Abstract
Background
Allergic airway diseases are more common in females than in males during early adulthood. A relationship between female hormones and asthma prevalence and severity has been suggested, but the cellular and molecular mechanisms are not understood.
Objective
To elucidate the mechanism(s) by which estrogens enhance the synthesis and release of mediators of acute hypersensitivity.
Methods
Two mast cell/basophil cell lines (RBL-2H3 and HMC-1) and primary cultures of bone marrow derived mast cells, all of which naturally express estrogen receptor-α, were examined. Cells were incubated with physiological concentrations of 17-β-estradiol with and without IgE and allergens. Intracellular Ca2+ concentrations and the release of β-hexosaminidase and leukotriene C4 were quantified.
Results
Estradiol alone induced partial release of the preformed, granular protein β-hexosaminidase from RBL-2H3, BMMC and HMC-1, but not from BMMC derived from estrogen receptor-α knock-out mice. The newly synthesized LTC4 was also released from RBL-2H3. Estradiol also enhanced IgE-induced degranulation and potentiated LTC4 production. Intracellular Ca2+ concentration increased prior to and in parallel with mediator release. Estrogen receptor antagonists or Ca2+ chelation inhibited these estrogenic effects.
Conclusion
Binding of physiological concentrations of estradiol to a membrane estrogen receptor-α initiates a rapid onset and progressive influx of extracellular Ca2+, which supports the synthesis and release of allergic mediators. Estradiol also enhances IgE-dependent mast cell activation, resulting in a shift of the allergen dose response.
Keywords: Estrogen, Estrogen receptor-α, Human, Rodent, Mast cells/basophils, Allergy
1. Introduction
Asthma and other allergic diseases of the airways are up to three times more common in women than in men during early to middle adulthood (De Marco et al., 2002; Mannino et al., 2002; Schatz and Camargo, 2003). A number of clinical and epidemiological studies suggest that female hormones contribute to these differences. A recent study found that women taking hormone replacement therapy had a higher risk of new onset asthma (Barr et al., 2004). Further, 30–40% of women who have asthma, experience worsening of their symptoms during the perimenstrual phase (perimenstrual asthma, PMA), when estrogen and progesterone concentrations are changing rapidly (Vrieze et al., 2003). Also, a recent study shows estrogen receptor (ER) polymorphisms are associated with airway hyperresponsiveness and lung function decline, particularly in females with asthma (Dijkstra et al., 2006).
However, very little is known about the mechanism(s) by which physiological concentrations of female hormones might promote the development of or increase the morbidity of asthma in women during their peak reproductive years (De Marco et al., 2002; Mannino et al., 2002; Schatz and Camargo, 2003; Skobeloff et al., 1992).
Serum concentrations of mast cell-derived leukotriene (LT) C4 are significantly higher during the perimenstrual phase inwomen with PMA, compared with those during the midcycle week. Further, LT receptor antagonists have been reported to reduce symptom scores and improve pulmonary function in PMA (Nakasato et al., 1999). These findings suggest a relationship between female hormones, mast cell-derived mediators of acute hypersensitivity, and asthma severity. However, the cellular and molecular mechanisms by which female hormones might alter mast cell and basophil function have not been elucidated.
Mast cells, in various tissues, express ERs (Harnish et al., 2004; Jiang et al., 2002; Nicovani and Rudolph, 2002; Zhao et al., 2001). Super-physiological concentrations of estradiol (E2, 1–10 μM) has been shown to induce (Spanos et al., 1996; Vliagoftis et al., 1992) or inhibit (Harnish et al., 2004) mast cell degranulation. Further, preincubating basophils or mast cells with physiological concentrations of E2 has been shown to increase the subsequent histamine release induced by cross-linking surface-bound IgE with antibodies (Cocchiara et al., 1990; Cocchiara et al., 1992). The effects of hysiological concentrations of E2 alone and the cellular and molecular mechanism(s) underlying estrogenic effects on mediator release from mast cells require further investigations.
In this study, we provide the first evidence that mast cells exposed to physiological concentrations of E2 undergo partial degranulation. While E2 alone had only a limited effect on LT synthesis, simultaneous exposure to E2 and allergen cross-linking of surface IgE had a synergistic effect on the synthesis and release of LTC4. The mechanisms underlying these independent and synergistic effects of E2 were elucidated by demonstrating the requirement for extracellular Ca2+ and non-genomic activity of ER-α.
2. Materials and methods
2.1. Cells and cell culture
RBL-2H3, a rat basophilic leukemia cell line (Morita and Siraganian, 1981), was obtained from the American type culture collection (Manassas, VA) and HMC-1, human mast cell line, from Dr. J.H. Butterfield (Mayo Clinic, Rochester, MN) (Butterfield et al., 1988). RBL-2H3 were grown in DMEM supplemented with 10% FCS (Hyclone, South Logan, UT) and HMC-1 in IMDM (Cellgro, Kansas City, MO) with 10% iron-supplemented calf serum (Hyclone, South Logan, UT). To avoid exposure to estrogens in our culture system, we used dextran/charcoal-treated FCS and phenol-red free media for the last 48 h of culture and throughout the experiments, as described (Lambert et al., 2005).
The primary cultures of bone marrow-derived mast cells (BMMC) were developed from the marrow of the femurs of C57B6 mice, as described (Odom et al., 2004). Wild type (WT) C57B6 mice were purchased from the Jackson Laboratory (Houston, TX) and ER-α (knockout) KO mice were obtained by breeding previously generated heterozygous ER-α KO mice (Lambert et al., 2005). After 4 weeks, the cultures contained 98% pure mast cells, as assessed by toluidine blue staining. BMMCs were used to confirm that the effects of E2 occurred through ER-α, by comparing the cells from WT and ER KO mice. All animal experimental protocols were approved by the University of Texas Medical Branch Institutional Animal Care and Use Committee.
2.2. Detection of mRNA for ERs in cultured cells
Mast cells and basophils have been reported to express ERs (Zhao et al., 2001). To confirm and type the ERs expressed by RBL-2H3, BMMC and HMC-1 cells, we used RT-PCR. RNA from these cells were purified using TRIzol Reagent (Invitrogen, Carlsbad, CA,) and cDNAs were synthesized with Superscript RT (Gibco BRL), using oligo dT (Pharmacia, Piscataway, NJ). PCR for RBL-2H3 and BMMC were performed with primers: 5′-GGGCTTTCCCCCAGCTCAAC-3′ and 5′-GCACACGGCACAGTAGCGAG-3′ for ER-α, and 5′-AACTACAGTGTTCCCAGCAGC-3′ and 5′-AGGACCAGACACCGTAATGA-3′ for ER-β, and 5′-GGAGAAACCTGCCAAGTATG-3′ and 5′-AGAGTGGGAGTTGCTGTTGA-3′ for the housekeeping gene, G3PDH, as described (Lambert et al., 2004). PCR for ER expression in HMC-1 was performed with primers: 5′-TGCCCTACTACCTGGAGAACG-3′ and 5′-GTCCTTCTCTTCCAGAGAC-3′ for ER-α and 5′-TCACATCTGTATGCGGAACCT-3′ and 5′-TAGCGATCTTGCTTCACACCA-3′, 5′-TCACATCTGTATGCGGAACCT-3′ and 5′-TAGCGATCTTGCTTCACACCA-3′, and 5′,5′-CGCTAGAACACACCTTACCTG-3′ and CTGTGACCAGAGGGTACAT-3′ for ER-β.
2.3. Mast cell activation experiments
The RBL-2H3 cells harvested by trypsinization, and HMC and BMMC cells by centrifugation were distributed into 96- or 24-well flat bottom plates, cultured for 2 days in estrogen-free medium to allow damaged membrane receptors to be resynthesized, and stimulated with various concentrations of E2 (Sigma–Aldrich) for various times. To examine the effects of cross-linking cell surface IgE on the release of mediators, the RBL-2H3 and BMMC were sensitized for 1 h with 100 ng/ml mouse monoclonal anti-DNP IgE antibody (Sigma–Aldrich) and HMC-1 cells with serum from a patient with house dust mite sensitivity. After washing away unbound IgE, the cells were stimulated with DNP–BSA (10 haptenes/1 carrier molecule) complexes (10 ng/ml, Biosearch Technologies Inc., Novato, CA) or dust mite allergen extract (DM, Dermatophagoides farinae, Hollister-Stier, Spokane, WA) in the presence or absence of E2. Patients’ sera were obtained according to a protocol approved by the Institutional Review Board of the University of Texas Medical Branch.
2.4. Assessment of degranulation by release of the granular protein β-hexosaminidase (β-hex)
Enzymatic assays for β-hex have been used extensively to assess the extracellular release of mast cell and basophil granule contents (Dastych et al., 1999). Cells (2 × 104) that had been cultured for 2 days in estrogen-free medium were stimulated by changing the media to 200 μl of Tyrode’s buffer (Dastych et al., 1999) containing various stimuli. β-hex release was quantified as previously described, (Dastych et al., 1999) using p-nitrophenyl-N-acetyl-β-D-glucopyranoside (8 nM, Sigma–Aldrich) as the substrate. The β-hex release into media was expressed as the percentage of the total amount of β-hex originally in the cells as determined by detergent lysis with Triton X-100. (% specific release = 100 × [experimental β-hex release − spontaneous β-hex release]/total cellular β-hex).
2.5. Measurement of LTC4 synthesis and release
Cells (1 × 105) were cultured for 2 days prior to stimulation. The LT concentration of each supernatant was quantified using an enzyme immunoassay kit, with anti-LTC4 and LTC4-acetylcholinesterase conjugate, according to the manufacturer’s recommendations (Cayman Chemical, Ann Arbor, MI).
2.6. Assessing the change in intracellular Ca2+ content by confocal microscopy
Imaging of intracellular Ca2+ fluxes was performed as previously described (Suzuki et al., 2003). Briefly, RBL-2H3 cells (5 × 104) were plated in the wells of chamber slides (Nalge Nunc Int, Naperville, IL) and cultured for 2 days. After pretreatment with 3 μM Fluo3-AM (Molecular Probes, Eugene, OR) for 30 min, the cells were washed to remove free Fluo3-AM and then stimulated. Ca2+ mobility in the cells was measured every 30 s by imaging under a Zeiss LSM laser scanning confocal microscope, using a Zeiss-40 lens (Zeiss, Thornwood, NY). We evaluated the fluorescence of 20 cells from the digital images of three separate experiments saved from each time point, using an LSM 5 Image Examiner (Zeiss).
2.7. Use of chemical inhibitors and stimulators
We also examined the effects of ER antagonists tamoxifen (Sigma–Aldrich) and ICI 182,780 (Tocris, Ellisville, MO), Ca2+ ionophore A23187 (Sigma–Aldrich), and EGTA (Sigma–Aldrich), an agent which chelates extracellular Ca2+, on the degranulation and release of newly formed LTC4 and the changes in intracellular Ca2+ concentration.
2.8. Statistical analyses
Data were expressed as the mean ± SEM. Statistical analysis was performed by one-way analysis of variance. Where differences between groups were present, they were identified by Scheffe’s test. A p value of <0.05 was defined as statistically significant. A repeated measures analysis, utilizing restricted maximum likelihood estimation (REML) was used to obtain parameter estimates, using the MIXED procedure in SAS® (Cary, 2000). Each set of measurements from the same batch were considered a correlated cluster of observations. Compound symmetry structures were used when possible for the covariance structure. Between-group comparisons were made using differences of least squares means.
3. Results
3.1. RBL-2H3, HMC-1 and BMMC cells express mRNA for ER-α, but not ER-β
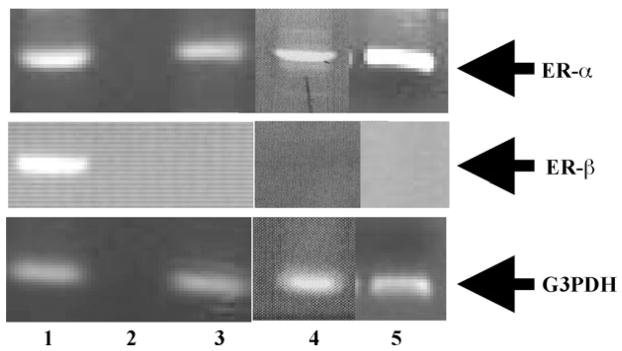
The amplicons from RT-PCR assays of mRNA from RBL-2H3, HMC-1 and BMMC cells were analyzed by gel electrophoresis (Fig. 1). The results indicate that these cells express mRNA for ER-α, but not ER-β. The negative results for ER-β were confirmed, using multiple sets of primers that amplify segments of the known alternate splicing variants of the ER-β transcripts (results not shown).
Fig. 1.
Expression of mRNA for ER-α in RBL-2H3, HMC-1 and BMMC cells; RT-PCR analysis of total RNA isolated from each of the cells. Lane 1: rat ovary = positive control; lane 2: no RNA = negative control; lane 3: RBL-2H3, lane 4: HMC-1 and lane 5: BMMCs.
3.2. Exposure to physiological doses of E2 alone induces the release of substantial amounts of a preformed granular protein β-hex and weakly induces LTC4 synthesis by mast cells
A series of experiments were performed to elucidate the effects of E2 alone and in combination with allergen cross-linking of surface IgE antibodies on mast cells. Synthesis and release of mediators of acute hypersensitivity by RBL-2H3 were assessed. All mediator measurements were performed in duplicate or triplicate and each figure presents the combined data from three independent experiments. A set of repeated measures mixed model fits of the time course data (Figs. 2A and B, 3A and 6A–C) showed significant group, time and (group × time) interaction effects (all p < 0.01), indicating group differences were dependant upon time, and the need to make comparisons between-groups across the time course. The significance of between-group differences, calculated using differences of least square means from the mixed models, are indicated in the figure legends.
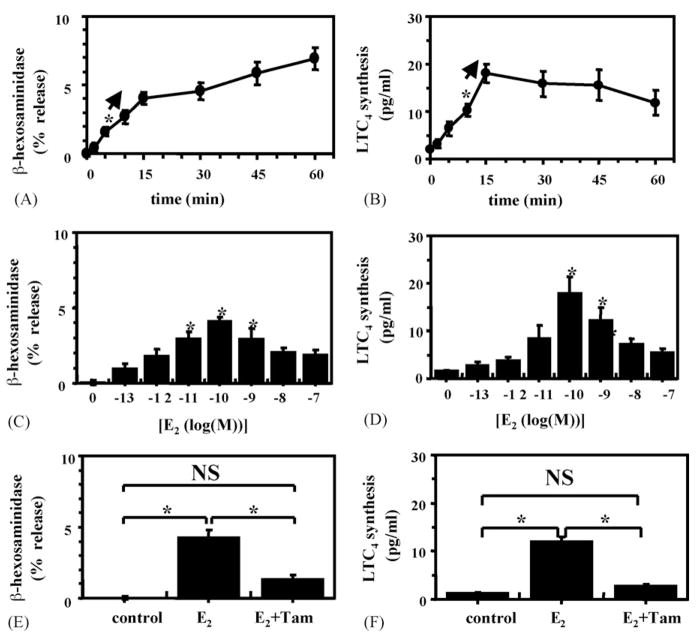
Fig. 2.
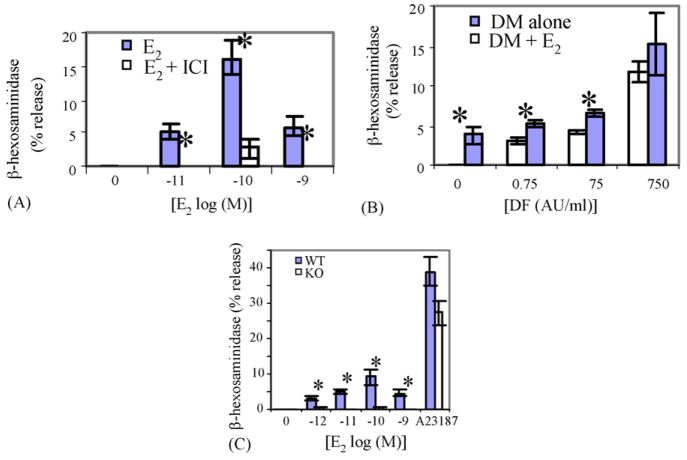
E2 promotes rapid β-hex release and LTC4 synthesis on RBL-2H3 cells: (A, C and E) represent β-hex release and (B, D and F) LTC4 release; (A and B) show time course after the addition of 100 pM E2; (C and D) dose responses 15 min after E2 addition; (E and F) effects of tamoxifen pretreatment. *p < 0.05 vs. control. Tam = tamoxifen, NS = not significant.
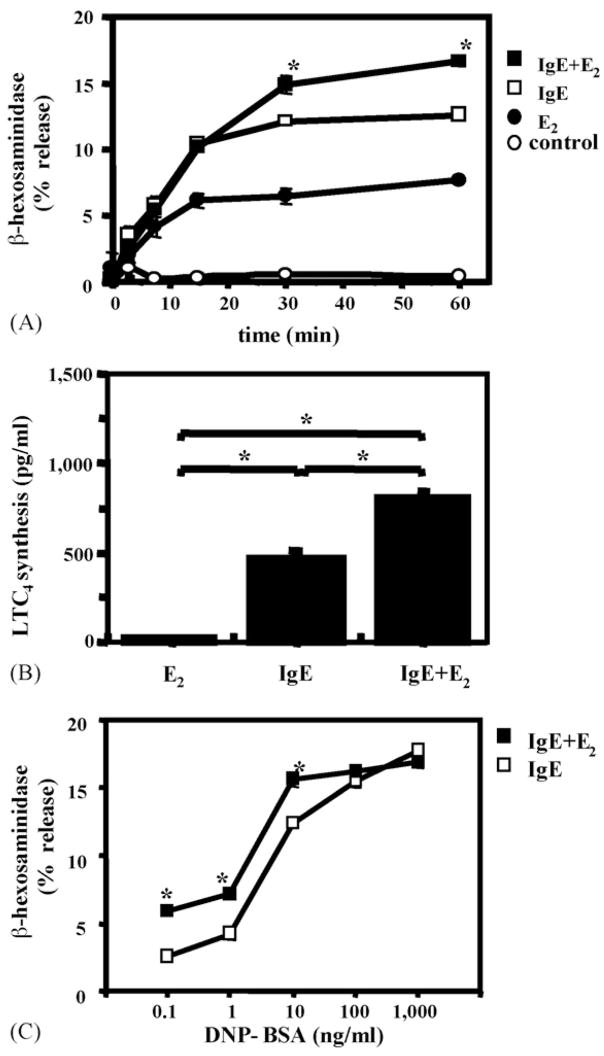
Fig. 3.
E2 enhances IgE-mediated β-hex release and potentiates LTC4 synthesis from RBL-2H3 cells: (A) time course of the effect of 100 pM E2 on β-hex release by IgE + allergen. *p < 0.05 for the effect of E2 at time points indicated; (B) effect of E2 on LTC4 synthesis. *p < 0.05 for the E2 effects. IgE = IgE anti-DNP + DNP–BSA; (C) dose response of E2 effects on β-hex release. *p < 0.05.
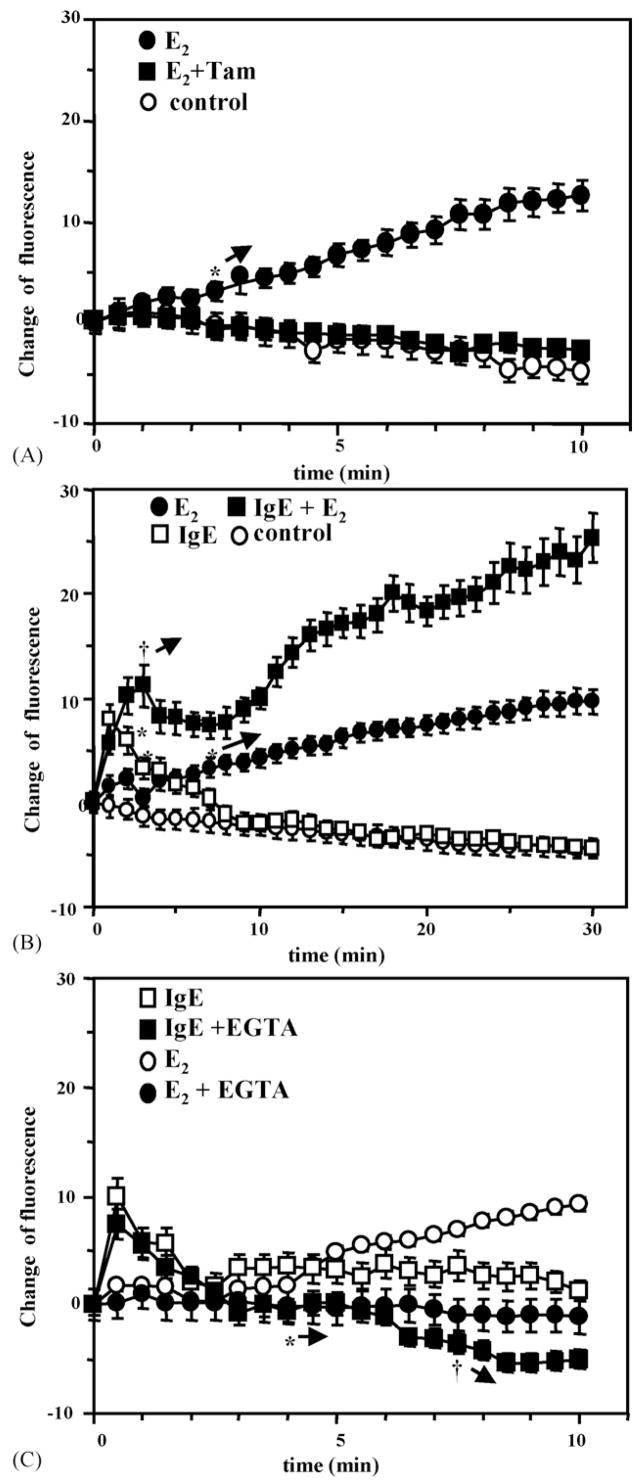
Fig. 6.
E2 increases intracellular Ca2+ and potentiates the effects of IgE cross-linking on mobilization of Ca2+ from RBL-2H3 cells: (A) E2 ± tamoxifen (Tam) on intracellular Ca2+ influx. *→: this and subsequent time points were different from the controls (p < 0.05); (B) E2 on allergen cross-linking of IgE. *p < 0.05 E2 vs. control. †p < 0.05. IgE vs. IgE + E2; (C) EGTA on E2-induced influx of intracellular Ca2+ after IgE cross-linking. *p < 0.05 between E2 vs. E2 + EGTA, †p < 0.05 between IgE and IgE + EGTA.
Incubating estrogen-starved RBL-2H3 cells with as little as 10 pM E2 induced a statistically significant release of β-hex, within 5 min (Fig. 2A). The time course and dose response of new synthesis and release of LTC4 from RBL-2H3 cells is shown in Figs. 2B and D, respectively. As little as 100 pM E2 induced a significant release of LTC4 by 10 min. The E2-induced β-hex release represented approximately 20% of that released by Ca2+ ionophore A23187. While E2 significantly enhanced LTC4 production, compared to control cells, this only represented approximately 1–2% of that induced by Ca2+ ionophore (data not shown). Fig. 2E and F shows that the ER antagonist tamoxifen inhibits E2-driven β-hex release.
3.3. IgE-mediated β-hex release and LTC4 synthesis are enhanced by E2
Cross-linking IgE anti-DNP antibodies on the surface of RBL-2H3 cells with DNP–BSA complexes (nominal allergen) rapidly induced β-hex release (Fig. 3A). Simultaneous addition of 100 pM E2 with the allergen had an additive effect on the degranulation after 30 min of incubation (Fig. 3A). Despite the small effect of E2 alone on LTC4 synthesis, E2 substantially potentiated the allergen-induced LTC4 synthesis (Fig. 3B).
To elucidate the nature of the E2 effects on allergen-induced degranulation, an allergen dose-response curves were generated on RBL-2H3 cells in the presence and absence of an optimal concentration of E2. The results indicated that the addition of E2 caused a statistically significant shift in the dose–response to DNA–BSA (Fig. 3C).
To confirm these effects of E2 in humans and in primary (non-transformed) mast cells, similar experiments were preformed on HMC-1 cells and BMMCs. The results, shown in Fig. 4A, indicate that physiological concentrations of E2 induced significant release of β-hex from the human mast cell line HMC-1. As in the case of RBL-2H3 cells, simultaneous introduction of E2 and allergen to IgE sensitized HMC-1 cells significantly enhanced the β-hex release (Fig. 4B). Fig. 4C shows that E2 alone induces a dose-dependent release of β-hex from WT BMMC cells.
Fig. 4.
E2 promotes release of β-hex from HMC-1 and BMMC: (A) dose response for E2 on β-hex release from HMC-1 and inhibition by ICI182,780. *p < 0.05 vs. no E2; (B) dose response of DM-induced β-hex release from HMC-1, with and without E2. *p < 0.05 with and without E2; (C) dose response of E2 on β-hex release from WT and ER-α KO BMMCs. *p < 0.05 between WT and KO.
3.4. E2 stimulation of β-hex release and LTC4 synthesis is mediated through an ER-α
Addition of 100 nM of the ER antagonist tamoxifen, 15 min prior to the addition of E2 to RBL-2H3 cells inhibited most of the β-hex release (Fig. 2E) and essentially all of the LTC4 synthesis (Fig. 2F). Similarly, ICI (100 nM), another ER antagonist, blocked E2-induced degranulation of HMC-1 cells (Fig. 4A).
The expression of mRNA for ER-α but not ER-β, in mast cells (Fig. 1) suggested that ER-α might be the isoform involved in these estrogenic effects. We therefore produced BMMCs from ER-α KO mice and tested the ability of E2 to release preformed mediators from these cells. The results, shown in Fig. 4C, indicate that ER-α is required for the E2-induced mediator release, while the responses of the KO BMMCs to Ca2+ ionophore and allergen were similar to those of the BMMCs from WT animals (data not shown).
3.5. E2 enhances Ca2+ ionophore-mediated degranulation and LTC4 synthesis
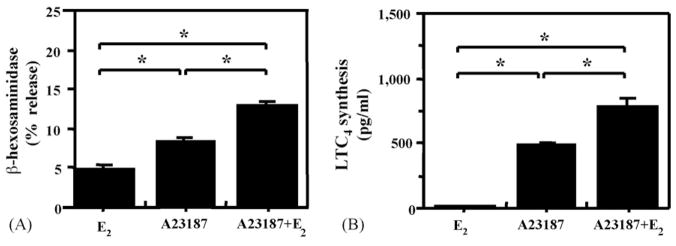
One mechanism that is common to the release of preformed mediators from mast cell granules and the activation of LT synthesis is an increase in intracellular Ca2+ concentration. In addition, estrogens have been shown to rapidly increase intracellular Ca2+ in other cells types via a membrane-associated, specific ER (Bulayeva et al., 2005; Wozniak et al., 2005). Thus, we evaluated the effects of E2 on the activation of RBL-2H3 cells by several different concentrations of the Ca2+ ionophore A23187. E2 significantly enhanced the effect of a sub-optimal concentration (10−7 M) of A23187 on the release of β-hex and LTC4 (Fig. 5). As anticipated, E2 did not enhance the effects of an optimal concentration (10−5 M) of A23187 (data not shown). These results support the concept that intracellular Ca2+ may be an important intermediate in the estrogenic effects on mediator release by mast cells.
Fig. 5.
E2 potentiates the effects of sub-optimal concentrations of A23187 on the release of-β-hex and LTC4 synthesis; RBL-2H3 cells were incubated with 100 pM E2 alone, 10−7 M A23187 alone or the combination of the two followed by measurement of: (A) β-hex release; (B) LTC4 release in the supernatant 15 min later. sp < 0.05 between indicated groups.
3.6. E2 alone increases intracellular Ca2+ in RBL cells and potentiates the effects of IgE cross-linking on intracellular Ca2+ concentrations
To directly evaluate the effects of E2 on intracellular Ca2+ concentration, RBL-2H3 cells were incubated with 100 pM E2 and the fluorescence of the intracellular Ca2+ dye Fluo 3-AM was monitored in individual cells. As shown in Fig. 6A, E2 alone significantly increased the intracellular Ca2+ after as little as 2.5 min incubation. The Ca2+ concentration increased progressively for at least 30 min in the experiments shown (Fig. 6B) and continued for at least 60 min (data not shown). Preincubation of cells with tamoxifen (100 nM) completely prevented the increase in intracellular Ca2+ (Fig. 6A), indicating that this prolonged effect of E2 was mediated by an ER.
The effects of IgE cross-linking by allergen on intracellular Ca2+ are shown in Fig. 6B. In contrast to the effect of E2, IgE cross-linking alone caused a more rapid increase in intracellular Ca2+, which returned to basal levels within 10 min, as previously described by others (Ishizaka et al., 1980; Smith et al., 1996). The combination of 100 pM of E2 and IgE cross-linking increased both the early transient and prolonged increase in intracellular Ca2+ concentration. However, the combination of these stimuli had more than an additive effect on the progressive increase in Ca2+. Fig. 6B shows the first 30 min of this progression. E2 enhanced the early phase of the Ca2+ rise after 3 min and IgE cross-linking enhanced the prolonged rise in Ca2+ seen in cells treated with the E2 alone. The gradual rise in intracellular Ca2+ concentrations continued for at least 60 min (data not shown).
3.7. Extracellular Ca2+ is required for E2 to induce and potentiate degranulation and LTC4 synthesis
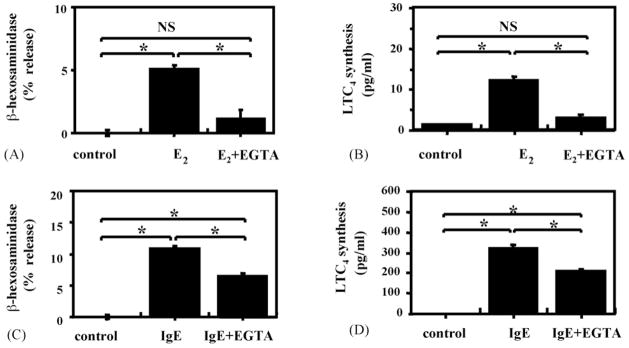
To distinguish between intracellular and extracellular pools as the source of Ca2+ for the E2-induced increase in intracellular free Ca2+, EGTA a chelator of extracellular Ca2+ was added to the extracellular media. The data shown in Fig. 6C indicate that EGTA completely abrogated the E2-induced slow rise in intracellular Ca2+, but not the early, rapid IgE-mediated Ca2+ flux. This finding allowed us to determine whether the slow increase in intracellular free Ca2+ is required for E2 to induce the synthesis and release of the mast cell mediators. The addition of EGTA to the culture media containing E2 reduced the estrogenic stimulation of β-hex release and LTC4 synthesis to the level of unstimulated cells, as shown in Fig. 7A and B. EGTA also significantly decreased IgE-mediated β-hex release and LTC4 synthesis (Fig. 7C and D). However, the residual release with IgE cross-linking was still significantly higher than that from unstimulated controls. Thus, the data shown in Figs. 6C, 7C and 7D are consistent with the current concept that IgE cross-linking causes a rapid release of Ca2+ from an intracellular pool, followed by recruitment of extracellular Ca2+ channels, leading to influx of extracellular Ca2+. Ca2+ from both pools is required for full mediator release. In contrast, E2’s effects on mediator release seem to be completely dependent on an influx of extracellular Ca2+.
Fig. 7.
Addition of EGTA inhibits E2-stimulated β-hex release and LTC4 synthesis from RBL-2H3 cells: (A and B) effects of EGTA on RBL-2H3 stimulated with E2; (C and D) EGTA effects on cells incubated with anti-DNP-IgE, followed by DNP–BSA; (A and C) β-hex release; (B and D) LTC4 synthesis were measured in the supernatants. *p < 0.05 between indicated groups. IgE = IgE anti-DNP + DNP−BSA, NS = not significant.
4. Discussion
The results of our experiments demonstrate that physiological concentrations of E2 rapidly stimulate estrogen-starved, murine and human mast cell lines (RBL-2H3 and HMC-1) and primary cultures of bone marrow mast cells (BMMCs) to release β-hex, a marker for the granules that contain preformed allergic mediators. The synthesis and release of LTC4 by RBL-2H3 cells was also stimulated by exposure to E2. The finding that tamoxifen inhibited these effects suggested that they were mediated through specific α or β ERs. Since we found mRNA for ER-α, but not ER-β in all of these mast cells, it was likely that ER-α was involved in this estrogenic effect on mast cells. This proposition was substantiated by the demonstration that BMMC from ER-α KO mice did not degranulate in response to E2. In addition to these direct effects, E2 also enhanced IgE-dependent synthesis and release of β-hex and LTC4. However, the interaction of these two stimuli on the release of these two types of mediators seemed to differ quantitatively. E2 alone induced the release of a substantial proportion of preformed mediators and was additive with the IgE-dependent release. In contrast, E2 alone had a statistically significant, but small effect on the synthesis and release of LTC4. However, E2 strongly potentiated the effects of IgE cross-linking on LTC4 release.
Our study also provides insight into the mechanisms of the estrogenic effects on the synthesis and release of allergic mediators from mast cells. While our results demonstrate that the effects of E2 on mast cells require the specific ER-α, the rapid onset of E2’s effects on mast cell activation indicates that it does not function through the classical (genomic) mechanisms, which require enhanced mRNA and protein synthesis over a 2 h or longer period (Simoncini et al., 2004). Rather our findings suggest that E2’s effects on mast cell mediator release are through a membrane-associated (non-genomic) form of ER-α. This type of ER stimulation has been shown to induce rapid cellular responses through G-protein activation, (Bulayeva et al., 2004; Collins and Webb, 1999; Doolan et al., 2000; Improta-Brears et al., 1999; Stefano et al., 1999; Watson et al., 1999) which can include various signaling pathways involving Ca2+ fluxes, and modulation of mitogen-activated protein kinases and adenyl cyclase (Bulayeva et al., 2004; Collins and Webb, 1999; Doolan and Harvey, 2003; Nadal et al., 2000; Song et al., 2004; Zivadinovic et al., 2005). Indeed, we found that intracellular Ca2+ was increased within the first 2.5 min after estrogenic stimulation of RBL. However, in contrast to the rapid rise of intracellular Ca2+ induced by IgE cross-linking, the E2-induced rise was completely inhibited by the extracellular Ca2+ chelator EGTA. EGTA also reduced the release of preformed mediators and substantially reduced new synthesis of mediators, indicating that the effects of E2 we observed depend on the influx of extracellular Ca2+.
We were also interested in understanding the mechanisms underlying the additive and synergistic effects of estrogens on the IgE-mediated release of preformed mediators and synthesis and release of LTs, respectively. Our results suggest that intracellular Ca2+ concentrations may explain, at least in part, these observations. The combination of E2 and IgE cross-linking not only increased the amplitude and duration of the early Ca2+ peak, but also caused a progressive rise in intracellular Ca2+ (not seen with IgE cross-linking alone). Addition of EGTA to the media abrogated the estrogenic effects on the early rise and resulted in a progressive fall in intracellular Ca2+ thereafter (data not shown). While the differential effects of intracellular Ca2+ on preformed (β-hex) and newly synthesized (LTC4) mediators are not explained directly by our results, it is interesting to speculate that the greater and more prolonged increase in intracellular Ca2+ is more effective in supporting the translocation of cytosolic phospholipase A2 (cPLA2) to the nuclear membrane (Durstin et al., 1994; Pouliot et al., 1996). This translocation has been shown to increase PLA2 activity and subsequent arachidonic acid turn-over after estrogenic stimulation (Fiorini et al., 2003; Wang et al., 2001). However, these same conditions may also enhance the lipoxygenase activity required to convert arachidonic acid to LTs. In addition, the other steps involved in the exocytosis of preformed peptides (movement of vesicles to the membrane, docking of the vesicles at membrane sites) may involve estrogenic effects other than Ca2+ elevation.
This study is the first to show that: (1) physiological concentrations of E2 alone induce mast cells to release a substantial component of their preformed mediators and potentiate the IgE-dependent synthesis and secretion of a potent LT, (2) E2’s effect on mast cells is mediated by a non-genomic effect of ER-α and (3) these E2 effects require influx of extracellular Ca2+. Perhaps, our finding with the greatest potential clinical relevance was that the addition of E2 significantly shifted the antigen dose response curve for mediator release. These findings may help to explain why women during their peak reproductive years have an increased incidence of asthma, and more specifically why women with PMA have increased concentrations of LTC4 in their blood during this phase of their menstrual cycle, when estrogens (and progesterone) concentrations are rapidly changing. Interestingly, the most active concentration of E2 (100 pM) in our cell culture media was similar to those in the plasma of women during the perimenstrual phase (Skobeloff et al., 1996).
Given the strict requirement for extracellular Ca2+ for the estrogenic effects on mediator release, blocking one or more types of Ca2+ channels on basophils and mast cells might abrogate estrogen’s effects on the release of allergic mediators. This approach might improve the management of asthma in women. However, a more complete understanding of the effects of E2, other estrogenic compounds (e.g. dietary estrogens and environmental estrogens) and other female hormones on each step of mast cell activation may suggest other therapeutic approaches for allergic diseases.
Acknowledgments
This work was supported by a seed grant from Center for Interdisciplinary Research in Women’s Health (T.M.H.) and John Sealy Memorial Endowment Fund for Biomedical Research from University of Texas Medical Branch (T.M.H.), Parker B. Francis Fellowship in Pulmonary Research from Francis Families Foundation (T.M.H.), Advanced Technology Program from Texas Higher Education Coordinating Board (R.M.G.) and the NIEHS Center for Environmental Science (#E06676, T.M.H., R.M.G.). We would like to thank Eugene P. Knutson and Dr. Thomas Albrecht for technical assistance with confocal microscopy and Dr. Yasuko Furumoto with BMMC. We also thank Dr. Melvyn S. Soloff, Dr. Philip T. Palade and Dr. Rafeul Alam for critical review and suggestions on the manuscript.
Abbreviations
- β-hex
β-hexosaminidase
- BMMC
bone marrow derived mouse mast cells
- DM
dust mite
- E2
estradiol
- ER
estrogen receptor
- HMC
human mast cell line
- KO
knockout
- LT
leukotriene
- PBS
phosphate buffered saline
- PMA
perimenstrual asthma
- RBL-2H3
rat basophilic cell line-2H3
- WT
wild type
References
- Barr RG, Wentowski CC, Grodstein F, Somers SC, Stampfer MJ, Schwartz J, Speizer FE, Camargo CA., Jr Prospective study of postmenopausal hormone use and newly diagnosed asthma and chronic obstructive pulmonary disease. Arch Intern Med. 2004;164:379–386. doi: 10.1001/archinte.164.4.379. [DOI] [PubMed] [Google Scholar]
- Bulayeva NN, Gametchu B, Watson CS. Quantitative measurement of estrogen-induced ERK 1 and 2 activation via multiple membrane-initiated signaling pathways. Steroids. 2004;69:181–192. doi: 10.1016/j.steroids.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulayeva NN, Wozniak AL, Lash LL, Watson CS. Mechanisms of membrane estrogen receptor-alpha-mediated rapid stimulation of Ca2+ levels and prolactin release in a pituitary cell line. Am J Physiol Endocrinol Metab. 2005;288:E388–E397. doi: 10.1152/ajpendo.00349.2004. [DOI] [PubMed] [Google Scholar]
- Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12:345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- Cary NC. SAS Publishing. 2000. [Google Scholar]
- Cocchiara R, Albeggiani G, Di Trapani G, Azzolina A, Lampiasi N, Rizzo F, Diotallevi L, Gianaroli L, Geraci D. Oestradiol enhances in vitro the histamine release induced by embryonic histamine-releasing factor (EHRF) from uterine mast cells. Hum Reprod. 1992;7:1036–1041. doi: 10.1093/oxfordjournals.humrep.a137790. [DOI] [PubMed] [Google Scholar]
- Cocchiara R, Albeggiani G, Di Trapani G, Azzolina A, Lampiasi N, Rizzo F, Geraci D. Modulation of rat peritoneal mast cell and human basophil histamine release by estrogens. Int Arch Allergy Appl Immunol. 1990;93:192–197. doi: 10.1159/000235300. [DOI] [PubMed] [Google Scholar]
- Collins P, Webb C. Estrogen hits the surface. Nat Med. 1999;5:1130–1131. doi: 10.1038/13453. [DOI] [PubMed] [Google Scholar]
- Dastych J, Walczak-Drzewiecka A, Wyczolkowska J, Metcalfe DD. Murine mast cells exposed to mercuric chloride release granule-associated N-acetyl-beta-D-hexosaminidase and secrete IL-4 and TNF-alpha. J Allergy Clin Immunol. 1999;103:1108–1114. doi: 10.1016/s0091-6749(99)70186-7. [DOI] [PubMed] [Google Scholar]
- De Marco R, Locatelli F, Cerveri I, Bugiani M, Marinoni A, Giammanco G. Incidence and remission of asthma: a retrospective study on the natural history of asthma in Italy. J Allergy Clin Immunol. 2002;110:228–235. doi: 10.1067/mai.2002.125600. [DOI] [PubMed] [Google Scholar]
- Dijkstra A, Howard TD, Vonk JM, Ampleford EJ, Lange LA, Bleecker ER, Meyers DA, Postma DS. Estrogen receptor 1 polymorphisms are associated with airway hyperresponsiveness and lung function decline, particularly in female subjects with asthma. J Allergy Clin Immunol. 2006;117:604–611. doi: 10.1016/j.jaci.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Doolan CM, Condliffe SB, Harvey BJ. Rapid non-genomic activation of cytosolic cyclic AMP-dependent protein kinase activity and [Ca(2+)](i) by 17-beta-oestradiol in female rat distal colon. Br J Pharmacol. 2000;129:1375–1386. doi: 10.1038/sj.bjp.0703193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolan CM, Harvey BJ. A Galphas protein-coupled membrane receptor, distinct from the classical oestrogen receptor, transduces rapid effects of oestradiol on [Ca2+]i in female rat distal colon. Mol Cell Endocrinol. 2003;199:87–103. doi: 10.1016/s0303-7207(02)00303-9. [DOI] [PubMed] [Google Scholar]
- Durstin M, Durstin S, Molski TF, Becker EL, Sha’afi RI. Cytoplasmic phospholipase A2 translocates to membrane fraction in human neutrophils activated by stimuli that phosphorylate mitogen-activated protein kinase. Proc Natl Acad Sci USA. 1994;91:3142–3146. doi: 10.1073/pnas.91.8.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorini S, Ferretti ME, Biondi C, Pavan B, Lunghi L, Paganetto G, Abelli L. 17Beta-eEstradiol stimulates arachidonate release from human amnion-like WISH cells through a rapid mechanism involving a membrane receptor. Endocrinology. 2003;144:3359–3367. doi: 10.1210/en.2002-221106. [DOI] [PubMed] [Google Scholar]
- Harnish DC, Albert LM, Leathurby Y, Eckert AM, Ciarletta A, Kasaian M, Keith JC., Jr Beneficial effects of estrogen treatment in the HLA-B27 transgenic rat model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G118–G125. doi: 10.1152/ajpgi.00024.2003. [DOI] [PubMed] [Google Scholar]
- Improta-Brears T, Whorton AR, Codazzi F, York JD, Meyer T, McDonnell DP. Estrogen-induced activation of mitogen-activated protein kinase requires mobilization of intracellular calcium. Proc Natl Acad Sci USA. 1999;96:4686–4691. doi: 10.1073/pnas.96.8.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T, Hirata F, Ishizaka K, Axelrod J. Stimulation of phospholipid methylation, Ca2+ influx, and histamine release by bridging of IgE receptors on rat mast cells. Proc Natl Acad Sci USA. 1980;77:1903–1906. doi: 10.1073/pnas.77.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YA, Zhang YY, Luo HS, Xing SF. Mast cell density and the context of clinicopathological parameters and expression of p185, estrogen receptor, and proliferating cell nuclear antigen in gastric carcinoma. World J Gastroenterol. 2002;8:1005–1008. doi: 10.3748/wjg.v8.i6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert KC, Curran EM, Judy BM, Lubahn DB, Estes DM. Estrogen receptor-{alpha} deficiency promotes increased TNF-{alpha} secretion and bacterial killing by murine macrophages in response to microbial stimuli in vitro. J Leukoc Biol. 2004;75:1166–1172. doi: 10.1189/jlb.1103589. [DOI] [PubMed] [Google Scholar]
- Lambert KC, Curran EM, Judy BM, Milligan GN, Lubahn DB, Estes DM. Estrogen receptor {alpha} (ER{alpha}) deficiency in macrophages results in increased stimulation of CD4+ T cells while 17{beta}-estradiol acts through ER{alpha} to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. J Immunol. 2005;175:5716–5723. doi: 10.4049/jimmunol.175.9.5716. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma—United States, 1980–1999. MMWR Surveill Summ. 2002;51:1–13. [PubMed] [Google Scholar]
- Morita Y, Siraganian RP. Inhibition of IgE-mediated histamine release from rat basophilic leukemia cells and rat mast cells by inhibitors of trans-methylation. J Immunol. 1981;127:1339–1344. [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci USA. 2000;97:11603–11608. doi: 10.1073/pnas.97.21.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasato H, Ohrui T, Sekizawa K, Matsui T, Yamaya M, Tamura G, Sasaki H. Prevention of severe premenstrual asthma attacks by leukotriene receptor antagonist. J Allergy Clin Immunol. 1999;104:585–588. doi: 10.1016/s0091-6749(99)70327-1. [DOI] [PubMed] [Google Scholar]
- Nicovani S, Rudolph MI. Estrogen receptors in mast cells from arterial walls. Biocell. 2002;26:15–24. [PubMed] [Google Scholar]
- Odom S, Gomez G, Kovarova M, Furumoto Y, Ryan JJ, Wright HV, Gonzalez-Espinosa C, Hibbs ML, Harder KW, Rivera J. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J Exp Med. 2004;199:1491–1502. doi: 10.1084/jem.20040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot M, McDonald PP, Krump E, Mancini JA, McColl SR, Weech PK, Borgeat P. Colocalization of cytosolic phospholipase A2, 5-lipoxygenase, and 5-lipoxygenase-activating protein at the nuclear membrane of A23187-stimulated human neutrophils. Eur J Biochem. 1996;238:250–258. doi: 10.1111/j.1432-1033.1996.0250q.x. [DOI] [PubMed] [Google Scholar]
- Schatz M, Camargo CA., Jr The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann Allergy Asthma Immunol. 2003;91:553–558. doi: 10.1016/S1081-1206(10)61533-5. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Mannella P, Fornari L, Caruso A, Varone G, Genazzani AR. Genomic and non-genomic effects of estrogens on endothelial cells. Steroids. 2004;69:537–542. doi: 10.1016/j.steroids.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Skobeloff EM, Spivey WH, Silverman R, Eskin BA, Harchelroad F, Alessi TV. The effect of the menstrual cycle on asthma presentations in the emergency department. Arch Intern Med. 1996;156:1837–1840. [PubMed] [Google Scholar]
- Skobeloff EM, Spivey WH, St Clair SS, Schoffstall JM. The influence of age and sex on asthma admissions. JAMA. 1992;268:3437–3440. [PubMed] [Google Scholar]
- Smith GD, Lee RJ, Oliver JM, Keizer J. Effect of Ca2+ influx on intracellular free Ca2+ responses in antigen-stimulated RBL-2H3 cells. Am J Physiol. 1996;270:C939–C952. doi: 10.1152/ajpcell.1996.270.3.C939. [DOI] [PubMed] [Google Scholar]
- Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci USA. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos C, el Mansoury M, Letourneau R, Minogiannis P, Greenwood J, Siri P, Sant GR, Theoharides TC. Carbachol-induced bladder mast cell activation: augmentation by estradiol and implications for interstitial cystitis. Urology. 1996;48:809–816. doi: 10.1016/S0090-4295(96)00239-7. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Prevot V, Beauvillain JC, Fimiani C, Welters I, Cadet P, Breton C, Pestel J, Salzet M, Bilfinger TV. Estradiol coupling to human monocyte nitric oxide release is dependent on intracellular calcium transients: evidence for an estrogen surface receptor. J Immunol. 1999;163:3758–3763. [PubMed] [Google Scholar]
- Suzuki Y, Yoshimaru T, Matsui T, Inoue T, Niide O, Nunomura S, Ra C. Fc epsilon RI signaling of mast cells activates intracellular production of hydrogen peroxide: role in the regulation of calcium signals. J Immunol. 2003;171:6119–6127. doi: 10.4049/jimmunol.171.11.6119. [DOI] [PubMed] [Google Scholar]
- Vliagoftis H, Dimitriadou V, Boucher W, Rozniecki JJ, Correia I, Raam S, Theoharides TC. Estradiol augments while tamoxifen inhibits rat mast cell secretion. Int Arch Allergy Immunol. 1992;98:398–409. doi: 10.1159/000236217. [DOI] [PubMed] [Google Scholar]
- Vrieze A, Postma DS, Kerstjens HA. Perimenstrual asthma: a syndrome without known cause or cure. J Allergy Clin Immunol. 2003;112:271–282. doi: 10.1067/mai.2003.1676. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Kim MP, Tiano HF, Langenbach R, Smart RC. Protein kinase C-alpha coordinately regulates cytosolic phospholipase A(2) activity and the expression of cyclooxygenase-2 through different mechanisms in mouse keratinocytes. Mol Pharmacol. 2001;59:860–866. doi: 10.1124/mol.59.4.860. [DOI] [PubMed] [Google Scholar]
- Watson CS, Norfleet AM, Pappas TC, Gametchu B. Rapid actions of estrogens in GH3/B6 pituitary tumor cells via a plasma membrane version of estrogen receptor-alpha. Steroids. 1999;64:5–13. doi: 10.1016/s0039-128x(98)00107-x. [DOI] [PubMed] [Google Scholar]
- Wozniak AL, Bulayeva NN, Watson CS. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect. 2005;113:431–439. doi: 10.1289/ehp.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XJ, McKerr G, Dong Z, Higgins CA, Carson J, Yang ZQ, Hannigan BM. Expression of oestrogen and progesterone receptors by mast cells alone, but not lymphocytes, macrophages or other immune cells in human upper airways. Thorax. 2001;56:205–211. doi: 10.1136/thorax.56.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinovic D, Gametchu B, Watson CS. Membrane estrogen receptor-alpha levels in MCF-7 breast cancer cells predict cAMP and proliferation responses. Breast Cancer Res. 2005;7:R101–R112. doi: 10.1186/bcr958. [DOI] [PMC free article] [PubMed] [Google Scholar]