Abstract
Caffeine, the world’s most common psychoactive substance, is used by approximately 90% of North Americans everyday. Little is known, however, about its benefits for memory. Napping has been shown to increase alertness and promote learning on some memory tasks. We directly compared caffeine (200mg) with napping (60–90 minutes) and placebo on three distinct memory processes: declarative verbal memory, procedural motor skills, and perceptual learning. In the verbal task, recall and recognition for unassociated words were tested after a 7hr retention period (with a between-session nap or drug intervention). A second, different, word list was administered post-intervention and memory was tested after a 20min retention period. The non-declarative tasks (finger tapping task and texture discrimination task) were trained before the intervention and then retested afterwards. Naps enhanced recall of words after a 7hr and 20min retention interval relative to both caffeine and placebo. Caffeine significantly impaired motor learning compared to placebo and naps. Napping produced robust perceptual learning compared with placebo; however, naps and caffeine were not significantly different. These findings provide evidence of the limited benefits of caffeine for memory improvement compared with napping. We hypothesize that impairment from caffeine may be restricted to tasks that contain explicit information; whereas strictly implicit learning is less compromised.
Introduction
Caffeine, the world’s most widely consumed stimulant (Nawrot et al. 2003), is an active ingredient in coffee, tea, chocolate, sodas, and energy drinks (the fastest growing sector of the American beverage industry)(Lovett 2005). Modern times have led to an increase in daily, often multiple doses of caffeine, a rise in the coffee business, and the addition of caffeine to common beverages such as soda, bottled water, and even chewing gum. Based on the available product usage and food consumption data, Barone and Roberts (Barone J 1996) estimated the mean daily intake was 4 mg/kg body weight (approximately 280mg for a 155 pound person; 16 ounces of Starbucks coffee contains 372 mg). For the 90th percentile of caffeine users, intakes approximated 5–7 mg/kg body weight (approximately 300–500mg).
This increasingly common use of caffeine in our society coincides with an increasingly common trend of individuals obtaining insufficient sleep on a regular basis. While it is difficult to ascertain the exact number of individuals who use caffeine as a substitute for sleep in society, the 2005–2007 National Sleep Foundation’s annual Sleep in America polls strongly suggest that Americans regularly consume caffeine as a substitute for sleep and/or as a result of insufficient sleep (Foundation 2005; Foundation 2006; Foundation 2006). These polls report consistent associations between low quantity or quality of sleep, decreased daytime functioning, and increased daytime caffeine consumption.
A number of studies have examined the benefits of daytime caffeine consumption in non-experimentally sleep-deprived individuals (Loke et al. 1985; Lieberman et al. 1987; Zwyghuizen-Doorenbos et al. 1990; Nehlig et al. 1992; Dimpfel et al. 1993; Spriet 1995; Lorist et al. 1996; Kaplan et al. 1997; Brice et al. 2002; Lieberman et al. 2002; Lorist et al. 2003; Cysneiros et al. 2007). The performance tasks used in these studies measure reaction time and motor speed, speed of information processing, vigilance and attention, immediate and delayed verbal memory, as well as mood and alertness (for review see (Nehlig et al. 1992; Lorist et al. 2003). Generally, caffeine enhances mood and alertness (Lieberman et al. 1987; Kaplan et al. 1997), vigilance and attention (Lieberman et al. 1987; Zwyghuizen-Doorenbos et al. 1990), speed of information processing (Kaplan et al. 1997; Cysneiros et al. 2007), reaction time and motor speed (Lieberman et al. 1987; Zwyghuizen-Doorenbos et al. 1990; Kaplan et al. 1997; Cysneiros et al. 2007). One study found 200 and 300 mg of caffeine benefited visual vigilance, choice reaction time, repeated acquisition, and self-reported fatigue and sleepiness, but did not improve marksmanship, a task that requires fine motor coordination and steadiness (Lieberman et al. 2002). Dimpfel et al. measured the effects of placebo, 200 and 400 mg of caffeine on human electroencephalogram (EEG) patterns at rest and during mental concentration tests. In addition to the finding that the effects of caffeine can be quantified with EEG spectral densities, they also found that subjects achieved the best results on concentration tests when given 200 mg of caffeine. In fact, subjects given 400mg tested below subjects in the placebo condition. Other studies have found similar improvements on cognitive tasks with as little as 70 mg of caffeine administration compared to placebo (Rogers et al. 1995).
While these studies show caffeine can enhance wakefulness and performance on attention and concentration tasks, little agreement can be found in the literature on caffeine and memory (Cattell 1930; Loke et al. 1985; Cysneiros et al. 2007). In their review, Nehlig and colleagues (1992) write ”In man, memory per se is not improved but response tends to be quicker and keener [with caffeine]”. An alternative explanation for the negative findings is that only a limited number of memory processes have been examined. A thorough examination of the effect of caffeine across a wide range of memory processes has not been completed. Thus, it is still an open question whether caffeine improves learning and memory (Spriet 1995; Nawrot et al. 2003), either more generally or in specific memory domains.
Naps, in contrast to caffeine, have been shown to enhance not only alertness and attention, but also some forms of memory consolidation. In particular, naps (daytime sleep between 5–90minutes) appear to improve performance on non-medio-temporal lobe dependent, procedural skills (Mednick et al. 2002; Mednick et al. 2003; Korman et al. 2007; Nishida et al. 2007). Mednick and colleagues reported that a mid-day nap can also reverse perceptual deterioration that builds with repeated within-day testing (Mednick et al. 2002). They further showed that naps with SWS and REM produced improvements in performance equivalent to that of a full night of sleep, whereas naps with only SWS restored deteriorated performance to baseline levels (Mednick et al. 2003). Walker and colleagues have demonstrated that naps improve procedural motor skill learning to the same degree as a full night of sleep, and that improvement on this task was correlated with Stage 2 and sleep spindle activity (Walker et al. 2004; Nishida et al. 2007). Tucker compared naps with non-REM sleep to a no-nap condition on a procedural memory task and a declarative, verbal-paired-associates task. They found that the non-REM naps produced improved performance in the declarative, but not the procedural task (Tucker et al. 2006). This is evidence that non-REM in naps can produce similar declarative memory improvements as nocturnal non-REM sleep (Plihal et al. 1999).
Prior studies of performance during nightshift work have directly compared caffeine and napping in on a variety of tasks (Schweitzer et al. 2006; Sagaspe et al. 2007). For example, recently, Sagaspe and colleagues compared the effects of a single 200mg dose of caffeine to a 30min nap and placebo on nocturnal driving in young and middle-aged participants. They found that both interventions significantly improved performance in both age groups, although napping was even more effective in younger compared to older participants. There are no studies, however, directly comparing the effects of caffeine and naps during the day in normally-rested individuals, and few that have compared caffeine and sleep at any time for cognitive processes beyond attention, vigilance, or driving. Here, we compared the effects of caffeine, a daytime nap, or placebo on three distinct memory processes: declarative verbal memory, procedural motor skills, and perceptual learning. For verbal memory, we tested recall and recognition in two different phases: 7hr retention with a between-session intervention (caffeine, placebo or nap), and 20min retention for a different list of words post-intervention. The non-declarative tasks (finger tapping task and texture discrimination task) were trained before the intervention and then retested afterwards.
Methods
Subjects
61 adults between ages 18–39 with no personal history of neurological, psychological or other chronic illness (non-smoking) gave informed consent to participate in the experiment, which was approved by the institutional review boards of the University of California San Diego. Subjects were low to moderate caffeine drinkers (no more that two cups of coffee per day). Since restricted nighttime sleep can have a deleterious effect on performance (Van Dongen et al. 2003), we required that subjects maintain a sleep schedule for one week prior to the study. For seven nights prior to the study, subjects were instructed to go to bed no later than midnight and to get up no later than 8am. They were asked to spend at least eight hours in bed each night. Subjects filled out sleep diaries and wore actigraphs as subjective and objective measures of sleep-wake activity. Subjects were restricted from consuming caffeine and alcohol 24 hours prior to and during the experimental day.
An uneven number of subjects were run in all three tasks due to technical error, subjects misunderstanding the task which led to unusable data, and adding the verbal task midway through the study. For the Verbal task, 11 placebo, 12 nappers and 12 caffeine subjects were run. For the Motor task, 18 placebo, 13 nappers and 18 caffeine subjects were run. For the Perceptual task, 19 placebo, 18 nappers and 18 caffeine subjects were run.
Study Procedures
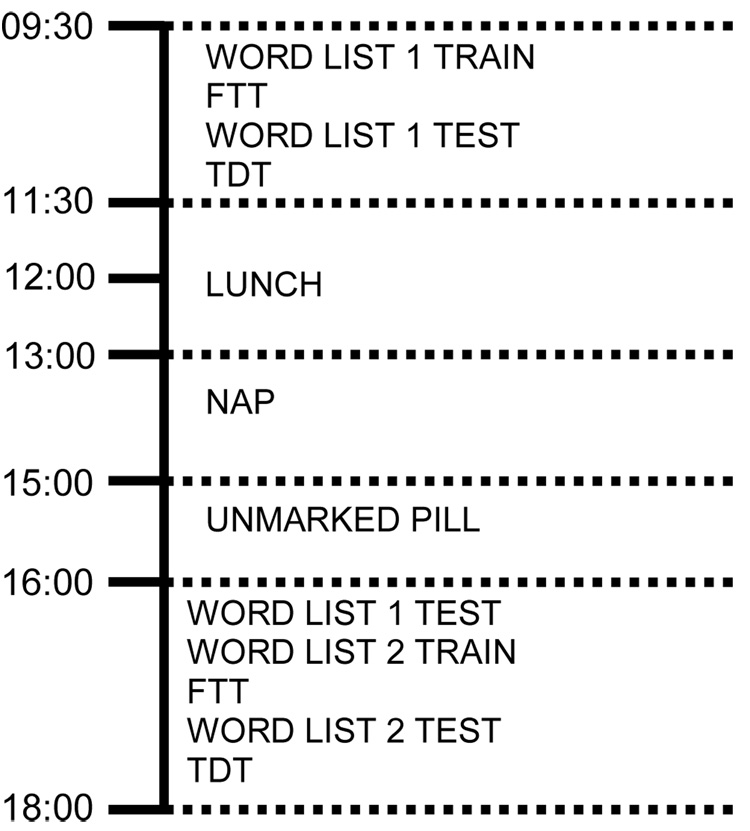
Figure 1 shows study timeline (an example task order scenario). Task order was counterbalanced across subjects. Subjects were in the lab under supervision during the entire experimental day. Subjects’ knowledge of testing procedure was limited to being told that they would be tested in the morning and afternoon on the all three tasks. At 0930, subjects were administered the initial verbal task and were trained on the finger tapping task (FTT) and texture discrimination task (TDT) (Session One). Lunch was served at noon. At 1300, subjects were randomly assigned to a nap or a drug group. Subjects either took a polysomnographically-recorded (PSG) nap (90-minutes of sleep maximum or up to two hours in bed) or listened to a book on tape with PSG monitoring. A summary of Nap PSG can be found in Table 2. At 1500, subjects in the drug groups were given an unmarked pill (200mg caffeine or placebo). Sixty minutes later (Session 2), subjects were tested on all three tasks, as described below.
Figure 1. Experimental timeline.
All subjects tested on Word List 1 in the morning. At 1pm, nappers slept with PSG monitoring. At 3pm non-nappers received an unmarked pill (200mg of caffeine or placebo). All subjects retested on Word List 1 after 7hr retention interval. All subjects were then trained and tested on Word List 2 with a 20min retention interval.
Table 2.
Polysomography of Naps (Mean and Standard Deviation)
| TST | Stage 1 | Stage 2 | SWS | REM |
|---|---|---|---|---|
| 69.38 ± 23 | 6.38 ± 4.1 | 41.57 ± 14 | 12.55 ± 13 | 8.88 ± 12 |
In addition, subjective sleepiness was measured before and after each test session with the Karolinska Sleepiness Scale (KSS). The KSS assesses subjects’ momentary state of alertness/sleepiness on a 1–9 scale (“extremely alert” to “extremely sleepy”). Before the first test session subjects also completed the Epworth Sleepiness Scale. The Epworth assesses trait daytime sleepiness with eight questions, each scored with a degree of severity ranging from 0 to 3. A score less than 10 is considered normal. Table 1 shows the demographic information, Epworth score, and actigraphy data from the week prior to experimental day, including Total Sleep Time (TST), Bedtime and Waketime.
Table 1.
Demographics and Actigraphy (Mean and Standard Deviation)
| Group | Age | N (# female) | Education (years) | Epworth | TST (minutes) | Bedtime | Waketime |
|---|---|---|---|---|---|---|---|
| Nap | 23.6 (.78) | 19 (12) | 15.1 (.40) | 5.81 (.58) | 417 (54) | 23:56 | 7:42 |
| Placebo | 22.2 (.84) | 21 (15) | 14.8 (.38) | 6.05 (.67) | 428 (45) | 00:05 | 8:02 |
| Caffeine | 25.5 (1.2) | 21 (15) | 15.5 (.27) | 6.37 (.62) | 401 (76) | 23:36 | 7:38 |
Verbal Task
We examined recall and recognition memory in two different phases of verbal memory: 7hr retention with a between-session intervention (caffeine, placebo or nap), and 20min retention for a different list of words post-intervention. During Session One, subjects were trained and tested on Word List 1. During training, the experimenter read aloud 24 unrelated words in three consecutive trials. Immediately after each trial, subjects were asked to recall the words. After a period of 20 minutes (during which non-verbal tasks were completed), subjects were given tests of free recall and recognition for Word List 1. No feedback on performance was given. In the recognition test, subjects were read aloud a list of 48 words (half the words were Word List 1 and half were lures) and determined which were on Word List 1.
At the start of the second test session, tests of recall and recognition were given for Word List 1 in order to test for 7hr retention. Afterwards, the entire verbal memory task was repeated with Word List 2 to test for 20min retention in recall and recognition memory. For each test session, free recall was measured as the number of words correctly recalled unprompted. Recognition memory performance was measured with d’ (index of discriminability between target and lure words). We used two of the word lists here that were previously developed for other studies of verbal learning in our lab (Stricker et al. 2006). Words were chosen from those normed for recallability by Christian et al. (1978) (Christian 1978), and each list was matched for recallability, word length, concreteness, and imagery. List order was counterbalanced across subjects.
Motor Task
The finger tapping task (FTT) was identical to that from Walker (Walker et al. 2002). The task required subjects to repeatedly complete, with their left (non-dominant) hand, the sequence 4-1-3-2-4 on a keyboard. Each block consisted of 30 seconds of key presses followed by 30 seconds of rest. The training session consisted of 12 blocks and the test session consisted of 3 blocks. The numeric sequence (4-1-3-2-4) was displayed at the top of the screen at all times to exclude any working memory component to the task. Each key press produced a white dot below, forming a row from left to right over the course of each key press sequence. Performance was measured as the number of correct sequences completed (score), and number of errors made (accuracy)
Perceptual Task
Participants performed a texture discrimination task similar to that developed by Karni and Sagi (Karni et al. 1991) and identical to that utilized in our previous studies (Mednick et al. 2002; Mednick et al. 2003; Mednick et al. 2005). Participants were asked to discriminate two targets per trial: a central letter (‘T’ or ‘L’), and a peripheral line array (vertical or horizontal orientation) in the lower left quadrant at 2.5–5.9 deg eccentricity from the center of the screen. The peripheral array consisted of three diagonal bars that were either positioned in a horizontal array or a vertical array against a background of horizontally oriented bars, which created a texture difference between the target and background.
An experimental trial consisted of the following sequence: central fixation cross, target screen for 32 ms, blank screen for a duration between 0 and 600 ms (the inter-stimulus-interval, or ISI), mask for 16 ms followed by the response time interval before the next trial. Subjects reported both the letter at central fixation (T or L) and the orientation of the peripheral, three-element array (horizontal or vertical) by making two key presses. The central task controlled for eye movements.
Each block consisted of 50 trials, each with the same ISI, and lasting approximately 2 minutes. A threshold was determined from the performance across 20 blocks, with a progressively shorter ISI, starting with 600 msec and ending with 0 msec. The specific sequence of ISIs across an entire session was [600, 500, 400, 350, 300, 250, 200, 175, 150, 125, 100, 80, 60, 40, 20, 0]. A psychometric function of percent correct for each block was fit with a Weibull function to determine the ISI at which performance yielded 80% accuracy.
Participants controlled the onset of each block and were instructed to take as many breaks as they needed between blocks. Once a block began, a new trial initiated every 2 seconds, regardless of whether or not the subject made a response. Training, which occurred at the beginning of the 9AM test session, consisted of 15 trials of an easy version of the task (ISI of 1000 – 1500 msec), and 50 trials of the easiest block of the actual task (ISI of 600 msec). This training ensured that participants understood the task and were discriminating the peripheral target between 90% and 100% correct on the easiest version of the task.
Analysis
Verbal Task
Our main outcome of interest involved the recall and recognition memory scores for the 7hr retention interval, since that interval included the different interventions. To examine that, we utilized a one-way analysis of variance (ANOVA) using three levels of the variable Group (Caffeine, Nap, Placebo), separately for recall and recognition. One concern with this approach, though, would be whether the three groups showed equal performance at baseline (i.e., 20min memory for Word List 1). Thus, we first evaluated that question with a similar 1-way ANOVA. If that analysis showed a significant main effect of group, we planned to control for baseline performance by examining the Session x Group interaction in a repeated measures ANOVA. However, since neither 20min recall nor recognition showed baseline differences (see Results, below), we utilized the 1-way ANOVAs for the 7hr retention interval to maximize power for our main effect of interest. Finally, to examine the impact of the intervention on the ability to encode new words, we conducted the same analysis for recall and recognition of Word List 2 at the 20min retention interval. Significant ANOVAs were followed-up by examining differences between groups at the specific time point with independent samples t-tests.
Motor Task
Prior to conducting the response time (RT) analyses described below, errors and a small number of extreme outlier trials (RTs of greater than 3000 ms) were excluded (Walker et al. 2002). We examined group differences across Session One and Two (i.e., learning) with a Repeated-Measures ANOVA, with Group as the between-subject variable, and Session (mean performance from last two blocks of the training vs. two blocks of test) as the within-subjects variable. This ANOVA was conducted for both Score and Accuracy.
Perceptual Task
We examined group differences across Session One and Two with a Repeated-Measures ANOVA, with Group as the between-subjects variable, and session thresholds as within-subjects variables (Mednick et al. 2003).
Subjective Sleepiness
Sleepiness was examined with a mixed-model repeated measured ANOVA with Group as the between variable and the four administrations as the within-factors. Also, we specifically examined the KSS rating from immediately after the treatment in a one-way ANOVA to examine acute treatment effects of subjective sleepiness.
Results
Verbal Task
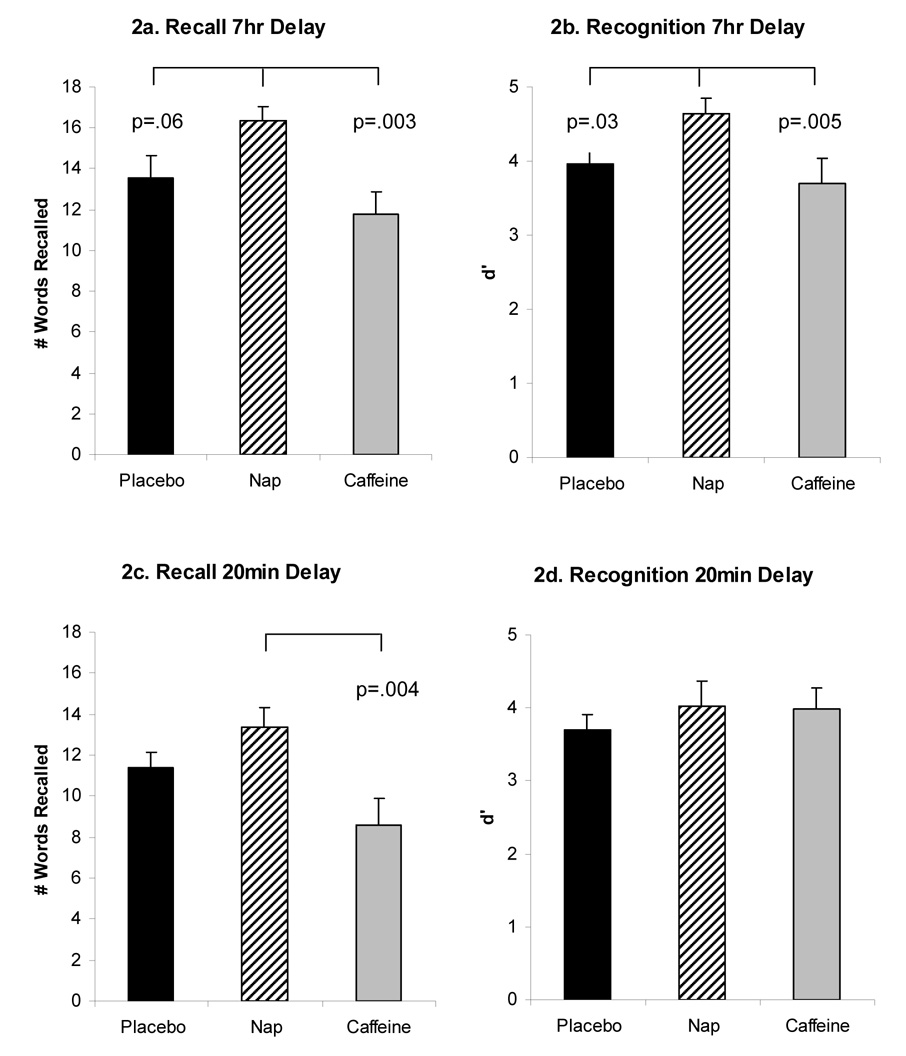
No significant differences were found between groups in Recall of Word List 1 at 20min (Recall means and standard deviations = 13.70(3.0), 15.25(3.33), 12.25(3.5) for placebo, nap, caffeine, respectively; F=2.36, p=.11, eta2=.12) or Recognition of Word List 1 at 20min (Recognition means and standard deviations = 4.5(.99), 4.9(.60), 4.5(.73), for placebo, nap, caffeine, respectively; F=.73, p=.49, eta2=.04). Recall memory for Word List 1 after 7hr retention interval showed significant group differences (F=5.41 p=.009, partial eta2=.25, Fig 2a). Post-hoc tests showed: a) the Nap group performed significantly better than the Caffeine group (p=.003); b) Nap performed marginally better than Placebo (p=.06.); and c) there were non-significant differences between Caffeine and Placebo (p=.22). Recognition memory for words after a 7hr retention interval also showed significant group differences for d’ (F=4.51 p=.019, partial eta2 =.22, Fig 2b). Post-hoc tests showed: a) Nap performed significantly better than Caffeine (p=.008); b) Nap better than Placebo (p=.03); and c) no difference between Caffeine and Placebo (p=.50).
Figure 2. Declarative Verbal Memory Task.
Verbal memory performance in Placebo (black bar), Nap (striped bar), and Caffeine groups (grey bar) (means and standard errors), with p-values of significant group differences. 7hr retention of Morning Words in Recall (2a) and Recognition in d’ (2b), 20min retention of Evening Words in Recall (2c) and Recognition in d’ (2d).
Recall after a 20min retention interval showed significant group differences (F=4.97 p=.01, partial eta2=.24, Figure 2c). Post-hoc tests showed: a) Nap performed significantly better than Caffeine (p=.004); b) no difference between Nap and Placebo (p=.21); and c) Caffeine performed marginally worse than Placebo (p=.08). For recognition memory after a 20min retention interval, no Group differences were found for d’ (F=.57 p=.57, partial eta2 =.03, Fig 2d). Data from the Verbal Task is shown in Figure 2.
Motor Task
A Repeated Measures ANOVA on Accuracy showed no significant interaction between group and accuracy (F=1.87, p=.16, partial eta2=.07). Accuracy was consistently high for all groups. Mean accuracy for the last two blocks of training was .97, .97, and .98 for the placebo, nap and caffeine groups, respectively. For the two blocks of the test session, these values were .98, .98, and .98.
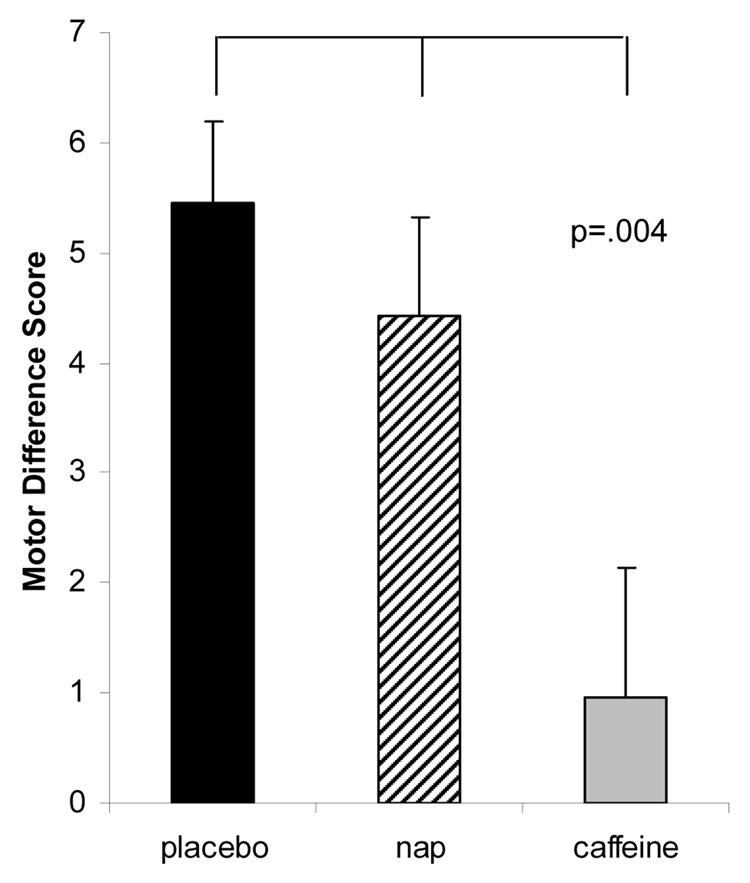
A Repeated Measures ANOVA on Score was statistically significant, (F=6.14, p=.004, partial eta2=.21). Post-hoc one-sample t-tests on the differences scores (last two blocks of train session minus first two blocks of test session) indicated the caffeine group showed significantly impaired learning (i.e., smaller increase in the number of sequences completed at Session 2) compared with placebo (p = .003), and nappers (p=.03). No difference was found in between nap and placebo (p=.38). Indeed the caffeine group did not show improvement across sessions (p=.43), whereas nappers (p=.000) and placebo (p=.000) groups showed significantly higher scores. Difference scores are shown in Figure 3.
Figure 3. Motor Skill Learning.
Differences Scores on Finger Tapping task represent increase in number of correct sequences completed in session two compared with session one in placebo (black bar), Nap (striped bar) and caffeine (grey bar) groups.
Perceptual Task
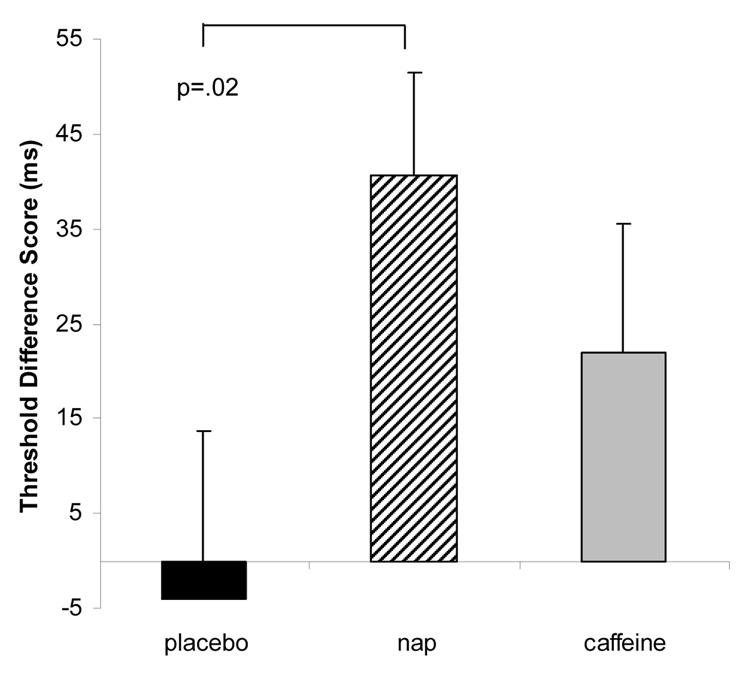
Performance improvement across the three groups was examined with a Repeated-Measures ANOVA. There was a marginally significant difference across three the groups (F=2.44, p=.09, eta2=.09). Post-hoc one-sample t-tests on the difference scores indicate that nappers showed the typical improvement on the TDT compared with placebo (p=.02). However, the caffeine group fell in-between naps and placebo and was not significantly different from either naps (p=.29) or placebo (p=.26). Difference scores are shown in Figure 4.
Figure 4. Perceptual Learning Task.
Threshold Difference Scores for Texture Discrimination task represents change in threshold from session one to session two in placebo (black bar), Nap (striped bar) and caffeine (grey bar) groups.
Subjective Sleepiness
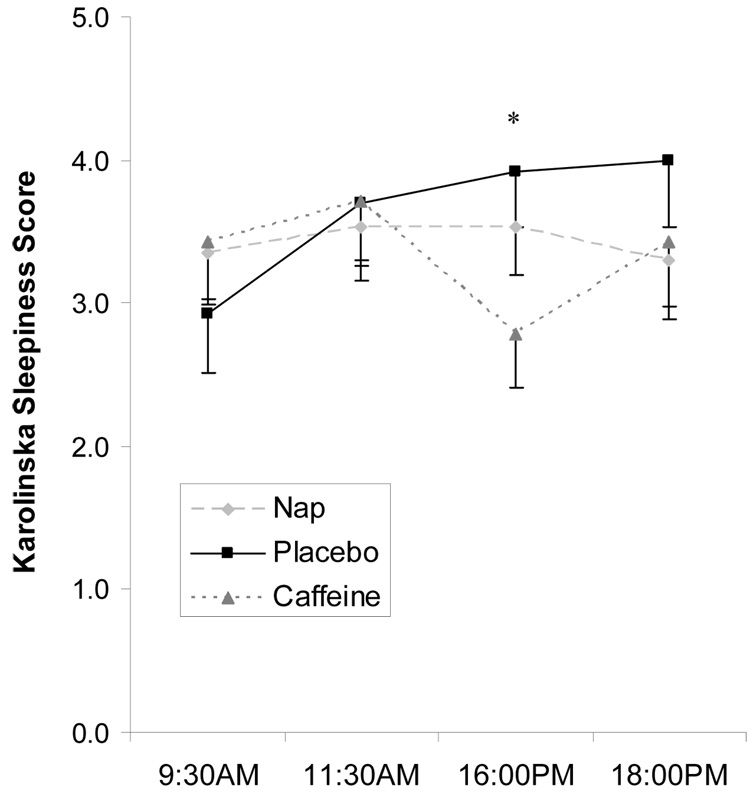
There was a marginally significant group effect on subjective sleepiness ratings across all four administrations (F=2.77, p=.07, eta2=.09). Compared to naps and placebo, caffeine subjects reported being more alert immediately prior to the testing session following the intervention. Sleepiness rating show significant group differences (F=3.90, p=.03, eta2=.20, Figure 5) during this third administration.
Figure 5.
Subjective sleepiness ratings across the day for each group (averages and standard errors). Caffeine group showed a short period of increased alertness immediately after a dose of caffeine (*).
Discussion
In this study, we find that a moderate dose of caffeine impaired motor sequence learning and declarative verbal memory compared to placebo and daytime sleep. These decreases were found despite the fact that caffeine increased subjective alertness, suggesting that the caffeine dose was sufficiently high to have some psychoactive effect. An afternoon nap, on the other hand, improved free recall memory relative to the caffeine group after both a 20min and a 7hr retention interval and produced greater learning on a motor sequence task than caffeine. Although napping produced improvements in the perceptual and motor tasks similar to that previously reported, we also found large amounts of learning in the placebo condition. In the perceptual task, the placebo group showed significantly better performance than previous studies have reported in the non-nap control groups (Mednick et al. 2003). Specifically non-nap controls typically show performance deterioration with repeated testing, whereas the placebo controls in the present study showed no deterioration. Furthermore, the level of improvements on the motor task in the placebo group is larger than control group performance in prior studies for both nocturnal sleep (Walker et al. 2002) and naps (Nishida et al. 2007). We hypothesize, at least for the motor and perceptual tasks, that the placebo condition produced a true “placebo effect” on these memory tasks.
Napping and Memory
Data from the Verbal Memory task suggest a sleep-dependent consolidation process occurs during the nap that allows for better recall and a finer discrimination between targets and distracters than can be achieved when sleep does not occur between study and test periods or by caffeine. It should be noted the present findings suggest a possible role for sleep during naps in declarative memory consolidation of unassociated, rather than associated, words. Recent research has shown that associative and non-associative declarative memory may rely on separate brain regions (Vargha-Khadem et al. 1997; Mayes et al. 2002; Turriziani et al. 2004). Specifically, these studies assign item memory formation (non-associative) to the parahippocampal gyrus (particularly rhinal cortices: anterior parahippocampal gyrus and parahippocampal cortex) and associative memory formation to the hippocampus. The majority of prior studies examining hippocampal-related, sleep-dependent memory have investigated memory for associated word-pairs (Gais et al. 2002; Drosopoulos et al. 2005; Backhaus et al. 2007). Instead, the present study examined item memory consolidation, which relies on parahippocampal and rhinal cortices. These findings expand the growing literature on the relationship between memory and sleep to suggest that sleep may benefit declarative memory consolidation not limited to processes subserved by the hippocampus itself. In addition to enhancing memory consolidation for previously studied words, naps improved the ability to learn a new list of words post-intervention when compared with caffeine.
Caffeine and Memory
In this study, caffeine decreased subjective sleepiness. This enhanced alertness, however, did not seem to transfer to motor learning and verbal memory. Although 200mg of caffeine is considered a moderate dose, other studies have also found similar doses impaired motor skill. In a complex test of hand-eye coordination in which subjects had to insert a stylus successively into three holes, 60 or 120mg of caffeine decreased, while 180 or 240mg of caffeine increased the time-to-task-completion (Hollingworth 1912). In another study, reading time of text increased with 2 or 4mg of caffeine per kilogram of body weight (MacPherson et al. 1996). Other studies have similarly shown that caffeine is unable to reverse the effects of sleep-deprivation on areas of higher level cognition, for example disadvantageous, high-risk decision making (Killgore WD 2007). A study of Navy Seals during the highly stressful training period (i.e. Hell Week) demonstrated that caffeine improved vigilance and speeded-reaction time (Lieberman et al. 2002). However, it was less effective for more complex cognitive tasks, such as working memory, marksmanship accuracy and time to sight the target. Other studies have noted a similar lack of efficacy for caffeine in higher cognitive tasks (Battig K 1984; Amendola CA 1998). This study extends these previous findings by being the first study, of which we are aware, to show reduced motor memory consolidation with caffeine.
Although caffeine is clearly effective in increasing arousal, the studies cited above indicate that the perceived cognitive benefit of caffeine may not universally translate to objective performance. High consumers of caffeine demonstrate faster simple and choice reaction times and report positive subjective effects in response to caffeine administration. Moderate to low users, on the other hand, do not demonstrate these enhancements (Attwood et al. 2007). Performance improvements from caffeine in some studies may thus represent a relief from withdrawal symptoms in high users. Consistent with this withdrawal hypothesis, we show no benefit to memory performance with caffeine, even in moderate consumers of caffeine (100–200mg per day). One could also argue based on these data that this relatively low dose of caffeine actually slightly impairs the ability to learn new information (Fig 2c, Fig 3). Such an impairment of performance, if replicated, runs counter to the general society assumption that caffeine typically benefits cognitive performance (in this case, verbal and motor memory).
Limitations and Caveats
One limitation of this study is that only one dose of caffeine was administered. Thus, the findings should not be generalized beyond this single dose of caffeine, roughly equivalent to two - three cups of coffee. Future studies using multiple doses may show a dose-response effect on motor and verbal memory. It is possible that a group receiving either a higher or lower (than 200 mg) dose of caffeine would exhibit more optimal arousal states and relatively improved performance relative to the placebo or nap group. Similarly, future studies may wish to use multiple doses of sleep (i.e., different lengths of sleep opportunity) to also test whether a dose response relationship exists with napping for verbal memory as reported for perceptual learning (Mednick et al. 2002; Mednick et al. 2003). Further, since the current study did not acquire plasma levels before or during the actual experiment, it is possible that subjects misrepresented their daily caffeine intake and/or ingested caffeine on the morning of the experimental day (Kennedy et al. 1991). Habitual caffeine usage has been shown to moderate performance enhancement abilities of caffeine (Attwood et al. 2007). The degree to which this caveat biases the data is lessened by the fact that subjects were randomized to their treatment group in the middle of the experimental day. Therefore, the likelihood of caffeine ingestion would be equal across groups. If anything, use of caffeine by some subjects on the day of the experiment should have minimized treatment effects, and thus minimize differences between our groups. If this were true, we may actually underestimate the differences between naps and caffeine here.
One possible explanation for the motor decrements reported in the present study is that caffeine impairs local motor movements. Typically, it is thought that at least 5mg/kg is needed to produce hand tremors. But a few studies have found even lower doses can induce tremors (Paroli 1972; Jacobson BH 1987). The moderate dose of 200mg may also have caused a global over stimulation to the nervous system, even without overt hand tremors, which impaired performance. Although subjects reported typically consuming 100–200mg of caffeine a day, this is likely absorbed through a caffeinated beverage. Oral administration of the pill may have increased arousal more suddenly than sipping a cup of coffee. This heightened increase in arousal may have impaired learning, as the Yerkes-Dodson law states that performance is poor at high and low arousal states.
It is possible that listening to a book on tape during the time interval reserved for sleep in the napping group may have caused interference in the caffeine and placebo groups for the verbal memory task. However, the fact that the book on tape started approximately 2 hours after the morning test session was completed may have reduced this possibility. If such interference did occur, the caffeine group appeared to suffer more from interference than the placebo group, since the caffeine group performed marginally worse than the placebo group for the 20min Recall of Word List 2. Finally, the choice of a language-based activity for this control period has the advantage of preventing (or at least reducing) rehearsal of Word List 1 in the drug groups, which would have potentially increased memory consolidation in those groups independent of the drug intervention.
Conclusion
Overall, a daytime nap generally improved performance across three different learning paradigms, while caffeine impaired (or at least did not benefit) performance. We hypothesize that the pattern of results demonstrated by the caffeine group may be explained by the relative level of explicit information in each memory task. The three tasks, perceptual learning, procedural motor skill, and verbal memory, each have varying levels of explicit information involved in learning. The perceptual learning task involves the least amount of explicit material, as demonstrated by the high degree of specificity shown in performance profiles (Mednick et al. 2005) and no conscious access to learning or deterioration (Mednick et al. 2002). The motor task, although procedural, shows a strong explicit component, in that explicit sequence knowledge has been shown to modify off-line consolidation (Robertson et al. 2004). Also, subjects report consciously practicing the specific sequence between training and test (Rickard 2007). The verbal task is by nature an explicit task in which subjects must consciously hold on to individual test words for later recall.
Explicitness in memory tasks has been shown to be related to the degree that the task engages the hippocampus (Greene 2007). Sleep-dependent memory improvement in hippocampal-related tasks appears to be reliant on SWS (Gais et al. 2000). In particular, Gais and Born have demonstrated that low acetylcholine during SWS is important for explicit verbal memory (Gais et al. 2004), but not implicit memory. Acetylcholine naturally decreases during sleep, whereas caffeine has been shown to increase hippocampal acetylcholine via antagonism of local adenosine A1 receptors (Carter et al. 1995). This increase in hippocampal acetylcholine by caffeine may block the consolidation process by blocking replay of new memories. Consistent with this conceptualization, we found that the greater the explicit component of each task, the worse the caffeine group performed.
Recent attention to the importance of overnight sleep for a variety of health and cognitive domains has demonstrated that no complete pharmacological alternative to a good night’s rest has been discovered. The present findings suggest that caffeine, the most common pharmacological intervention for sleepiness, may not be an adequate substitute for the memory enhancements of daytime sleep, either.
Acknowledgments
We would like to acknowledge Kathy Resovsky, Ryan Wong, Arlene Schlosser for help conducting the study; Robert Stickgold and John Wixted for their thoughtful comments. Research was supported by DARPA award # N0014-06-1-0660 , the UCSD GCRC M01 RR00827, K01 MH080992-01, R01-AG024506
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amendola CA GJ, Lieberman HR. Caffeine’s effects on performance and mood are independent of age and gender. Nutr Neurosci. 1998;1:269–280. doi: 10.1080/1028415X.1998.11747237. [DOI] [PubMed] [Google Scholar]
- Attwood AS, Higgs S, et al. Differential responsiveness to caffeine and perceived effects of caffeine in moderate and high regular caffeine consumers. Psychopharmacology (Berl) 2007;190(4):469–477. doi: 10.1007/s00213-006-0643-5. [DOI] [PubMed] [Google Scholar]
- Backhaus J, Born J, et al. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem. 2007;14(5):336–341. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone J rH. Caffeine Consumption. Food Chem Toxicol. 1996;34:119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- Battig K BR, Martin JR, Feierabend JM. The effects of caffeine on physiological functions and mental performance. Experientia. 1984;40:1218–1223. doi: 10.1007/BF01946650. [DOI] [PubMed] [Google Scholar]
- Brice CF, Smith AP. Effects of caffeine on mood and performance: a study of realistic consumption. Psychopharmacology (Berl) 2002;164(2):188–192. doi: 10.1007/s00213-002-1175-2. [DOI] [PubMed] [Google Scholar]
- Carter AJ, O'Connor WT, et al. Caffeine enhances acetylcholine release in the hippocampus in vivo by a selective interaction with adenosine A1 receptors. J Pharmacol Exp Ther. 1995;273(2):637–642. [PubMed] [Google Scholar]
- Cattell RB. The effects of alcohol and caffeine on intelligent and associate performance. Br J Med Psychol. 1930;10:20–33. [Google Scholar]
- Christian J, Bickley W, Tarka M, Clayton K. Measures of free recall of 900 English nouns: Correlations with imagery, concreteness, meaningfulness, and frequency. Memory and Cognition. 1978;6:379–390. [Google Scholar]
- Cysneiros RM, Farkas D, et al. Pharmacokinetic and pharmacodynamic interactions between zolpidem and caffeine. Clin Pharmacol Ther. 2007;82(1):54–62. doi: 10.1038/sj.clpt.6100211. [DOI] [PubMed] [Google Scholar]
- Dimpfel W, Schober F, et al. The influence of caffeine on human EEG under resting conditions and during mental loads. Clin Investig. 1993;71(3):197–207. doi: 10.1007/BF00180102. [DOI] [PubMed] [Google Scholar]
- Drosopoulos S, Wagner U, et al. Sleep enhances explicit recollection in recognition memory. Learn Mem. 2005;12(1):44–51. doi: 10.1101/lm.83805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundation NS. Sleep in America Poll. 2005 [Google Scholar]
- Foundation NS. Sleep in America Poll. 2006 [Google Scholar]
- Foundation NS. Sleep in America Poll. 2006 [Google Scholar]
- Gais S, J Born. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci U S A. 2004;101(7):2140–2144. doi: 10.1073/pnas.0305404101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Molle M, et al. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22(15):6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Plihal W, et al. Early sleep triggers memory for early visual discrimination skills. Nature Neuroscience. 2000;3:1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- Greene AJ. Human hippocampal-dependent tasks: is awareness necessary or sufficient? Hippocampus. 2007;17(6):429–433. doi: 10.1002/hipo.20296. [DOI] [PubMed] [Google Scholar]
- Hollingworth HL. The influence of caffeine on mental and motor efficiency. Arch. Psychol. 1912;3:1–166. [Google Scholar]
- Jacobson BH EB. Effects of caffeine on simple reaction time and movement time. Avia Space Environment Med. 1987;58:1153–1156. [PubMed] [Google Scholar]
- Kaplan GB, Greenblatt DJ, et al. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J Clin Pharmacol. 1997;37(8):693–703. doi: 10.1002/j.1552-4604.1997.tb04356.x. [DOI] [PubMed] [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proceedings of the National Academy of Science of the United States of America. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy JS, von Moltke LL, et al. Validity of self-reports of caffeine use. J Clin Pharmacol. 1991;31(7):677–680. doi: 10.1002/j.1552-4604.1991.tb03756.x. [DOI] [PubMed] [Google Scholar]
- Killgore WD LE, Kamimori GH, Balkin TJ. Caffeine effects on risky decision making after 75 hours of sleep deprivation. Aviat Space Environ Med. 2007;78(10):957–962. doi: 10.3357/asem.2106.2007. [DOI] [PubMed] [Google Scholar]
- Korman M, Doyon J, et al. Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci. 2007;10(9):1206–1213. doi: 10.1038/nn1959. [DOI] [PubMed] [Google Scholar]
- Lieberman H, Tharion W, et al. Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Psychopharmacology. 2002;164(3):250–261. doi: 10.1007/s00213-002-1217-9. [DOI] [PubMed] [Google Scholar]
- Lieberman HR, Tharion WJ, et al. Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Sea-Air-Land. Psychopharmacology (Berl) 2002;164(3):250–261. doi: 10.1007/s00213-002-1217-9. [DOI] [PubMed] [Google Scholar]
- Lieberman HR, Wurtman RJ, et al. The effects of low doses of caffeine on human performance and mood. Psychopharmacology (Berl) 1987;92(3):308–312. doi: 10.1007/BF00210835. [DOI] [PubMed] [Google Scholar]
- Loke WH, Hinrichs JV, et al. Caffeine and diazepam: separate and combined effects on mood, memory, and psychomotor performance. Psychopharmacology (Berl) 1985;87(3):344–350. doi: 10.1007/BF00432719. [DOI] [PubMed] [Google Scholar]
- Lorist MM, J Snel, et al. Acute effects of caffeine on selective attention and visual search processes. Psychophysiology. 1996;33(4):354–361. doi: 10.1111/j.1469-8986.1996.tb01059.x. [DOI] [PubMed] [Google Scholar]
- Lorist MM, Tops M. Caffeine, fatigue, and cognition. Brain Cogn. 2003;53(1):82–94. doi: 10.1016/s0278-2626(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Lovett R. Coffee: The demon drink? New Scientist. 2005;24:2518–2522. [Google Scholar]
- MacPherson J, Sternhagen S, et al. Effect of caffeine, impulsivity, and gender on the components of text processing and recall. Experimental and Clinical Psychopharmacology. 1996;4(4):438–446. [Google Scholar]
- Mayes AR, Holdstock JS, et al. Relative sparing of item recognition memory in a patient with adult-onset damage limited to the hippocampus. Hippocampus. 2002;12(3):325–340. doi: 10.1002/hipo.1111. [DOI] [PubMed] [Google Scholar]
- Mednick S, Nakayama K, et al. Sleep-dependent learning: a nap is as good as a night. Nat Neurosci. 2003;6(7):697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- Mednick SC, Arman AC, et al. The time course and specificity of perceptual deterioration. Proc Natl Acad Sci U S A. 2005;102(10):3881–3885. doi: 10.1073/pnas.0407866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SC, Nakayama K, et al. The restorative effect of naps on perceptual deterioration. Nat Neurosci. 2002;5(7):677–681. doi: 10.1038/nn864. [DOI] [PubMed] [Google Scholar]
- Nawrot P, Jordan S, et al. Effects of caffeine on human health. Food Addit Contam. 2003;20(1):1–30. doi: 10.1080/0265203021000007840. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Daval JL, et al. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev. 1992;17(2):139–170. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE. 2007;2(4):e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroli E. Psychopharmacological aspects of coffee and of caffeine. Minerva Med. Guil. 1972;63:3319–3323. [PubMed] [Google Scholar]
- Plihal W, Born J. Effects of early and late nocturnal sleep on priming and spatial memory. Psychophysiology. 1999;36:571–582. [PubMed] [Google Scholar]
- Rickard TC, Cai DJ. 2007 [Google Scholar]
- Robertson EM, Pascual-Leone A, et al. Awareness modifies the skill-learning benefits of sleep. Curr Biol. 2004;14(3):208–212. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Richardson NJ, et al. Overnight caffeine abstinence and negative reinforcement of preference for caffeine-containing drinks. Psychopharmacology (Berl) 1995;120(4):457–462. doi: 10.1007/BF02245818. [DOI] [PubMed] [Google Scholar]
- Sagaspe P, Taillard J, et al. Aging and nocturnal driving: better with coffee or a nap? A randomized study. Sleep. 2007;30(12):1808–1813. doi: 10.1093/sleep/30.12.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer PK, Randazzo AC, et al. Laboratory and field studies of naps and caffeine as practical countermeasures for sleep-wake problems associated with night work. Sleep. 2006;29(1):39–50. doi: 10.1093/sleep/29.1.39. [DOI] [PubMed] [Google Scholar]
- Spriet LL. Caffeine and performance. Int J Sport Nutr. 1995;(5 Suppl):S84–S99. doi: 10.1123/ijsn.5.s1.s84. [DOI] [PubMed] [Google Scholar]
- Stricker JL, Brown GG, et al. The impact of sleep deprivation and task difficulty on networks of fMRI brain response. J Int Neuropsychol Soc. 2006;12(5):591–597. doi: 10.1017/S1355617706060851. [DOI] [PubMed] [Google Scholar]
- Tucker MA, Hirota Y, et al. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006 doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Turriziani P, Fadda L, et al. Recognition memory for single items and for associations in amnesic patients. Neuropsychologia. 2004;42(4):426–433. doi: 10.1016/j.neuropsychologia.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, et al. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, et al. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35(1):205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, et al. Practice with Sleep Makes Perfect: Sleep-Dependent Motor Skill Learning. Neuron. 2002;35(1):205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44(1):121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Zwyghuizen-Doorenbos A, Roehrs TA, et al. Effects of caffeine on alertness. Psychopharmacology (Berl) 1990;100(1):36–39. doi: 10.1007/BF02245786. [DOI] [PubMed] [Google Scholar]