Abstract
Heroin administration alters the induction of nitric oxide, a molecule known to play a critical role in immune function. Previous research has shown that these alterations can be conditioned to environmental stimuli that have been associated with drug administration. Little is known about the brain areas that mediate these effects; however, the basolateral amygdala (BLA) has been implicated in the formation of stimulus-reward associations within models of drug abuse. The present study sought to determine whether inactivation of the BLA would alter heroin's conditioned effects on the expression of inducible nitric oxide synthase (iNOS) and the pro-inflammatory cytokines, TNF-α and IL-1β, in the rat. The conditioning procedure involved repeated pairing of heroin with placement into a standard conditioning chamber. To test the conditioned response, animals were returned to the previously drug-paired environment six days after the final conditioning session. Prior to testing, animals received intra-BLA microinfusions of a mixture of the GABA agonists, muscimol and baclofen. Following removal from the chambers on test day, all animals received subcutaneous lipopolysaccharide to induce systemic expression of iNOS, TNF-α and IL-1β. Analyses using real-time RT-PCR indicated that inactivation of the BLA blocked the suppressive effect of heroin-associated environmental stimuli on iNOS induction and on the expression of the pro-inflammatory cytokines TNF-α and IL-1β in spleen and liver tissue. This study is important because it is the first to demonstrate that heroin's conditioned effects on pro-inflammatory mediators require the basolateral amygdala. These findings may have significant implications for the treatment of heroin users.
Keywords: Rat, iNOS, lipopolysaccharide, TNFα, IL-1β
Introduction
Exposure to drug cues is one of the key contributing factors to relapse mainly because these cues induce a wide variety of complex, classically conditioned physical and behavioral responses (Unnithan et al 1992; Derbas et al 2001). There has been increasing evidence from studies conducted on human subjects showing that drug-paired stimuli can cause intense craving, feelings of being ‘high’ and altered neural activity in drug users (Sideroff & Jarvik 1980; Ehrman et al. 1992). Current research in the area of drug addiction has focused on the critical task of reducing relapse by attempting to curb cue-induced drug seeking behavior, however, it is also imperative to take into account the widespread effects these cues may have on immune functioning and the ability of previous drug users to combat infectious disease while in recovery.
Research in our laboratory and others has shown that not only will administration of heroin suppress a number of basic immune parameters but many of these suppressive effects on the immune system may also be conditioned to environmental cues that predict drug availability. For instance, rats re-exposed to a previously morphine–paired environment display alterations in basic immune parameters similar to those seen upon actual morphine administration including decreased mitogen responsiveness of lymphocytes, decreased NK cell activity and suppressed IL-2 production (Coussons et al 1992; Saurer et al 2008). In line with these studies, it has been shown that heroin administration results in a profound reduction in LPS-induced nitric oxide expression (Lysle & How 2000) and this effect on nitric oxide may be conditioned to environmental stimuli that predict drug availability (Lysle & Ijames, 2002). In addition to effects on nitric oxide, there is evidence that opiate administration may also alter the production of the pro-inflammatory cytokines, TNF-α and IL-1β (Chao et al 1993; Pacifici et al 2000). Given the critical nature of these pro-inflammatory mediators in the initial response to infection it is imperative that we understand how heroin and heroin related cues may alter their effectiveness.
Little is known about the brain areas involved in these conditioned effects, however, the basolateral amygdala (BLA) has been implicated in the formation of stimulus-reward associations within models of drug abuse. For example, inactivation of the BLA blocks the ability of drug cues to re-instate drug seeking in rats that had been previously taught to self-administer cocaine (McLauglin & See 2003; Fuchs et al 2005). In a similar study, inactivation of the BLA was shown to abolish cue-induced reinstatement of drug seeking in rats taught to administer heroin (Fuchs & See 2002). The current experiment sought to establish the role of the basolateral amygdala in the conditioned effects of heroin on nitric oxide by quantifying iNOS expression in spleen and liver tissue following inactivation of the BLA. In addition, IL-1β and TNF-α were measured in these same tissues to determine whether exposure to stimuli previously paired with heroin would alter the expression of these cytokines and if inactivation of the BLA would have an effect on these conditioned alterations.
Materials and Methods
Animals
Male Lewis rats, weighing 225-250 g, were purchased from Charles River Laboratories (Raleigh, N.C., USA). Upon arrival, animals were housed individually in plastic cages in a colony room with a reversed light-dark (12h) cycle maintained through artificial illumination. Animals were allowed access to food and water ad libitum throughout the experiment except for the time spent in the conditioning chambers when food and water were not available. All animals were given a 2-week habituation period before the start of experimental manipulations and were handled regularly during this time. All procedures described were approved by the IACUC of the University of North Carolina at Chapel Hill and conformed to National Institutes of Health (NIH) Guidelines on the Care and Use of Laboratory Animals.
Drug Administration
Heroin (diacetylmorphine) was obtained from NIDA (Bethesda, MD) and dissolved in 0.9 % sterile saline. For all experiments, animals received a subcutaneous injection of heroin at a dose 1 mg/kg immediately prior to placement in the conditioning chamber on each of the five conditioning trial days. This dose was selected based on prior experiments in our laboratory showing that a 1 mg/kg dose of heroin alters LPS-induced iNOS mRNA expression in spleen and liver tissue and induces conditioning (Lysle & How, 2000; Lysle & Ijames, 2002; Szczytkowski & Lysle, 2007).
Surgeries and microinjections
Animals were anesthetized with 0.35 ml intramuscular injections of 1:1 (vol/vol) ketamine hydrochloride (100 mg/ml) mixed with xylazine (20 mg/ml) and placed into the stereotaxic apparatus. Animals were implanted with 26-gauge bilateral guide cannula (Plastics One, Roanoke, VA) directed towards the BLA (AP -2.5, ML±5.0, DV -6.6) or Caudate control region (AP -2.4, ML±4.5, DV -4.0). Coordinates are expressed as millimeters from bregma (Paxinos & Watson, 1986).
Animals were given a two week recovery period before the start of conditioning trials. On test day, animals received bilateral intracranial injections (0.3 μl/side infused over 1 min) of saline vehicle or a combination of GABA agonists, muscimol (0.03 nmol)/baclofen (0.3 nmol), 30 minutes prior to re-exposure to the conditioning chambers to determine whether the brain areas inactivated are necessary for the conditioned alterations in pro-inflammatory mediators. Injectors extended 2 mm beyond the tip of the cannula and were left in place for 1 minute after the injection to allow for proper infusion.
Procedures
Acquisition of conditioned response
All animals received five 60-min training sessions in which they were administered a subcutaneous injection of heroin (1 mg/kg) upon placement into a standard conditioning chamber which served as the conditioned stimulus. Training sessions were separated by 48 hours. The conditioning chambers (BRS/LVE, Laurel, Md., USA) were contained in a room separate from the animal colony. Chambers were fitted with a metal grid floor design and cedar bedding to create an environment distinct from that of the home cage and to provide both olfactory and tactile cues for conditioning. All conditioning took place during the dark phase of the light cycle and the conditioning chambers were kept dark to minimize effects on circadian rhythms.
Testing of expression of conditioned response
The test day took place six days following the final conditioning session. Thirty minutes prior to testing, two groups of animals (Mus/Bac-BLA; Mus/Bac-Caud) received intra-BLA (or intra-caudate) microinfusions of the combination of GABA agonists, muscimol and baclofen, to temporarily inactivate the BLA (or caudate). Control animals received intra-BLA (or intra-caudate) microinfusions of saline vehicle (Saline-BLA; Saline-Caud). To test the expression of the conditioned response, two groups of animals were re-exposed to the conditioned stimulus (i.e.; the conditioning chambers) without drug to determine whether the conditioned stimulus alone would induce alterations in pro-inflammatory mediators. Animals re-exposed to the conditioning chambers on test day are indicated in the figures as CS. The remaining animals (home cage, HC) were returned to the home cage following microinfusions and served as controls. Experimental treatment of each group is outlined in Table 1. After the 60-min re-exposure, the animals were removed from the chambers and given a subcutaneous injection of LPS (1000 μg/kg) to induce iNOS production in spleen and liver tissue. Home cage animals also received LPS at this time. Six hours after LPS administration all animals were sacrificed and samples of spleen, liver and blood were collected for analysis. The 6-hr timepoint was selected based on previous research in our laboratory showing maximal iNOS induction at six hours following LPS administration (Lysle & How, 2000).
Table 1.
Schematic representation of the behavioral and pharmacological manipulations employed for each treatment group.
| Treatment groups by experiment | ||||
|---|---|---|---|---|
| BLA: | Days 1-9 | Test Day (D15): | BLA Microinfusion | CS Exposure |
| HC Saline-BLA | Conditioning | Saline | Home Cage | |
| CS Saline-BLA | Conditioning | Saline | CS Re-Exposure | |
| HC Mus/Bac-BLA | Conditioning | Muscimol/Baclofen | Home Cage | |
| CS Mus/Bac-BLA | Conditioning | Muscimol/Baclofen | CS Re-Exposure | |
|
| ||||
| Caudate: | Days 1-9 | Test Day (D15): | Caudate Microinfusion | CS Exposure |
| HC Saline-Caud | Conditioning | Saline | Home Cage | |
| CS Saline-Caud | Conditioning | Saline | CS Re-Exposure | |
| HC Mus/Bac-Caud | Conditioning | Muscimol/Baclofen | Home Cage | |
| CS Mus/Bac-Caud | Conditioning | Muscimol/Baclofen | CS Re-Exposure | |
Real Time RT-PCR
To determine iNOS expression, real time RT-PCR was performed on tissue samples from the spleen and liver. Total RNA was extracted from a section of each of the tissues using TRI-Reagent (Molecular Research Center, Cincinnati, OH), a modification of the original method described by Chomczynski and Sacchi (1987). RNA was quantified spectrophotometrically (GeneQuant II, Pharmacia-Biotech, Piscataway, NJ, USA). For the RT-PCR, reverse transcription is performed using Oligo(dT)18 primer and Moloney Murine Leukemia Virus-Reverse transcriptase following the protocol of the Advantage RT-for-PCR Kit from Clontech (Palo Alto, CA, USA).
PCR amplifications were performed using the Fast Start™ DNA Master SYBR Green I Real-Time PCR Kit (Roche) and the LightCycler instrument (Roche). A master mix containing all reaction components was prepared for all reactions, with each reaction using a 20 ml mix placed in glass capillary tubes specifically designed for use in the LightCycler system. The PCR primer set for iNOS, 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ and 5′-GGGTGTCAGAGTCTTGTGCCTTTGG-3′ was synthesized by the Nucleic Acids Core Facility (Lineberger Cancer Center, UNC-Chapel Hill). Copy numbers were generated from an external standard curve. Amplifications were carried out for 40 cycles and curves showing fluorescence at each cycle were determined by the computer software (Roche). Samples were pre-incubated for 10 minutes at 95° C to activate the Fast-Start Taq DNA polymerase. The cycle temperatures were 95, 60, and 72°C for the denaturing, annealing, and extending, respectively. The cycle times were 15, 5, and 25 s for the denaturing, annealing, and extending, respectively. Fluorescence level was determined at the end of the extending phase for each cycle of PCR. The analysis of the fluorescence level in standards and samples over the course of 40 cycles was used to derive the number of copies of the target molecule in each sample. Additionally, assessments of housekeeping gene expression, cyclophilin, were made to assure comparable quality of RNA among samples. The sequence of the cyclophilin primers was 5′-CCAAGACTGAGTGGCT-3′ and 5′-AGATTACAGGGTATTGCG-3′. The data are expressed as a copy number of iNOS (per 10ng cDNA) based on the standard curve using the Lightcycler software (Roche). Furthermore, to confirm the nature of amplification product, a melt curve analysis was conducted after the final PCR cycle. This analysis involved denaturing the products by slowly heating them to 95°C, during which fluorescence is continuously measured.
ELISA
For IL-1β and TNF-α protein determinations, protein was extracted from a section of each tissue using freeze/thaw lysis in tris-buffer containing antiproteinases. Protein was quantified spectrophotometrically (Bio-Tek, Model EL312 kinetic reader, Winooski, VT, USA) using Bio-Rad protein dye. The BioSource International, Inc. (Carlsbad, CA) rat IL-1β and TNF-α ELISA test kits were used to determine the levels of IL-1β or TNF-α protein in each tissue sample. Briefly, samples and standards were added to microtiter wells coated with antibody that recognizes IL-1β or TNF-α and incubated at room temperature. Wells were washed extensively and then incubated with biotinylated antibody, followed by a second wash and then incubation with Streptavidin-HRP. After the final washing, a chromagen substrate solution was added which reacted with the bound enzyme to produce color. The color intensity developed proportionally to the amount of IL-1β or TNF-α present in each sample. The enzyme reaction was stopped after 30 minutes, and the absorbance at 450 nm was measured with a Bio-Tek (Winooski, VT) Model EL312 kinetic reader. A standard curve was obtained by plotting the absorbance versus the corresponding concentrations of the supplied standards.
Nitrite/Nitrate Assay
The level of nitrite/nitrate in plasma samples was assessed using the Greiss reagent assay. Nitrate and nitrite are formed non-enzymatically when nitric oxide is exposed to oxygen, thus plasma levels of these products indicate the level of nitric oxide production. Total nitrite/nitrate levels was determined by the conversion of nitrate to nitrite utilizing nitrate reductase in the presence of NADPH and flavin adenine dinucleotide, and then an assessment using Greiss reagent. Briefly, 6 μl of plasma diluted in 44 μl of dH2O was incubated in the dark for 90-min with 10 μl of nitrate reductase (1.0 unit/ml), 20 μl of a 0.31 M phosphate buffer (ph 7.5), 10 μl of 0.86 mM NADPH (Sigma), and 10 μl of a 0.11 mM flavin adenine dinucleotide in individual wells of a 96-well plate. Then, 200 ml of Greiss reagent consisting of a 1:1 (v/v) solution 1% sulfanilamide in 5.0% phosphoric acid and 0.1% N-(1-napthyl) ethyl-enedamine dihydrochloride in distilled water was added to the samples. The color developed for ten minutes at room temperature after which the absorbance was determined using a spectrophotometer set at 550 nm. All reactions were carried out in triplicate. The total micromolar concentration of nitrite was determined for each sample based on a standard curve. Recovery of nitrate was greater than 95% using this assay.
Statistical Analysis
Analysis of variance was performed on all data sets. When the overall ANOVA showed significant effects, post hoc comparisons were made using Tukey's test to compare individual treatment groups. All analyses were conducted with the level of significance set at p < 0.05.
Histology
To confirm proper cannula placement Alcian blue dye was infused via the cannula following sacrifice. Brains were then extracted and post-fixed in a 4% paraformaldehyde solution. Following fixation the brains were transferred to a 30% sucrose solution for cryoprotection and then frozen at -80° C until further analysis. Coronal sections (50 μm) were taken and stained with cresyl violet for verification of cannula placement. Animals with cannula placement outside of the targeted region were removed from the analyses. Figure 1 shows a schematic diagram illustrating the most ventral point of the injection cannula for each animal in the study. Figure 2 is a representative photomicrograph showing the injection cannula tract to the BLA.
Figure 1.
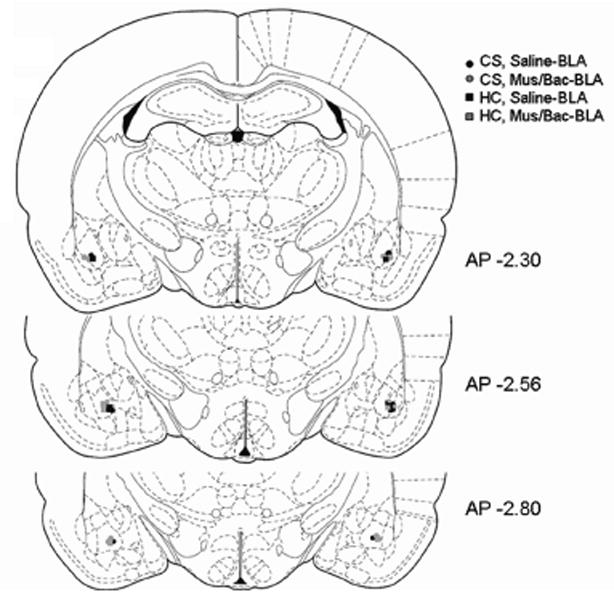
BLA injection cannula placement was verified by cresyl violet stained coronal sections. Symbols in the diagram represent the most ventral point of the injection cannula for each animal in the study based on the atlas of Paxinos and Watson (1998). AP coordinates indicate the distance from bregma in mm. (CS, Saline-black circles; CS, Mus/Back-gray circles; HC, Saline-black squares; HC, Mus/Bac-gray squares; n=4 per group)
Figure 2.
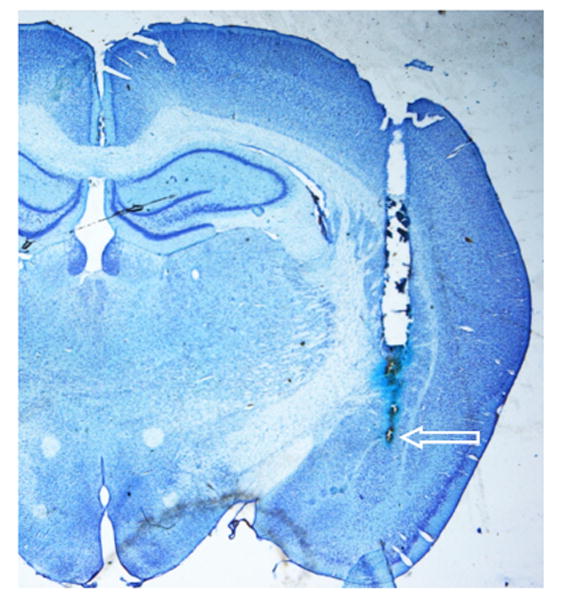
Representative photomicrograph showing the cannula tract and site of injection within the BLA on a cresyl violet stained coronal section. The arrow indicates the most ventral point of the injector cannula tract.
Results
Effects of BLA inactivation on conditioned heroin-induced suppression of nitric oxide expression
The first study investigated whether inactivation of the BLA or caudate control region immediately before testing (exposure to the previously drug-paired environment) would alter the conditioned effects of heroin on nitric oxide production. Six days following the final conditioning session, half of the animals were re-exposed to the previously heroin-paired environment for 60 min (Conditioned Stimulus, CS) while the other half remained in the home cage and served as control groups (Home Cage, HC). In order to temporarily inactivate the brain regions under investigation, animals in the Mus/Bac-BLA and Mus/Bac-Caud groups received microinfusions of the muscimol/baclofen combination directly into the BLA or Caudate, respectively, 30 min prior to testing. The animals in the Saline-BLA and Saline-Caud groups received microinfusions of saline into these same areas.
Figure 3A shows the results of each treatment on LPS-induced expression of iNOS mRNA in the spleen and liver following inactivation of the BLA. Analysis of iNOS copy number in spleen and liver revealed a significant main effect of treatment [F(4,15)=10.711, P<0.01; F(4,15)=9.432, p<0.01]. Moreover, there was a significant difference in iNOS mRNA copy number between the animals exposed to the conditioned stimulus on test day who had received intra-BLA saline (CS, Saline-BLA) and the animals who remained in the home cage on test day and received intra-BLA saline (HC, Saline-BLA). These differences were evident in both tissues (p<0.05) which supports our earlier findings indicating that exposure to a previously heroin-paired environment alters the expression of iNOS mRNA. Most importantly, there were no significant differences between the HC, Saline-BLA group and the experimental group that received intra-BLA microinjections of muscimol/baclofen before exposure to the conditioned stimulus on test day (CS, Mus/Bac-BLA) demonstrating the ability of BLA inactivation to reduce the conditioned response. Furthermore, there were no significant differences between the HC, Saline-BLA group and the group that received intra-BLA muscimol and baclofen but remained in the home cage on test day (HC, Mus/Bac-BLA) indicating that these results are specific to administration of drug and not due to ancillary effects of the microinjections. There were also no differences found in expression of the housekeeping gene, cyclophilin, between any of the groups indicating that the alterations in mRNA were specific for iNOS and not the result of an overall reduction in RNA expression or production (data not shown).
Figure 3.
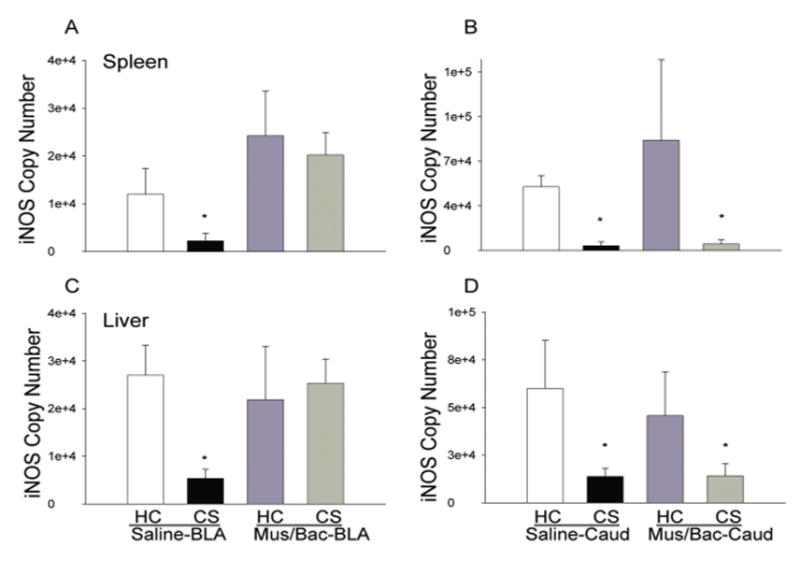
Effect of treatments on LPS-induced expression of iNOS mRNA in the spleen and liver as determined by real-time RT-PCR. Re-exposure to the previously heroin-paired environment (CS, conditioned stimulus) reduced iNOS mRNA in both spleen and liver tissue and this reduction was attenuated by inactivation of the BLA (A) but not affected by inactivation of the caudate control region (B). Data are expressed as iNOS copy number per 10 ng cDNA based on a standard curve using Roche LightCycler software. (*) Asterick indicates a significant difference (p<0.05) from the HC (Home cage), Saline-BLA/Caud group.
Figure 3B also shows the effects of each treatment on LPS-induced expression of iNOS mRNA in the spleen and liver following inactivation of the caudate control region. Analysis of iNOS copy number in spleen and liver revealed a significant main effect of treatment [F(4,15)=6.458, p<0.05; F(4,15)=6.868, p<0.01]. There was a significant difference between the CS, Saline-Caud group and the HC, Saline-Caud group in both tissues (p<0.05) which is in agreement with the data from our previous research. Most importantly, there was also found to be a significant difference between the HC, Saline-Caud group and the experimental group that received intra-caudate microinjections of muscimol/baclofen that was re-exposed to the conditioned stimulus on test day (CS, Mus/Bac-Caud) indicating that this region is not involved in the conditioned effects of heroin on iNOS mRNA expression (p<0.05). Furthermore, there were no significant differences between the HC, Saline-Caud group and the HC, Mus/Bac-Caud group indicating that these results are specific to administration of drug and not due to ancillary effects of the microinjections.
The data in Figure 4 show the effects of BLA (A) or caudate control region (B) inactivation on serum nitrite/nitrate levels. Nitrite and nitrate are byproducts of nitric oxide production and levels of these markers serve as an indirect analysis of nitric oxide levels in the blood. For the BLA inactivation experiment, examination of nitrite/nitrate levels in the serum revealed a significant main effect of treatment [F(4,15)=13.939, p<0.01] and a significant difference between the CS, Saline-BLA group and the HC, Saline-BLA group (p<0.05) indicating that exposure to a previously heroin paired environment reduces the production of nitric oxide. There were no significant differences between the HC, Saline-BLA group and the CS, Mus/Bac-BLA group demonstrating that inactivation of the BLA reduces the conditioned effects of heroin on nitric oxide. Taken together, these findings show that inactivation of the BLA will reduce the conditioned effects of heroin on nitric oxide production.
Figure 4.
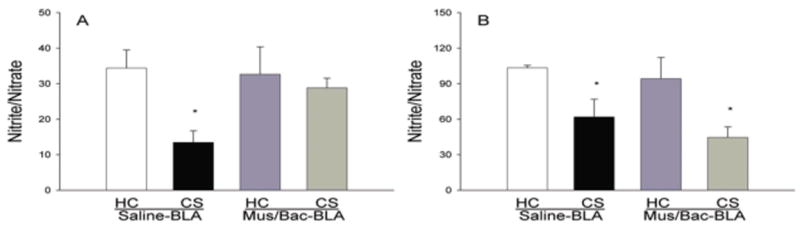
Effect of treatments on serum nitrite/nitrate levels as determined by the Greiss reagent assay. Re-exposure to the previously heroin-paired environment (CS, conditioned stimulus) reduced serum levels of nitrite/nitrate and this reduction was attenuated by inactivation of the BLA (A) but not affected by inactivation of the caudate control region (B). The data are expressed as the micromolar concentration of nitrite/nitrate. (*) Asterick indicates a significant difference (p<0.05) from the HC (Home cage), Saline-BLA/Caud group.
For the caudate control region experiment, analysis of variance revealed a significant main effect of treatment [F(4,15)=16.767, p<0.01] and a significant difference between both the CS, Saline-Caud group and the HC, Saline-Caud group (p<0.01) and the CS, Mus/Bac-Caud group and the HC, Saline-Caud group (p<0.01). These results indicate that exposure to a previously heroin-paired environment reduces the production of nitric oxide and this effect is not attenuated by inactivation of this region of the caudate.
Effects of BLA inactivation on conditioned heroin-induced suppression of pro-inflammatory cytokines
Figure 5 shows that the suppressive effect of heroin on the pro-inflammatory cytokine, IL-1β, can be conditioned to environmental stimuli and this conditioned effect is reduced by inactivation of the BLA. This effect was evident at both the protein and mRNA level and in both spleen and liver tissue. Analysis revealed a main effect of treatment on both mRNA and protein levels in the spleen [F(4,15)=6.546, p<0.01; F(4,15)=38.871, p<0.01] and liver [F(4,15)=7.993, p<0.01; F(4,15)=21.964, p<0.01]. In line with our previous experiments, the CS, Saline-BLA group exhibited significantly lower levels of IL-1β mRNA and protein in both the spleen and liver (p<0.05) as compared to the HC, Saline-BLA group. The CS, Mus/Bac-BLA group was not significantly different from the HC, Saline-BLA group indicating that inactivation of the BLA was able to reverse the conditioned effects of heroin on IL-1β mRNA and protein expression.
Figure 5.
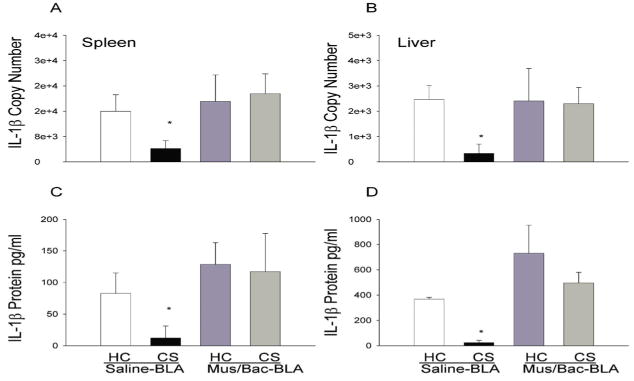
Effect of treatments on LPS-induced expression of IL-1β mRNA and protein in the spleen (A, C) and liver (B, D) as determined by real-time RT-PCR. Re-exposure to the previously heroin-paired environment (CS, conditioned stimulus) reduced IL-1β mRNA and protein in both spleen and liver tissue and this reduction was attenuated by inactivation of the BLA. The RT-PCR data (A, B) are expressed as IL-1β copy number per 10 ng cDNA based on a standard curve using Roche LightCycler software. The ELISA data (C, D) are expressed as picograms of protein per ml. (*) Asterick indicates a significant difference (p<0.05) from the HC (Home cage), Saline-BLA group.
Figure 6 shows that the suppressive effect of heroin on the pro-inflammatory cytokine, IL-1β, can be conditioned to environmental stimuli and this conditioned effect is not altered by inactivation of the caudate control region. This effect was evident at both the protein and mRNA level in the spleen and at the protein level in liver tissue. Since the time of tissue collection was optimized for iNOS analysis it is not surprising that there is no significant effect at this timepoint in liver IL-1β mRNA levels. Since the effect is evident at the protein level this suggests that it might also have been present at the mRNA level at an earlier timepoint. Analysis revealed a main effect of treatment on both mRNA and protein levels in the spleen [F(4,15)=20.141, p<0.01; F(4,15)=21.585, p<0.01] and on protein in the liver [F(4,15)=7.993, p<0.01]. In line with our previous experiments, the CS, Saline-Caud group exhibited significantly lower levels of IL-1β mRNA and protein in the spleen and in protein in the liver (p<0.05) as compared to the HC, Saline-Caud group. The CS, Mus/Bac-BLA group also exhibited significantly lower IL-1β protein and mRNA (except for liver mRNA which shows the same trend but is not significant) when compared with the HC, Saline-Caud group indicating that inactivation of the caudate control region was not able to reverse the conditioned effects of heroin on IL-1β mRNA and protein expression.
Figure 6.
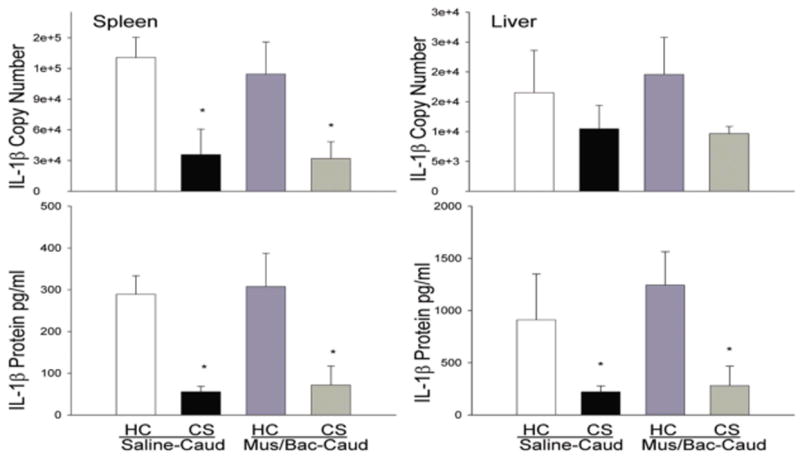
Effect of treatments on LPS-induced expression of IL-1β mRNA and protein in the spleen (A, C) and liver (B, D) as determined by real-time RT-PCR. Re-exposure to the previously heroin-paired environment (CS, conditioned stimulus) reduced IL-1β mRNA and protein in the spleen and IL-1β protein in liver tissue and this reduction was not altered by inactivation of the caudate control region. The RT-PCR data (A, B) are expressed as IL-1β copy number per 10 ng cDNA based on a standard curve using Roche LightCycler software. The ELISA data (C, D) are expressed as picograms of protein per ml. (*) Asterick indicates a significant difference (p<0.05) from the HC (Home cage), Saline-Caud group.
Figure 7 shows that the suppressive effect of heroin on the pro-inflammatory cytokine, TNF-α, can also be conditioned to environmental stimuli and, again, this conditioned effect is reduced by inactivation of the BLA. This effect was evident at both the protein and mRNA level in the spleen and liver with the exception of TNF-α mRNA in the liver. As with IL-1β, the lack of a significant effect in liver TNF-α mRNA is not surprising and the significance at the protein level suggests that an effect may have been evident at an earlier timepoint. Analysis revealed a main effect of treatment on both mRNA and protein levels in the spleen [F(4,15)=6.946, p<0.01; F(4,15)=27.644, p<0.01] and on protein levels in the liver [F(4,15)=7.313, p<0.01]. In line with our previous experiments, the CS, Saline-BLA group exhibited significantly lower levels of TNF-α mRNA in the spleen (p<0.05) and significantly lower levels of TNF-α protein in both the spleen and liver (p<0.05) when compared to the HC, Saline-BLA group.
Figure 7.
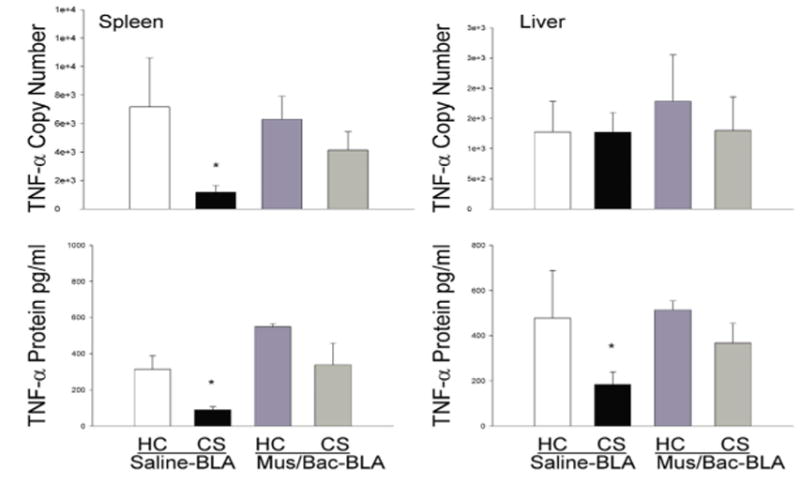
Effect of treatments on LPS-induced expression of TNF-α mRNA and protein in the spleen (A, C) and liver (B, D) as determined by real-time RT-PCR. Re-exposure to the previously heroin-paired environment (CS, conditioned stimulus) reduced TNF-α mRNA and protein in the spleen and TNF-a protein in liver tissue and this reduction was attenuated by inactivation of the BLA. The RT-PCR data (A, B) are expressed as TNF-α copy number per 10 ng cDNA based on a standard curve using Roche LightCycler software. The ELISA data (C, D) are expressed as picograms of protein per ml. (*) Asterick indicates a significant difference (p<0.05) from the HC (Home cage), Saline-BLA group.
Figure 8 shows that the suppressive effect of heroin on the pro-inflammatory cytokine, TNF-α, is not altered by inactivation of the caudate control region. The suppressive effect of conditioning was evident at both the protein and mRNA level. Analysis revealed a main effect of treatment on both mRNA and protein levels in the spleen [F(4,15)=7.605, p<0.01.; F(4,15)=7.779, p<0.01] and liver [F(4,15)=11.494, p<0.01; F(4,15)=12.655, p<0.01]. In line with our previous experiments, the CS, Saline-Caud group exhibited significantly lower levels of TNF-α mRNA and protein in the spleen and in the liver when compared to the HC, Saline-Caud group. Inactivation of the caudate control region had no effect on this suppression as the CS, Mus/Bac-Caud group also showed significantly lower levels of TNF-α protein and mRNA as compared to the HC, Saline-Caud group (p<0.05).
Figure 8.
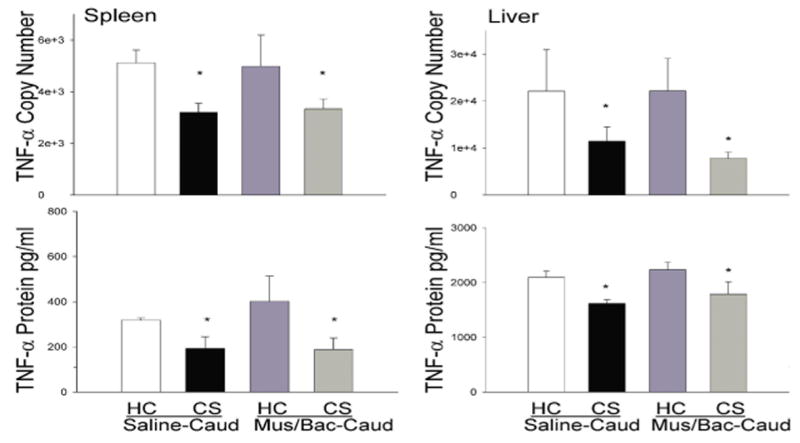
Effect of treatments on LPS-induced expression of TNF-α mRNA and protein in the spleen (A, C) and liver (B, D) as determined by real-time RT-PCR. Re-exposure to the previously heroin-paired environment (CS, conditioned stimulus) reduced TNF-α mRNA and protein in both spleen and liver tissue and this reduction was not attenuated by inactivation of the caudate control region. The RT-PCR data (A, B) are expressed as TNF-α copy number per 10 ng cDNA based on a standard curve using Roche LightCycler software. The ELISA data (C, D) are expressed as picograms of protein per ml. (*) Asterick indicates a significant difference (p<0.05) from the HC (Home cage), Saline-Caud group.
Discussion
Previous research in our laboratory has shown that exposure to a previously drug-paired environment induces alterations in nitric oxide production similar to those observed upon actual drug administration (Lysle & Ijames, 2002; Szczytkowski & Lysle, 2007). The data presented here are the first to demonstrate that the suppressive effects of heroin on the pro-inflammatory cytokines, TNF-α and IL1-β, may also be conditioned to environmental stimuli. Both Interleukin-1 beta (IL-1β) and Tumor necrosis factor-alpha (TNF-α) are pro-inflammatory cytokines involved in host defense. When secreted, both IL-1β and TNF-α have widespread effects in the host including altering the thermoregulatory setting in the hypothalamus to produce fever and increasing the expression of adhesion factors on endothelial cells to promote the transmigration of leukocytes to sites of infection. In addition, it appears that both TNF-α and IL-1β are highly involved in LPS-induced septic shock. Rats with LPS-induced septic shock exhibited a peak in TNF-α mRNA in plasma at one hour post-infection with levels beginning to decline by two hours after LPS administration. Similarly, IL-1β peaked at one hour post-LPS and gradually returned to baseline over nine hours (Lin et al, 2006). The samples collected in this study were taken at 6 hours post-LPS injection to optimize for LPS-induced nitric oxide production. The ability of these manipulations to detect a significant change in IL-1β and TNF-α demonstrates the robustness of these effects. Furthermore, given the time course of these effects the fact that some of the data failed to reveal significant differences between treatment groups in levels of TNF-α or IL-1β mRNA in the liver is not surprising. However, the significant reduction in TNF-α and IL-1β protein in liver tissue detected by the ELISA suggests that mRNA levels for these cytokines may have been previously suppressed in the group that was re-exposed to the conditioned stimulus on test day but may have rebounded by the 6-hour timepoint at which they were analyzed.
Given the multifaceted and complex involvement of these cytokines, as well as nitric oxide, in the initial immune response to infectious challenge it is essential to understand the mechanisms and neural circuitry through which heroin's conditioned alterations of these pro-inflammatory mediators are controlled. The results presented here provide the first evidence that inactivation of the BLA can reduce or even eliminate the conditioned suppressive effects of heroin on nitric oxide induction and on the expression of the pro-inflammatory cytokines, TNF-α and IL-1β. This attenuation appears to encompass several organ systems as it is found in both spleen and liver tissues as well as in serum levels of nitrite/nitrate. In addition, the attenuation is apparent at both the mRNA and protein levels showing that the expression as well as the production of these mediators is altered. The use of several control groups further demonstrates that these results are specific to the manipulations used and not related to ancillary effects of the conditioning or surgical procedures. Moreover, in order to ensure that these results were specific to the BLA, a control experiment was conducted in which an area of the caudate situated dorsal to the BLA was inactivated. The inactivation of this region of the caudate did not produce an attenuation of the conditioned response demonstrating that the results are specific to the BLA. These findings are consistent with prior investigations showing that heroin-induced alterations in immune status may be conditioned to drug-paired stimuli (Lysle & Ijames, 2002) and that these alterations are indeed a form of associative learning as they are susceptible to both extinction and latent inhibition (Szczytkowski & Lysle, 2007). In these previous investigations, animals received subcutaneous injections of heroin upon placement in a distinctive environment. Upon re-exposure to that environment, in the absence of drug, the subjects exhibited a reduction in nitric oxide production similar to what is observed with heroin treatment. Repeated exposure to the distinctive environment without drug, either pre- (latent inhibition) or post-conditioning (extinction) abolished this conditioned effect. The present study extends these previous findings by showing that the basolateral amygdala, an area known to be involved in associative learning processes, is required for the expression of these conditioned effects.
These results suggest that there exists a neural circuit, of which the BLA is an integral component, which mediates these conditioned alterations in immune measures. Given the wealth of research showing the involvement of the BLA in conditioned responses, it is not surprising that this area would be required for the expression of conditioned suppression of pro-inflammatory mediators. Numerous investigators have demonstrated a role of the BLA in associative learning. For example, lesions of the BLA block acquisition of classical eye blink conditioning (Blankenship et al., 2005), inhibit conditioned fear responses to aversive stimuli in rats (Lee et al., 2005), and block acquisition of the motivational value of an appetitive US (Setlow et al., 2002). In addition, learning of an inhibitory avoidance task involves neuronal activity within the BLA (Chang et al., 2005) as does olfactory fear conditioning (Sevelinges et al., 2004) and conditioning of odorant attractiveness in female mice (Moncho-Bogani et al., 2005). Furthermore, the BLA has been implicated in the formation of new, and in the utilization of already established, stimulus-reward associations within models of drug abuse. For example, inactivation of the BLA inhibits the reacquisition of heroin seeking in a test of conditioned place preference (Rizos et al., 2005). Similarly, it has been demonstrated that inactivation of the BLA abolishes the expression of CS-induced reinstatement of heroin-seeking behavior in rats (Fuchs & See, 2002). In addition, it has been shown that animals receiving morphine paired with a distinct environment exhibit Fos protein expression, a marker of neural activation, within both the BLA and the central nucleus of the amygdala during a test of conditioned place preference (Harris & Ashton-Jones, 2003).
The exact mechanism through which conditioned drug cues exert their effects on pro-inflammatory mediators remains unknown as does the means by which the BLA alters these effects. Studies suggest that it is unlikely the BLA itself has a direct effect on immune parameters as lesions and/or inactivation of this area do not seem to induce changes in immune reactivity (Jurkowski et al 2001; Grijalva et al 1990). This is consistent with the data reported here since inactivation of the BLA, by itself, did not alter the induction of pro-inflammatory mediators. While the BLA may not directly alter immune parameters, previous work in our laboratory and others has shown several brain areas to which the BLA is connected that have direct effects on the immune system including the central amygdala and the nucleus accumbens. For instance, Hayley et al (2002) reported increased monoamine utilization in the central amygdala in response to systemic administration of TNF-a suggesting that this area of the amygdala is sensitive to peripheral changes in cytokine production. In addition, research has shown that systemic LPS administration induces c-fos expression in the central amygdala (Rivest & LaFlamme, 1995; Sagar et al 1995; Tkacs & Li, 1999) and infusion of dopamine D1 agonists directly into this area results in increased Con-A stimulated splenocyte proliferation (Nistico et al 1994; Caroleo et al 1998). The BLA sends extensive projections to the central amygdala and the communication between these two areas is thought to create a circuit by which many conditioned responses are mediated (Pare et al 1995; Royer et al 1999; Likhtik et al 2008). Likewise, studies have shown that interactions between the BLA and the nucleus accumbens may be necessary for the establishment and expression of some conditioned responses including cocaine seeking induced by conditioned drug cues (Setlow et al, 2002; Di Ciano & Everitt, 2004). In addition, infusion of dopamine D1 antagonists into the nucleus accumbens shell blocks morphine-induced suppression of NK cell cytotoxicity while infusion of a D1 agonist recreates this suppression (Saurer et al 2006). These data suggest that dopamine activity within the nucleus accumbens is both necessary and sufficient for the induction of immune alterations. Since inactivation of the BLA attenuates the conditioned effects of heroin on pro-inflammatory mediators it is possible that information regarding the conditioned stimulus is routed through the BLA to the nucleus accumbens and it is actually the nucleus accumbens that directly influences immune alterations.
Considering the increased susceptibility to infection that occurs with opioid use it is possible that the conditioned alterations in certain immune parameters that occur in response to exposure to drug-paired cues might also compromise immune function and increase susceptibility to disease. The host's initial response to an immune challenge is a complex and highly regulated process. Some of the key mediators involved in the initial response to potential pathogens are inducible nitric oxide, IL-1β, and TNF-α. Each of these immune system components is responsible for numerous processes that together make up the response that is necessary to combat infectious agents and to prepare the site of infection for repair and recovery. These results are important, not only because they provide the first demonstration that the suppression of pro-inflammatory cytokines can be conditioned to environmental stimuli paired with heroin administration, but also because they demonstrate that the expression of the conditioned effects of heroin on pro-inflammatory mediators require the basolateral amygdala. These effects and the brain areas that control them need to be factored into the comprehensive treatment of opiate users.
Acknowledgments
This work was supported by United States Public Service Grant DA13371 and Research Scientist Awards (DA00334 and DA21467) from the National Institute on Drug Abuse. The authors would like to thank Stephanie Ijames, Dr. Timothy Saurer and Dr. Rita Fuchs Lokensgard for their expert technical assistance.
Abbreviations
- BLA
Basolateral amygdala
- iNOS
inducible nitric oxide synthase
- CS
conditioned stimulus
- HC
home cage
- NPY
neuropeptide Y
References
- Blankenship MR, Huckfeldt R, Steinmetz JJ, Steinmetz JE. The effects of amygdala lesions on hippocampal activity and classical eyeblink conditioning in rats. Brain Res. 2005;1035:120–30. doi: 10.1016/j.brainres.2004.11.061. [DOI] [PubMed] [Google Scholar]
- Caroleo MC, Arbirio M, Di Francesco P, Pulvirenti L, Garaci E, Nistico G. Cocaine induced T cell proliferation in the rate: role of amygdala dopamine D1 receptors. Neurosci Lett. 1998;256(2):61–4. doi: 10.1016/s0304-3940(98)00758-7. [DOI] [PubMed] [Google Scholar]
- Chang CH, Liang KC, Yen CT. Inhibitory avoidance learning altered ensemble activity of amygdaloid neurons in rats. Eur J Neurosci. 2005;21:210–8. doi: 10.1111/j.1460-9568.2004.03821.x. [DOI] [PubMed] [Google Scholar]
- Chao CC, Molitor TW, Close K, Hu S, Peterson PK. Morphine inhibits the release of tumor necrosis factor in human peripheral blood mononuclear cell cultures. Int J Immunopharmacol. 1993;15:447–53. doi: 10.1016/0192-0561(93)90057-6. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O'Brien CP. Abstinent Opiate Abusers Exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. Br J Addict. 1986;81:655–660. doi: 10.1111/j.1360-0443.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Coussons ME, Dykstra LA, Lysle DT. Pavlovian conditioning of morphine-induced alterations of immune status. J Neuroimmunol. 1992;39:219–30. doi: 10.1016/0165-5728(92)90256-k. [DOI] [PubMed] [Google Scholar]
- Derbas AN, al-Haddad MK. Factors associated with immediate relapse among Bahraini heroin abusers. East Mediterr Health J. 2001;7(3):473–80. [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24(32):7167–73. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology. 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 2002;160:425–33. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- Grijalva CV, Levin ED, Morgan M, Roland B, Martin FC. Contrasting effects of centromedial and basolateral amygdaloid lesions on stress-related responses in the rat. Physiol Behav. 1990;48(4):495–500. doi: 10.1016/0031-9384(90)90289-g. [DOI] [PubMed] [Google Scholar]
- Harris GC, Ashton-Jones G. Enhanced morphine preference following prolonged abstinence: association with increased Fos expression in the extended amygdala. Neuropsychopharmacology. 2003;28(2):292–299. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- Hayley S, Wall P, Anisman H. Sensitization to the neuroendocrine, central monoamine and behavioural effects of murine tumor necrosis factor-alpha: peripheral and central mechanisms. Eur J Neurosci. 2002;15(6):1061–76. doi: 10.1046/j.1460-9568.2002.01936.x. [DOI] [PubMed] [Google Scholar]
- Jurkowski M, Trojniar W, Borman A, Ciepieleweski Z, Siemion D, Tokarski J. Peripheral blood natural killer cell cytotoxicity after damage to the limbic system in the rat. Brain Behav Immun. 2001;15(1):93–113. doi: 10.1006/brbi.2000.0602. [DOI] [PubMed] [Google Scholar]
- Lee JL, Dickinson A, Everitt BJ. Conditioned suppression and freezing as measures of aversive Pavlovian conditioning: effects of discrete amygdala lesions and overtraining. Behav Brain Res. 2005;159:221–33. doi: 10.1016/j.bbr.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454(7204):642–5. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin NT, Yang FL, Lee RP, Cheng TC, Chen HI. Nitric oxide synthase mediates cytokine release: The time course in conscious and septic rats. Life Sciences. 2006;78:1038–43. doi: 10.1016/j.lfs.2005.05.091. [DOI] [PubMed] [Google Scholar]
- Lysle DT, How T. Heroin modulates the expression of inducible nitric oxide synthase. Immunopharmacology. 2000;46:181–192. doi: 10.1016/s0162-3109(99)00172-1. [DOI] [PubMed] [Google Scholar]
- Lysle DT, Ijames SG. Heroin-associated environmental stimuli modulate the expression of inducible nitric oxide synthase in the rat. Psychopharmacology. 2002;164:416–422. doi: 10.1007/s00213-002-1208-x. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Moncho-Bogani J, Martinez-Garcia F, Novejarque A, Lanuza E. Attraction to sexual pheromones and associated odorants in female mice involves activation of the reward system and basolateral amygdala. Eur J Neurosci. 2005;21:2186–98. doi: 10.1111/j.1460-9568.2005.04036.x. [DOI] [PubMed] [Google Scholar]
- Nistico G, Caroleo MC, Arbitrio M, Pulvirenti L. Dopamine D1 receptors in the amygdala enhance the immune response in the rat. Ann NY Acad Sci. 1994;741:316–23. doi: 10.1111/j.1749-6632.1994.tb39674.x. [DOI] [PubMed] [Google Scholar]
- Pacifici R, di Carlo S, Bacosi A, Pichini S, Zuccaro P. Pharmocokinetics and cytokine production in heroin and morphine-treated mice. Int J Immunopharmacol. 2000;22:603–14. doi: 10.1016/s0192-0561(00)00023-0. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y, Pare JF. Intra-amygdaloid projections of the basolateral and basomedial nuclei in the cat: Phaseolus vulgaris-leucoagglutinin anterograde tracing at the light and electron microscope level. Neuroscience. 1995;69(2):567–83. doi: 10.1016/0306-4522(95)00272-k. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1986. [Google Scholar]
- Rivest S, Laflamme N. Neuronal activity and neuropeptide gene transcription in the brains of immune-challenged rats. J Neuroendocrinol. 1995;7(7):501–25. doi: 10.1111/j.1365-2826.1995.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Rizos Z, Ovari J, Leri F. Reconditioning of heroin place preference requires the basolateral amygdala. Pharmacol Biochem Behav. 2005;82:300–305. doi: 10.1016/j.pbb.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19(23):10575–83. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar SM, Price KJ, Kasting NW, Sharp FR. Anatomic patterns of Fos immunostaining in rat brain following systemic endotoxin administration. Brain Res Bull. 1995;36(4):381–92. doi: 10.1016/0361-9230(94)00217-o. [DOI] [PubMed] [Google Scholar]
- Saurer TB, Carrigan KA, Ijames SG, Lysle DT. Suppression of natural killer cell activity by morphine is mediated by the nucleus accumbens shell. J Neuroimmunol. 2006;173(12):3–11. doi: 10.1016/j.jneuroim.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Setlow B, Gallagher M, Holland PC. The basolateral complex of the amygdala is necessary for acquisition but not expression of CS motivational value in appetitive Pavlovian second-order conditioning. Eur J Neurosci. 2002;15:1841–53. doi: 10.1046/j.1460-9568.2002.02010.x. [DOI] [PubMed] [Google Scholar]
- Setlow B, Holland PC, Gallgher M. Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive Pavlovian second-order conditioned responses. Behav Neurosci. 2002;116(2):267–75. doi: 10.1037//0735-7044.116.2.267. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Gervais R, Messaoudi B, Granjon L, Mouly AM. Olfactory fear conditioning induces field potential potentiation in rat olfactory cortex and amygdala. Learn Mem. 2004;11:761–9. doi: 10.1101/lm.83604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideroff SI, Jarvik ME. Conditioned responses to a videotape showing heroin-related stimuli. Int J Addict. 1980;15(4):529–36. doi: 10.3109/10826088009040035. [DOI] [PubMed] [Google Scholar]
- Szczytkowski JL, Lysle DT. Conditioned effects of heroin on the expression of inducible nitric oxide synthase in the rat are susceptible to extinction and latent inhibition. Psychopharmacology. 2007;191:879–89. doi: 10.1007/s00213-006-0673-z. [DOI] [PubMed] [Google Scholar]
- Tkacs NC, Li J. Immune stimulation induces Fos expression in brainstem amygdala afferents. Brain Res Bull. 1999;48(2):223–31. doi: 10.1016/s0361-9230(98)00167-1. [DOI] [PubMed] [Google Scholar]
- Unnithan S, Gossop M, Strang J. Factors associated with relapse among opiate addicts in an out-patient detoxification programme. Br J Psychiatry. 1992;161:654–7. doi: 10.1192/bjp.161.5.654. [DOI] [PubMed] [Google Scholar]


