping flies obtained 155 ± 5 minutes of nighttime sleep that was consolidated into bouts of 101 ± 17 min. Sleeping flies exhibited learning scores identical to those achieved after a full nights sleep but at an opposite circadian phase (schematic). In contrast, the flies that were not allowed to sleep in the evening were significantly impaired at the same circadian time. (*: p<0.05).
To determine whether the decrement in performance was the consequence of the stimulus used to keep the animal awake rather than sleep loss per se, we conducted several control experiments. First we exposed flies to the perturbations induced by our apparatus for 6 h between zeitgeber time ZT0 and ZT5:59. Keeping flies awake during this time does not result in subsequent changes in sleep [8]. As expected, exposure to the stimulus in the absence of sleep loss did not result in an additional learning deficit (Figure 1C). Currently, all studies that have kept flies awake, including sleep deprivation by ‘gentle handling’, have used methods that share common features. To exclude the possibility that these methods impair performance we invented a novel sleep deprivation apparatus. The Sleep Interrupting Device (SLIDE) consists of a thin plastic floor inserted into the tubes underneath flies that can be manipulated like a treadmill (Figure 1D; Supplemental Figure 4). When flies are kept awake using this approach learning is impaired (Figure 1E).
Sleep fragmentation in humans and rodents is associated with learning impairments [11, 12]. To determine whether sleep fragmentation also deteriorates learning in flies, we took advantage of the observation that ≈10–15% of Cs flies spontaneously exhibit fragmented sleep while maintaining normal total sleep time (Figure 1F). Learning was impaired in flies with fragmented sleep compared to their siblings with consolidated sleep (Figure 1G). Thus, even in the absence of mechanical stimulation sleep fragmentation is associated with learning impairments. Flies with consolidated and fragmented sleep displayed similar Control Metrics (TCT, PI and QSI) indicating that they did not differ in sensory thresholds or motoric ability (Supplementary Table 1). Importantly, experimentally induced sleep fragmentation impairs learning in otherwise sleep consolidated flies (Figure1H, I) indicating that sleep fragmentation impairs learning in flies as it does in humans.
Sleepiness does not impair learning
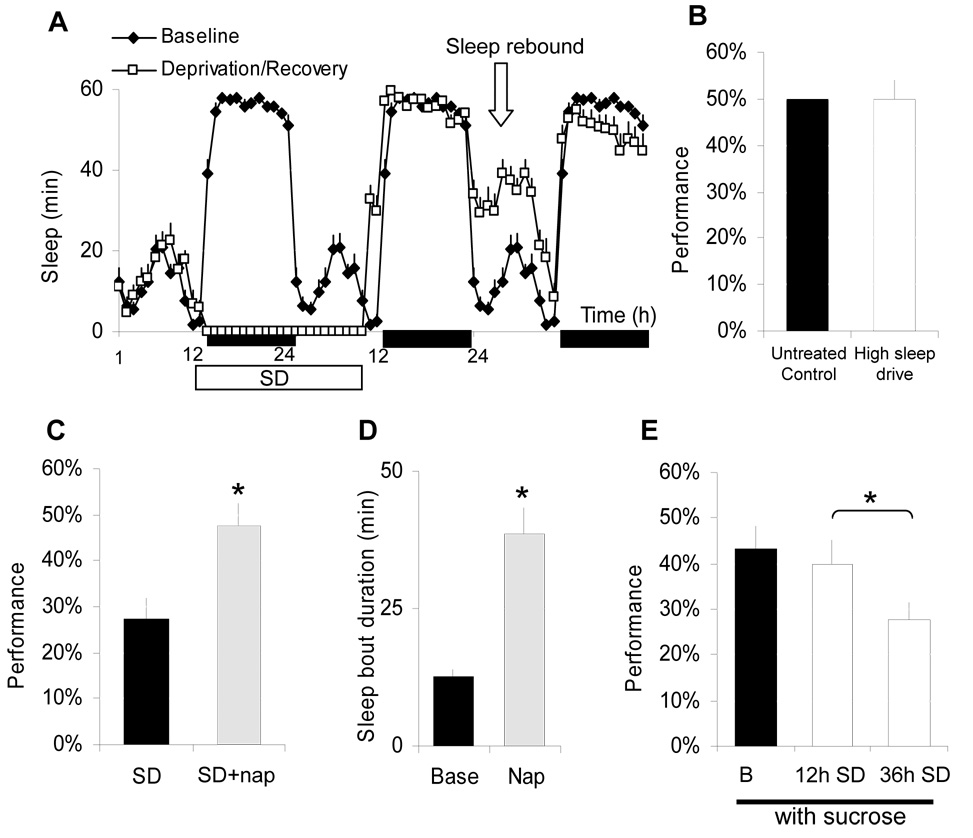
Performance decrements observed in sleep deprived humans have, at times, been attributed to the intrusion of sleep into periods of waking rather than cognitive impairment per se. Are the learning impairments in our flies simply due to high sleep drive? To test this hypothesis, we designed a protocol that allowed us to separate the effects of extended wakefulness from increased sleepiness. When flies are deprived of sleep for 22 h and released into recovery in the evening, sleep rebound is only observed the following morning (Figure 2A). If sleep drive impairs performance, flies released into recovery at night should show a deficit when tested the next morning. As seen in Figure 2B, flies with high sleep drive exhibit normal performance indicating that the amount of prior waking rather than interference due to sleepiness is responsible for learning deficits.
Figure 2. The effect of sleep drive and recovery sleep on learning.
A, Sleep min/h for each hour of the 24-h day in female Cs flies maintained on a 12:12 LD schedule for 3 days; the dark bar represents lights-out, the white bar indicates sleep deprivation. When recovery from 22h SD begins in the evening at ZT10 sleep homeostasis is delayed until the next morning. B, Although sleep rebound is a condition with high sleep-drive, flies show normal learning compared to untreated circadian-matched controls. C, Learning is restored in flies that had been sleep deprived for 12 h and allowed to sleep spontaneously for 2 h between ZT0 and ZT2 (white) compared to sleep deprived siblings (black). D, The duration of rebound sleep during the ‘nap’ was 98±2 min vs. 27±6 min in untreated flies and the average sleep bout duration was significantly longer than spontaneous sleep observed in untreated flies at this circadian time. E, When the dark vial contained dry filter paper with 10% sucrose 12 h SD does not disrupt learning (white). However, sucrose in the dark vial could not prevent learning impairments following 36 h SD (grey). Learning was assessed between ZT0-3 for both 12 h and 36 h SD groups (*: p<0.05).
Is a full night of sleep required to restore learning? As seen in Figure 2C, performance after sleep deprivation was restored to baseline level when flies were allowed to ‘nap’ for two hours. In contrast to spontaneous daytime sleep which is characterized by short sleep bouts, the naps following sleep deprivation resemble nighttime sleep (Figure 2D). Thus, as in humans [13], ‘naps’ improve learning in flies. Environmental and social factors can alter motivation and temporarily reduce the negative impact of sleep deprivation on performance[14]. For example, sleep deprived subjects who were given a monetary reward for correct responses were able to maintain performance longer than controls [14]. To evaluate this relationship in flies, we modified the assay by placing a piece of dry filter paper previously soaked in a sucrose solution in the dark vial. Under baseline conditions the presence of sucrose did not alter performance (Figure 2E). However, after 12 h of sleep deprivation flies tested with dry sucrose in the dark alley performed as well as flies that had obtained a full nights’ sleep. These beneficial effects were lost when sleep deprivation was extended to 36 h indicating that deficits cannot be entirely compensated by motivational factors.
Extended waking impairs learning
It has been hypothesized that in humans neurobehavioral deficits accrue when wake time extends beyond a minimal interval measured in hours [3]. In flies, daytime sleep is characterized by short bouts (Figure 3A, inset-text). Interestingly, learning is highest in the morning and declines as the amount of waking accrues during the biological day (Figure 3B). Control Metrics are similar over the course of the day indicating that the decrements in performance cannot be explained by circadian modulation of sensory thresholds (Supplementary Table 1). However, circadian factors have been shown to influence learning [15]. Thus we combined sleep deprivation with the ‘napping’ protocols described above to vary the duration of waking at a given circadian time. We utilized three experimental conditions and in each instance performance was evaluated at ZT4 (Figure 3C schematic). Performance at ZT4 was dependent upon prior wake duration (Figure 3C) suggesting that learning is impaired as a function of time spent awake. Interestingly, daytime sleep appears to be less restorative than consolidated sleep observed during the ‘nap’ (Figure 3C). Since performance is reduced by the end of the day (Figure 3D), these data suggest that consolidated sleep is required after each waking day to restore optimal learning. Indeed, learning is restored in the evening after 3 h of spontaneous sleep (ZT12–ZT15) but remains impaired in circadian matched siblings that were kept awake until ZT15 (Figure 3D).
Learning requires the Mushroom bodies
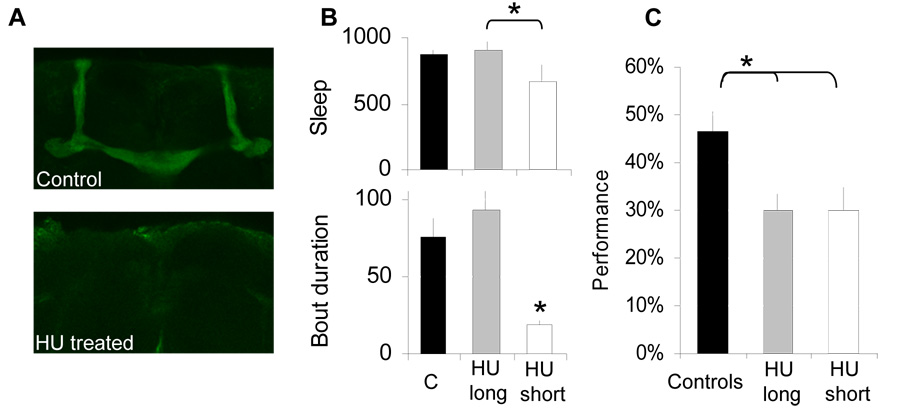
No neural substrate has been identified for Aversive Phototaxic Suppression. A likely candidate is the mushroom bodies (MB) given its role in many, but not all learning/memory tests [16]. MBs play a role in olfactory memory acquisition [17] and play a role in decision making under conflicting situations [18]. The MBs have recently been shown to regulate sleep [19, 20], and inhibitory control [21]. They can be ablated in the fly by feeding larvae hyroxyurea (HU) (Figure 4A, Supplemental Figure 5). Although ablating the MBs disrupts sleep, a minority of HU flies exhibit normal sleep thereby allowing us to determine whether performance is influenced by the MBs independently of sleep time (Figure 4B). As seen in Figure 4C, learning is impaired in the absence of MBs in all short and long sleeping flies; Control Metrics were unaffected (supplementary Table 1). HU also results in a reduction of antennal lobe size [22] raising the possibility that the learning impairment may be due to deficits in olfactory processing. However, smell-blind (sbl-1) flies that are olfactory defective [23] perform as well as Cs flies, indicating that olfactory input is not required in this assay (supplemental Figure 6).
Figure 4. Learning requires the mushroom bodies.
A, HU treatment results in ablation of the MBs: whole mount immuno-stainings of representative vehicle-control and HU treated brains with anti-fasciclin-2. B, Sleep time and sleep bout duration are reduced in MB ablated flies (HU short), but a minority of individual have normal sleep (HU long) compared to their vehicle-control (C). C, Performance is impaired in MB ablated flies regardless of sleep time. (*: p<0.05).
Extended waking alters DA signaling
To determine whether sleep deprivation induced impairments in learning can be explained through alterations in DA signaling, we evaluated DA levels. As seen in Figure 5A,B whole-head DA levels are significantly elevated following sleep deprivation and are associated with the transcriptional down regulation of the Drosophila dopamine 1-like receptor (dDA1) [24]. Down regulation of dDA1 transcripts is also seen in flies with spontaneously fragmented sleep (Figure 5B). The pharmacology of DA agonists has been characterized and these drugs are known to be biologically active in flies [25–27]. As seen in Figure 5C, Ritalin®, methamphetamine, L-DOPA and the D1 agonist SKF82958 rescued performance after sleep deprivation; none of these treatments enhanced learning in baseline conditions (Supplemental Figure 7). Control Metrics were unaffected by pharmacologic manipulations (Supplementary Table 1). Thus globally enhancing dopamine signaling overcomes deficits in learning in flies as it does in humans.
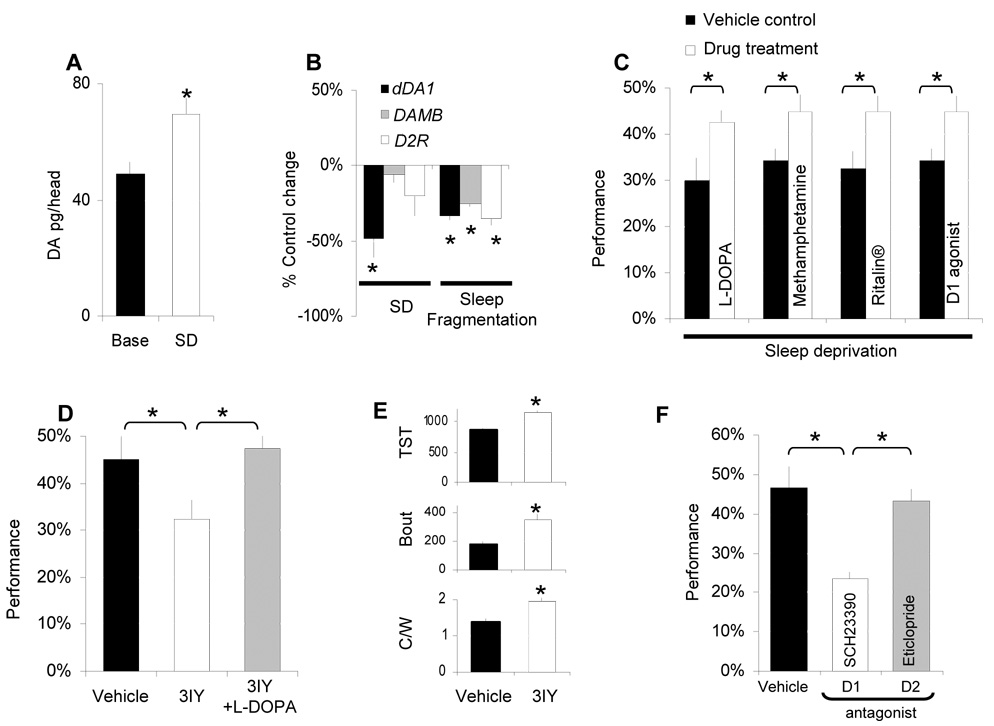
Figure 5. DA, extended waking and learning.
A, Whole head DA levels, measured by HPLC, are increased following 12 h SD compared to untreated circadian matched controls. B, dDA1 transcripts are down regulated by 12 h SD whereas mRNA levels for the other D1 like receptor DAMB and for the D2 receptor (D2R) remain stable. All three receptors are transcriptionally down regulated in the flies with spontaneous sleep fragmentation described in Figure 1F (data are presented as % change from controls, one-sample t-test). C, Performance impairments after 12hSD are reversed when flies are fed methamphetamine (1mg/mL), L-DOPA (5mg/mL), Ritalin (2.5mg/mL) or the D1 agonist SKF-82958 (3mg/mL). Learning was evaluated between ZT0-ZT3:59; D, Blocking DA synthesis by feeding flies 3IY (10mg/mL) for 36 h results in performance impairments. Co-administering flies 3IY and L-DOPA (10mg/mL) rescues learning. E, Total sleep time (TST in minutes), average sleep bout duration (Bout in minutes) and locomoter activity during waking (C/W) are significantly increased in flies on 3IY. F, Feeding flies the D1 receptor antagonist SCH-23390 (1mg/mL) but not the D2 antagonist eticlopride (1mg/mL) for 2 h (ZT0-ZT2) before the test impairs performance. (*:p<0.05).
To determine the extent to which DA is involved in this learning assay we conducted additional genetic and pharmacological experiments. DA levels were reduced by feeding flies the tyrosine hydroxylase (TH) inhibitor 3-iodo L-tyrosine (3IY) [28]. Performance was impaired in flies fed 3IY and this impairment could be rescued by co-administering L-DOPA (Figure 5D). Consistent with previous reports 3IY consolidated sleep without reducing the intensity of locomotor activity (Figure 5E) [25]. PI and QSI were unaffected by drug treatment while flies fed 3IY took significantly longer to complete 16 trials (Supplementary Table 1). Although TCT was increased in flies fed 3IY it was not outside the range seen in Cs flies and thus cannot explain the deficit (Supplementary Figure 8). In addition, disrupting synaptic output from dopaminergic neurons by expressing a temperature sensitive allele of shibire (UAS-shits1) also impairs learning (Supplementary Figure 9)[29]. Thus reducing DA signaling, using either pharmacology or genetics, impairs performance in APS.
Learning requires the dDA1 receptor
Although a recent study has shown that the dDA1 receptor is important for Pavlovian conditioning [30], its role in other learning paradigms is unknown. Since the pharmacology of the four Drosophila DA receptors has been investigated (e.g. [27]) we began by administering either a D1 (SCH23390) or D2 (eticlopride) antagonist for 2 h before evaluating learning. SCH23390 and eticlopride have been shown to activate separate behaviors in flies [26] and while SCH23390 blocks both D1-like receptors (dDA1 and dopamine receptor in mushroom bodies (DAMB)) [27, 31], eticlopride does not [27]. Both D1 and D2 antagonists modified sleep at this dose indicating they are biologically active (supplementary Figure 10A,B). However, only the D1 antagonist disrupted performance (Figure 5F). Importantly, the induction of Gαs in flies fed the D1 agonist SKF 82958 was blocked by co-administering the D1 antagonist (supplementary Figure 10 C).
Because the D1 agonist and D1 antagonist are active at both the dDA1 and the DAMB receptors we evaluated learning in flies mutant for dDA1. The dDA1 receptor is heavily expressed in MB neuropile and is required for olfactory learning (Figure 6A)[30]. dumb2 is a hypomorphic allele which reduces dDA1 expression in the mushroom bodies (Figure 6B, and [30]). The P-element insertion PL00420 (dumb3) removes most of dDA1 expression in the MBs while inducing ectopic expression in glia and the optic lobes (Figure 6C). We find that both alleles have reduced learning (Figure 6 D–E). dumb2 and dumb3 mutants exhibited normal sleep, PI and QSI but dumb3 flies had 12% longer TCT, (Supplementary Table 1 and supplementary Figure 11). To confirm that this phenotype maps to the dDA1 locus we crossed dumb2 and dumb3 with flies carrying a deficiency (Df) of the dDA1 locus, Df(3R)red1. Learning was significantly reduced in the resulting dumb2/Df and dumb3/Df flies indicating that the impairments were due to disruption of dDA1 expression (Figure 6D–E). Finally, we administered the D1 agonist SKF 82958 to dumb2 and dumb3 flies and assessed learning. As seen in Figure 6 D, E, performance could not be rescued by the D1 agonist. Since the D1 agonist did not restore learning in either the dumb2 or dumb3 mutants it is unlikely that the previous improvement in learning after sleep deprivation was due to nonspecific effects of SKF82958 at other receptors.
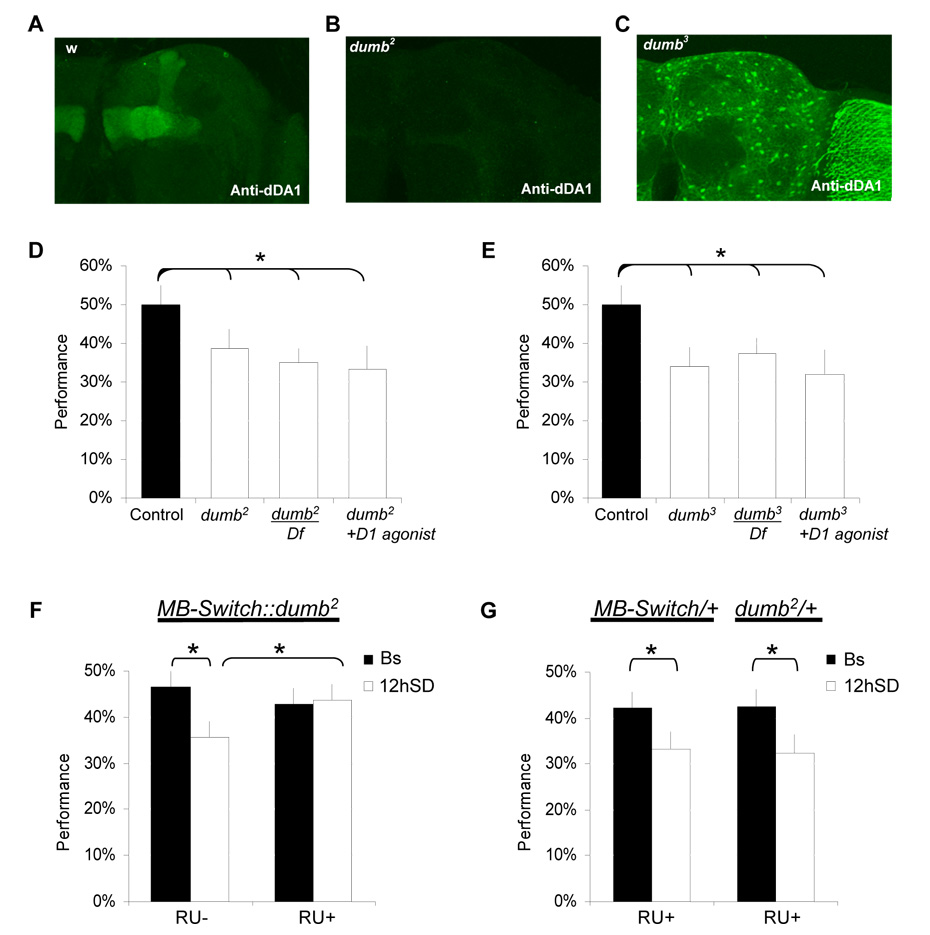
Figure 6. The D1-like receptor dDA1 is required for learning after sleep deprivation.
A–C, dDA1 immunolocalization in controls (w1118), dumb2 and dumb3 mutant brains. In both dumb2 and dumb3 dDA1 expression in the mushroom bodies is strongly reduced. dDA1 is ectopically expressed in glia and in the optic lobes in dumb3 mutant brains. D, The D1 like receptor hypomorph mutant dumb2 shows performance decrements compared to wild type controls (C). Impairments are still observed in dumb2/Df(3R)red1 (dumb2/Df) and in dumb2 flies fed with a D1 agonist SKF-82958. E, The misexpression mutant dumb3 also shows performance decrements as homozygotes or over Df(3R)red1 (dumb3/Df) compared to wild type controls. Feeding dumb3 a D1 agonist does not improve performance. F, Learning was evaluated in MB-Switch::dumb2 flies fed 100µg/mL RU486 (RU+) or vehicle (RU-) for 2 days. Both RU+ and RU-groups show normal performance under baseline conditions. However, MB-Switch::dumb2 RU+ flies show normal learning after SD in contrast to vehicle-control siblings (RU-). G, RU486 does not prevent learning impairments following sleep deprivation in MB-Switch/+ and dumb2/+ parental lines. Learning was evaluated in all flies between ZT0-ZT3:59. (*:p<0.05).
Local changes in dDA1 receptor protect learning during extended waking
Imaging studies in humans suggest that performance decrements following waking may not be due to global brain impairments [4] and thus may reflect a molecular vulnerability in specific neuronal circuits. To determine whether waking impairs learning by modifying dDA1 globally or in specific circuits we manipulated dDA1 only in the MBs. The piggyBac inserted into the first intron of the dDA1 gene in the dumb2 mutants contains a UAS that can be used to induce functional dDA1 receptor [30]. We used the gene-switch system (MBSwitch) to avoid potential developmental defects [32]. As seen in Figure 6F, MB-Switch/+; dumb2/+ flies fed RU486 maintained learning after extended wakefulness while their vehicle fed siblings were impaired. Interestingly RU486 treated MB-Switch/+; dumb2/+ had no effect on baseline learning in the absence of sleep loss and baseline sleep was not altered (Supplemental Figure 12). As expected, the parental lines (dumb2/+ and MB-Switch/+) learn normally when exposed to RU486 and are impaired following extended waking (Figure 6G). Furthermore RU486 has no effect on learning in Cs flies, either under baseline condition or during extended waking (supplemental Figure 13).
Discussion
These data provide direct evidence that extended waking disrupts learning and is amenable to genetic dissection in Drosophila. Importantly, manipulating dDA1 only in the MBs, which represent ≈2 % of the total number of neurons in the Drosophila central nervous system, was sufficient to prevent the learning deficits associated with extended waking. These data support the hypothesis that extended waking can deteriorate the function of specific brain areas that are critical for adaptive behavior.
Sleep deprivation experiments are inherently problematic in that it is frequently difficult to determine whether an observed outcome is due to the lack of sleep or the methods used to keep the organism awake [33]. Thus, we conducted several control experiments to evaluate potential confounding variables before we turned our attention to elucidating underlying mechanisms. We found that while learning is disrupted when extended waking is achieved by mechanical stimulation, mechanical stimulation in the absence of sleep loss produced no deficits in learning. Importantly, spontaneous waking and sleep fragmentation impair learning without mechanical stimulation. Together these data indicate that it is the extended waking per se that disrupts learning.
In Drosophila, dopaminergic neurons project arborizations to the MB neuropile [34] where they influence aversive learning [29]. Although a recent study has shown that the dDA1 receptor is important for olfactory conditioning [30], its role in other learning paradigms is unknown. Our results extend the role of dDA1 receptor beyond olfactory learning. It is worth noting that a role for D1 receptor in short term memory and response inhibition has been reported in humans [35], nonhuman primates [36] and rodents [37]. Previous studies have shown that DA in the MBs plays a role in decision making under conflicting situations [18] and may signal the aversive stimulus to the MBs in olfactory conditioning [34, 38]. Interestingly, flies in the APS also face a conflicting choice between their pre-potent attraction towards light and the aversive stimulus. Thus the modulation of DA signaling observed during extended waking may disrupt performance by multiple mechanisms. Interestingly, children with Attention Deficit Hyperactivity Disorder (ADHD) exhibit both disorganized DA signaling and difficulty with response inhibition [39]. Moreover, sleep problems are highly prevalent in ADHD and when present, are associated with poorer child outcomes [40].
Conclusion
In conclusion, sleep deprivation impairs short-term memory and response inhibition in the genetic model organism Drosophila melanogaster. Our data demonstrate that waking is particularly deleterious for DA-circuits that are crucial for maintaining adaptive behavior. Because optimal performance can only occur within a narrow range of DA signaling and DA signaling is easily disrupted by waking, we propose that an important role of sleep may be to restore DA homeostasis. Nonetheless, it is likely that sleep loss impacts the brain by altering a number of molecular pathways. Together these experiments pave the way for the identification of the underlying molecular mechanisms.
Methods
Fly stocks, sleep and sleep deprivation
We obtained dumb2 (f02676) and dumb3(PL00420) from the Exelexis Drosophila collection (Harvard Medical School), UAS-shits1 from M. Heisenberg (University of Wurzburg, Germany), Hi5 and Lo geotaxic lines from D. Toma (Neuroscience Institute, San Diego), Sbl1 from Joel Levine (University of Toronto), TH-GAL4 from S Birman (Université de la Méditerranée). Flies were cultured at 25°C, 50% humidity, in 12hr:12hr Light:Dark cycle, on food containing yeast, dark corn syrup, molasses, dextrose and agar. Three day old flies were placed into 65mm glass tubes and monitored using the Trikinetics activity monitoring system as previously described [8] (www.Trikinetics.com). Flies were sleep deprived using the SNAP [8]. Unless otherwise stated flies were sleep deprived using the SNAP from ZT 12 (Lights-out) to ZT 0 (Lights-on) and until each fly was tested for learning.
Sleep Interrupting DEvice (SLIDE)
As with the SNAP, flies were housed in 65mm tubes and their activity was continuously monitored in a Trikinetics monitor. Plastic floors were inserted into the tubes underneath the flies. The floor is connected to a motor that controls the distance the floor travels in addition to its speed and acceleration (see Supplementary Figure 4 for details).
Learning
The learning paradigm requires flies to inhibit a potent attraction towards light and has been previously described [7]. Both dark and lighted vials are covered with filter paper. The filter paper in the lighted vial is wetted with 320 µl of a 10-1M quinine hydrochloride solution (Sigma). After entering the dark or lighted vial, the choice is recorded and the fly is quickly removed from the vial and placed back at the entrance of the maze. During the test the light and quinine/humidity appear equally on both the right and left. For an experiment, learning was evaluated by the same experimenter who was blind to genotype and condition. Unless otherwise stated, all flies were tested in the morning between ZT0 and ZT4. Learning scores are normally distributed (Supplemental Figure 2). Thus, Statistical analyses were performed using Systat (Systat, Chicago, IL). Differences were assessed using either a Student’s t-test or Analyses of variance (ANOVA) which were followed by planned pair-wise comparisons with a Tukey correction. Unless stated otherwise, all experiments are n ≥ 10.
Geotaxis
Groups of 10 flies were placed at the bottom of a 12×75 mm vial. After gentle tapping down, the number of flies crossing a 4cm mark after 20 s was scored. 30 flies were evaluated per condition (baseline and sleep deprived).
Photosensitivity
Photosensitivity was evaluated in the T-maze over 10 trials in the absence of filter paper. The lightened and darkened chambers appeared equally on both the left and right. PI is the average of the scores obtained for 5 flies ±SEM. Photosensitivity in the T-maze was also evaluated at low (240 lux), medium (5900 lux), and max (9500 Lux) light intensities. Fast phototaxis was evaluated in groups of 10 flies placed at the bottom of a 12×75 mm vial. The vial was then positioned horizontally toward a bright (9000lux) fiber-light lamp. Flies crossing a 4 cm mark after 10 s were scored. 30 flies were evaluated per condition.
Quinine/humidity sensitivity
Flies were placed at the bottom of a 14 cm cylindrical tube which was uniformly lighted (n=5). Each half of the apparatus contained separate pieces of filter paper which could be wetted with quinine or kept dry. The QSI was determined by calculating the time that the fly spent on the dry side of the tube when the other side had been wetted with quinine, during a 5 min period.
Courtship
Five-day old naïve males were exposed to virgin females for 10 minutes. The Courtship Index (CI) was calculated by dividing the time spent courting (the sum of the lengths of all of the courtship bouts) by the total length of the test.
Mushroom body ablation
0–1 h larvae were fed yeast paste (controls), or yeast paste containing HU for 4 h using standard protocols [20]. The efficiency of the ablation procedure was evaluated using standard whole mount immunohistochemistry (Supplemental Figure 5).
Drugs
L-DOPA (5mg/ml in 1%agar 1%sucrose), Methamphetamine (1mg/ml), Ritalin (2.5mg/ml), SKF-82958 (3mg/ml), were fed to flies for 2 hours before lights off and during sleep deprivation. Flies were fed 3IY (10mg/ml), 3IY + L-DOPA (both 10mg/ml) diluted in 1% agar 5% sucrose for 36h. Flies were fed SCH 23390 (1mg/ml), and eticlopride (1mg/ml) diluted in 1% agar 1%sucrose 2 h before testing (ZT0-ZT2). dumb2 and dumb3 flies were fed SKF-82958 3mg/ml diluted in 1% agar 1% sucrose for 2 h before testing. RU486 (mifepristone, Sigma) was diluted in Ethanol (50mg/ml) and then diluted in food (100µg/ml). Flies were fed RU486 for 48h prior to testing.
HPLC
For each condition two independent replicates of 20 flies were frozen and whole heads collected. Brains were quickly dissected in ice cold PBS and transferred to 500µl of HPLC buffer. HPLC was conducted by Dr. Raymond F Johnson, Neurochemistry Core Lab, Nashville.
Immunohistochemistry
Brains were dissected in cold Phosphate Buffered Saline and processed for standard whole mount immunostaining. The following antibodies were used: mAb1D4 anti-fas2 (Hybridoma Bank, University of Iowa) at 1:100, and mouse anti-dDA1 ( a Gift from K-A Han) at 1:200, Alexa 488 conjugated anti mouse IgG (Molecular probes). Confocal stacks were processed using Metamorph software.
QPCR
QPCR was conducted as previously described using total RNA extracted from 20 heads; all groups were collected at the same circadian time (ZT0-1) [41]. cDNA from comparable reverse transcription reactions were used as a starting material to run four QPCR replicates. Expression values for RP49 were used to normalize results and two independent groups of flies were collected and processed independently for each analysis.
Supplementary Material
Acknowledgements
We thank Mathew Thimgan, Jeff Donlea Isabelle Raymond Shaw Troy Zars and David Van Essen for helpful comments, Gabbi Merlin for lab assistance and Eric Lebourg for assistance Extended waking disrupts short term memory 28 with the learning assay. This study was funded in part by 1 R01 NS051305-01A1, the McDonnell Center for Cellular and Molecular Neurobiology, and the NIH Neuroscience Blueprint Core Grant (#NS057105).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rogers NL, Dorrian J, Dinges DF. Sleep, waking and neurobehavioural performance. Front Biosci. 2003;8:s1056–s1067. doi: 10.2741/1174. [DOI] [PubMed] [Google Scholar]
- 2.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 3.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 4.Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–657. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 5.Balling A, Technau GM, Heisenberg M. Are the structural changes in adult Drosophila mushroom bodies memory traces? Studies on biochemical learning mutants. J Neurogenet. 1987;4:65–73. [PubMed] [Google Scholar]
- 6.McGuire SE, Deshazer M, Davis RL. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog Neurobiol. 2005;76:328–347. doi: 10.1016/j.pneurobio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Le Bourg E, Buecher C. Learned suppression of photopositive tendencies in Drosophila melanogaster. Anim Learn Behav. 2002;30:330–341. doi: 10.3758/bf03195958. [DOI] [PubMed] [Google Scholar]
- 8.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 9.Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 10.Toma DP, White KP, Hirsch J, Greenspan RJ. Identification of genes involved in Drosophila melanogaster geotaxis, a complex behavioral trait. Nat Genet. 2002;31:349–353. doi: 10.1038/ng893. [DOI] [PubMed] [Google Scholar]
- 11.Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, Brown RE, Strecker RE. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnet MH. Cognitive effects of sleep and sleep fragmentation. Sleep. 1993;16:S65–S67. doi: 10.1093/sleep/16.suppl_8.s65. [DOI] [PubMed] [Google Scholar]
- 13.Lahl O, Wispel C, Willigens B, Pietrowsky R. An ultra short episode of sleep is sufficient to promote declarative memory performance. J Sleep Res. 2008;17:3–10. doi: 10.1111/j.1365-2869.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- 14.Horne JA, Pettitt AN. High incentive effects on vigilance performance during 72 hours of total sleep deprivation. Acta Psychol (Amst) 1985;58:123–139. doi: 10.1016/0001-6918(85)90003-4. [DOI] [PubMed] [Google Scholar]
- 15.Decker S, McConnaughey S, Page TL. Circadian regulation of insect olfactory learning. Proc Natl Acad Sci U S A. 2007;104:15905–15910. doi: 10.1073/pnas.0702082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf R, Wittig T, Liu L, Wustmann G, Eyding D, Heisenberg M. Drosophila mushroom bodies are dispensable for visual, tactile, and motor learning. Learn Mem. 1998;5:166–178. [PMC free article] [PubMed] [Google Scholar]
- 17.Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang S, Guo A. Choice behavior of Drosophila facing contradictory visual cues. Science. 2001;294:1543–1547. doi: 10.1126/science.1058237. [DOI] [PubMed] [Google Scholar]
- 19.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 20.Pitman JL, McGill JJ, Keegan KP, Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441:753–756. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- 21.Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 22.Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Levine JD, Funes P, Dowse HB, Hall JC. Resetting the circadian clock by social experience in Drosophila melanogaster. Science. 2002;298:2010–2012. doi: 10.1126/science.1076008. [DOI] [PubMed] [Google Scholar]
- 24.Cirelli C, LaVaute TM, Tononi G. Sleep and wakefulness modulate gene expression in Drosophila. J Neurochem. 2005;94:1411–1419. doi: 10.1111/j.1471-4159.2005.03291.x. [DOI] [PubMed] [Google Scholar]
- 25.Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15:1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 26.Yellman C, Tao H, He B, Hirsh J. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc Natl Acad Sci U S A. 1997;94:4131–4136. doi: 10.1073/pnas.94.8.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng G, Hannan F, Reale V, Hon YY, Kousky CT, Evans PD, Hall LM. Cloning and functional characterization of a novel dopamine receptor from Drosophila melanogaster. J Neurosci. 1996;16:3925–3933. doi: 10.1523/JNEUROSCI.16-12-03925.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neckameyer WS. Dopamine and mushroom bodies in Drosophila: experience-dependent and -independent aspects of sexual behavior. Learn Mem. 1998;5:157–165. [PMC free article] [PubMed] [Google Scholar]
- 29.Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci. 2007;27:7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gotzes F, Balfanz S, Baumann A. Primary structure and functional characterization of a Drosophila dopamine receptor with high homology to human D1/5 receptors. Receptors Channels. 1994;2:131–141. [PubMed] [Google Scholar]
- 32.Mao Z, Roman G, Zong L, Davis RL. Pharmacogenetic rescue in time and space of the rutabaga memory impairment by using Gene-Switch. Proc Natl Acad Sci U S A. 2004;101:198–203. doi: 10.1073/pnas.0306128101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: an update of the 1989 paper. Sleep. 2002;25:18–24. doi: 10.1093/sleep/25.1.18. [DOI] [PubMed] [Google Scholar]
- 34.Riemensperger T, Voller T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 35.Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139:263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 36.Sawaguchi T. The effects of dopamine and its antagonists on directional delayperiod activity of prefrontal neurons in monkeys during an oculomotor delayed-response task. Neurosci Res. 2001;41:115–128. doi: 10.1016/s0168-0102(01)00270-x. [DOI] [PubMed] [Google Scholar]
- 37.Rinaldi A, Mandillo S, Oliverio A, Mele A. D1 and D2 Receptor Antagonist Injections in the Prefrontal Cortex Selectively Impair Spatial Learning in Mice. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301176. [DOI] [PubMed] [Google Scholar]
- 38.Unoki S, Matsumoto Y, Mizunami M. Roles of octopaminergic and dopaminergic neurons in mediating reward and punishment signals in insect visual learning. Eur J Neurosci. 2006;24:2031–2038. doi: 10.1111/j.1460-9568.2006.05099.x. [DOI] [PubMed] [Google Scholar]
- 39.Holroyd CB, Baker TE, Kerns KA, Muller U. Electrophysiological evidence of atypical motivation and reward processing in children with attention-deficit hyperactivity disorder. Neuropsychologia. 2008 doi: 10.1016/j.neuropsychologia.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Sung V, Hiscock H, Sciberras E, Efron D. Sleep problems in children with attention-deficit/hyperactivity disorder: prevalence and the effect on the child and family. Arch Pediatr Adolesc Med. 2008;162:336–342. doi: 10.1001/archpedi.162.4.336. [DOI] [PubMed] [Google Scholar]
- 41.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.