SUMMARY
Sindbis virus (SINV) is a mosquito-borne virus in the genus Alphavirus, family Togaviridae. Like most alphaviruses, SINVs exhibit lytic infection (apoptosis) in many mammalian cell types, but are generally thought to cause persistent infection with only moderate cytopathic effects in mosquito cells. However, there have been several reports of apoptotic-like cell death in mosquitoes infected with alphaviruses or flaviviruses. Given that apoptosis has been shown to be an antiviral response in other systems, we have constructed recombinant SINVs that express either pro-apoptotic or anti-apoptotic genes in order to test the effects of inducing or inhibiting apoptosis on SINV replication in mosquito cells. Recombinant SINVs expressing the pro-apoptotic genes reaper (rpr) from Drosophila or michelob_x (mx) from Aedes aegypti caused extensive apoptosis in cells from the mosquito cell line C6/36, thus changing the normal persistent infection observed with SINV to a lytic infection. Although the infected cells underwent apoptosis, high levels of virus replication were still observed during the initial infection. However, virus production subsequently decreased compared to persistently infected cells, which continued to produce high levels of virus over the next several days. Infection of C6/36 cells with SINV expressing the baculovirus caspase inhibitor P35 inhibited actinomycin D-induced caspase activity and protected infected cells from actinomycin D-induced apoptosis, but had no observable effect on virus replication. This study is the first to directly test whether inducing or inhibiting apoptosis affects arbovirus replication in mosquito cells.
INTRODUCTION
Each year several million people die of arthropod-borne diseases including malaria, yellow fever, and dengue fever (Hill et al., 2005). Sindbis virus (SINV) (genus Alphavirus; family Togaviridae) is an arthropod-borne virus (arbovirus) having a positive-sense, single-stranded RNA genome of 11.7 kb, with a 5′cap and a 3′ poly(A) tail (Strauss & Strauss, 1994). SINV is an important tool to study the interaction between viruses and mosquitoes because full-length infectious cDNA clones are available which have been engineered to allow expression of foreign genes, and because SINV can infect Aedes aegypti, a mosquito vector which is important in the transmission of dengue and yellow fever viruses.
SINVs generally cause acute cell death in most types of mammalian cells, and infected cells display typical characteristics of apoptosis (Levine et al., 1993, Nava et al., 1998). However, SINVs are generally thought to cause only moderate cytopathic effect in mosquito cells with a persistent infection (Karpf & Brown, 1998). Expression of the apoptotic inhibitory gene bcl-2 can convert the pattern of SINV infection in mammalian cells from lytic to persistent (Levine et al., 1993). In addition, the ability of SINV to cause apoptosis in neurons correlates with pathogenesis in mice (Lewis et al., 1996). The reasons why SINV infection does not cause apoptosis in mosquito cells are still unknown. Cell and species specificity of SINV-induced cell death implies that cellular and viral regulators of apoptosis play important roles in determining the outcome of SINV infection. However, it is important to keep in mind that most of the information in this area comes from studies performed using mosquito cell lines. Less is known about SINV infection in vivo, and the possibility remains that SINV could cause apoptosis in certain cell types in mosquitoes, or in certain mosquito species. Indeed, there are a number of reports of cell death in mosquitoes infected with arboviruses (including the alphaviruses SINV, Semiliki Forest virus, and Eastern and Western equine encephalitis viruses, as well as the flavivirus West Nile virus), some of which are consistent with apoptosis (Bowers et al., 2003, Girard et al., 2005, Mims et al., 1966, Weaver et al., 1992, Weaver et al., 1988). In addition, correlation between apoptosis and resistance to West Nile virus infection has been observed in midgut cells of a refractory lab strain of Culex pipiens pipiens (Vaidyanathan & Scott, 2006), and apoptosis that occurs in the salivary glands of Culex pipiens quinquefasciatus late in infection also correlates with reduced transmission potential for West Nile virus (Girard et al., 2005, Girard et al., 2007). However, despite these intriguing observations, no causative data exist that directly link apoptosis to effects on viral vector competence in mosquitoes.
Apoptosis is executed by initiator and effector caspases (cysteinyl aspartate-specific proteases), which become activated following an apoptotic stimulus and cleave a number of cellular substrates. Caspases are negatively regulated by cellular IAP (inhibitor of apoptosis) proteins, and IAPs are themselves negatively regulated by IAP antagonists. IAP antagonists are characterized by sharing a highly conserved N-terminal motif, an IAP-binding motif (IBM). Drosophila Reaper (Rpr) and Ae. aegypti Michelob_x (Mx) are examples of IAP antagonists which contain an IBM and function as pro-apoptotic proteins (Pronk et al., 1996, Zhou et al., 2005). On the other hand, the baculovirus caspase inhibitor P35 is a potent inhibitor of effector caspases from a wide variety of organisms (Clem, 2007). Following cleavage of P35 by an active caspase, a covalent bond is formed between P35 and the active site cysteine of the caspase (Fisher et al., 1999, Xu et al., 2001).
Recombinant SINV expression systems have been developed by inserting an additional copy of the viral subgenomic promoter in the genome to facilitate expression of foreign genes (Foy et al., 2004, Hahn et al., 1992, Olson et al., 2000, Pierro et al., 2003, Raju & Huang, 1991). The SINV infectious clones 5′dsMRE16ic and TE5′2J each contain a duplicated subgenomic promoter upstream of the normal subgenomic promoter in the viral genome. TE5′2J was generated from the mouse neurovirulent TE12 SINV strain, while 5′dsMRE16ic was engineered from the MRE16 SINV strain (Foy et al., 2004, Pierro et al., 2003, Pierro et al., 2007). TE5′2J viruses replicate well in cell lines, but poorly infect mosquito midguts after oral infection. In contrast, 5′dsMRE16ic viruses are able to efficiently infect and disseminate from midgut epithelial cells after oral infection (Foy et al., 2004, Myles et al., 2004). In this study, we have used these SINV constructs to express pro-apoptotic and anti-apoptotic proteins in order to begin testing whether apoptosis can play a role in governing interactions between alphaviruses and mosquitoes.
METHODS
Cell culture
BHK-21 cells were propagated in Dulbecco’s Modification of Eagle’s Medium (DMEM) (Mediatech) supplemented with 10 % heat-inactivated fetal bovine serum (FBS) (Invitrogen). Aedes albopictus C6/36 cells were propagated in Leibovitz’s medium (Gibco) with 10 % FBS. BHK-21 cells were cultured at 37 °C with 8 % CO2, and C6/36 cells were maintained at 27 °c.
Recombinant virus construction
The coding regions of the mx, rpr and p35 cDNAs were amplified by PCR and cloned into the SINV DNA infectious clones p5′dsMRE16ic (MRE) (Foy et al., 2004, Myles et al., 2004) or pTE5′2J (TE) (Pierro et al., 2003) in the sense and antisense orientation. Additional clones were constructed containing in-frame fusions with the HA epitope tag at the C- (Mx and Rpr) or N-terminus (P35), sites which have been shown previously to not affect protein function. The insert sequences of all of the plasmids were verified by nucleotide sequencing. The GFP-expressing viruses MRE/GFP and TE/GFP have been previously described (Foy et al., 2004, Pierro et al., 2003).
Virus production
Capped transcripts of SINV RNA were produced using AmpliScribe™ SP6 High Yield Transcription Kit (EPICENTRE Biotechnologies) and m7 G(5′)ppp(5′)G Cap Analog (Ambion). Ten μl of each transcript reaction were transfected into BHK-21 cells using Lipofectamine™ 2000 (Invitrogen) and 100 μl Opti-MEM® I Reduced Serum Medium (Opti-MEM) (Invitrogen). After 3 days, medium containing virus was harvested, aliquoted and stored at -80 °c. Virus titers were determined by tissue culture infectious dose (TCID50) assay in BHK-21 cells. The TCID50 of each sample was converted to p.f.u. ml-1 by multiplying by 0.69 (O’Reilly et al., 1994). All of the virus stocks used in this study came directly from transfected BHK-21 cells without any further passage, and were only frozen and thawed once before use.
Virus growth curves and TCID50 assay
One million C6/36 cells were infected at an m.o.i. of 0.1 or 10 in a 6-well plate. After a one hour absorption period with Leibovitz’s medium, the cells were washed three times with PBS, and 2 ml of Leibovitz’s medium containing 10 % FBS was added into each well. At 0, 1, 2, 3, 4, and 5 days p.i., 100 μl of cell medium containing virus was collected and frozen at -80 °c until being subjected to TCID50 assay as described above. In the non-cumulative assay, after each time point the cells were washed three times with PBS and the medium was replaced.
Caspase assay
To detect caspase activity, 1 × 105 cells were infected at an m.o.i. of 0.01 or 1. At 6, 12, and 24 hpi, cells were harvested and centrifuged at 500 × g for 5 min. Cell pellets were washed with PBS and resuspended in 100 μl lysis buffer (20 mM HEPES KOH, pH 7.5, 50 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 250 mM sucrose). One complete mini EDTA-free protease inhibitor tablet (Roche Molecular Biochemicals) was added per 50 ml lysis buffer. Cells were lysed by four cycles of freezing-thawing and 50 μg of protein was mixed in 100 μl reaction buffer (100 mM HEPES buffer, pH 7.4 containing 2 mM DTT, 0.1 % CHAPS, 1 % sucrose) with 200 μM Ac-DEVD-AFC (MP Biomedicals), an effector-type caspase substrate, and incubated for 15 min at 37 °c. The fluorescence (excitation 405 nm, emission 535 nm) in the reactions was monitored over 1 hr at 25 °c using a Victor3 1420 Multilabel counter (Perkin-Elmer), and the values of the final measurements are shown.
TUNEL staining and flow cytometry analysis
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) was performed using the In Situ Cell Death Detection Kit, TMR red (Roche Applied Science). Two million C6/36 cells were infected at an m.o.i. of 0.1. Cells were harvested and washed 3 times with PBS, then pelleted by centrifugation at 500 x g for 5 min, and resuspended in 2 % paraformaldehyde freshly prepared in PBS for 1 hr at room temperature. After washing once with PBS, cell pellets were resuspended in fresh permeabilization solution (0.1 % Triton X-100 in 0.1 % sodium citrate) for 2 min on ice. Cells were washed twice with PBS and resuspended in 50 μl TUNEL reaction mixture (5 μl enzyme solution with 45 μl label solution) for 1 hour at 37 °c. Cells were washed twice with PBS and resuspended in 250 μl PBS with 1 μM TO-PRO-3 (Invitrogen) for nuclear counterstaining. Cells were detected using FL2 and FL4 in a FACSCalibur (Becton Dickinson), and data were analyzed with WinList5.0 (Verity Software House).
DNA fragmentation assay
C6/36 cells (2 × 106) were infected at an m.o.i. of 1. At 24 hpi, cells were harvested and pelleted as described above. The cell pellet was resuspended in 100 μl lysis buffer (10 mM Tris-HCl, pH 8.0; 100 mM NaCl; 25 mM EDTA; 0.5 % SDS; 0.1 mg/ml Proteinase K). The lysate was extracted twice with phenol/chloroform and ethanol precipitated. The precipitate was washed with 75 % ethanol and resuspended in 100 μl TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.5) containing 100 μg ml-1 RNase. Twenty μl of each sample were analyzed by agarose gel electrophoresis and the bands were visualized by ethidium bromide staining. To visualize nuclei, cells (48 hpi) were stained with 5 μg ml-1 Hoechst 33258 for 20 min before observation by UV microscopy.
Cell viability assay (MTT assay)
To determine cell viability, C6/36 cells (1 × 105) were infected at an m.o.i. of 0.01 in a 96-well-plate. Every 24 h, the cell medium was replaced with fresh medium. At each time point, cells were centrifuged at 500 x g for 5 min and washed once with PBS. Cells were incubated with 100 μl 1 % [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) (Sigma) for 4 h at 27 °c. Cells were washed with PBS again, and 150 μl acidic isopropanol (0.04 M HCl in absolute isopropanol) was added, followed by rocking on a shaking platform for 15 min at room temperature. Absorbance was determined at 550 nm.
For the actinomycin D (ActD)-induced cell death experiment, C6/36 cells (4 × 105) were infected at an m.o.i. of 1 in a 24-well-plate. At 24 hpi, 1 μg ml-1 ActD (Clontech Laboratories) and/or 100 μM z-VAD-FMK (MP Biomedicals) were added. After 24 h of ActD treatment, cell viability was determined by MTT assay as described above.
Immunoblotting
C6/36 cells (2 × 106) were infected at an m.o.i. of 1. At 6 hpi, 100 μM z-VAD-FMK was added to the medium. At 24 hpi, cells were collected in 100 μl of SDS-PAGE loading buffer, heated at 100 °c for 5 min and resolved by 15 % SDS-PAGE, and then transferred to PVDF. Proteins were detected with a 1:1000 dilution of anti-HA antibody (Covance) or anti-β actin antibody, and a 1:10,000 dilution of goat anti-mouse IgG—horseradish peroxidase (Bio-Rad) and SuperSignal West Pico Chemiluminescent substrate (Pierce).
RESULTS
Construction of recombinant SINVs and expression in C6/36 cells
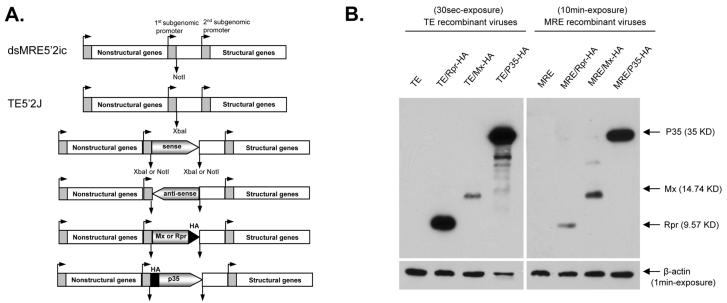
The IAP antagonist genes michelob_x (mx) from Ae. aegypti and reaper (rpr) from Drosophila melanogaster have been shown to induce apoptosis when expressed in insect cells (Pronk et al., 1996, Zhou et al., 2005), while expression of the baculovirus p35 gene blocks apoptosis by inhibiting caspases (Clem & Miller, 1994). In order to test whether inducing or inhibiting apoptosis would have an effect on SINV replication, a series of recombinant SINVs were constructed by inserting the coding regions of mx, rpr, or p35 into the TE5′2J (TE) and 5′dsMRE16ic (MRE) SINV infectious clones in sense or antisense orientation, and in sense orientation with an HA epitope (Fig. 1A and B). To examine protein expression, C6/36 cells infected with viruses expressing HA-tagged proteins were harvested at 24 hpi and analyzed by western blotting. All of the foreign genes were expressed in infected C6/36 cells, with the level of expression being generally higher from the TE viruses than from the MRE viruses (Fig. 1C; note the longer exposure of the MRE blot).
Figure 1.
Recombinant SINVs and foreign gene expression in C6/36 cells by SINV. (A) Schematic of recombinant SINVs constructed in this study. Constructs expressing the sense, antisense, or epitope tagged forms of each gene under the 5′ subgenomic promoter were prepared in both the MRE and TE infectious clones. In the tagged versions, the HA-tag was inserted at the N-terminus of P35, and at the C-terminus of Mx and Rpr. (B) Detection of foreign gene expression by immunoblotting. C6/36 cells were infected with the indicated HA-tagged recombinant and wild-type viruses (m.o.i. = 1). Lysates were prepared at 24 hpi, and analyzed by immunoblotting using anti-HA antibody. Antibody against β-actin was used as a loading control.
SINVs expressing IAP antagonists induce apoptosis
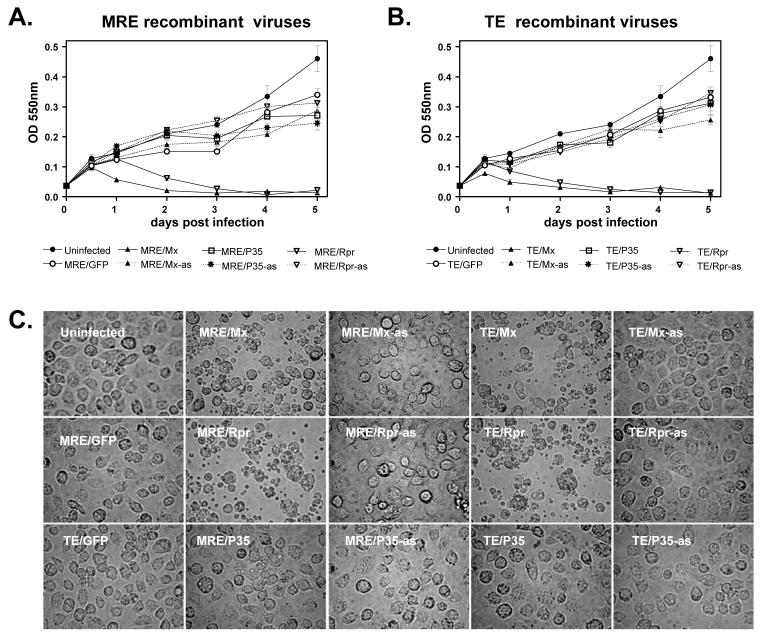
In initial experiments, C6/36 cells infected with viruses expressing Mx or Rpr in the sense orientation underwent lysis within the first 24-48 hpi, while cells infected with all of the other viruses did not lyse, but instead exhibited typical signs of persistent infection. To quantify the death of C6/36 cells infected with SINVs expressing Mx or Rpr, cell viability was quantified by MTT assay, which measures metabolic activity. C6/36 cells that were infected with viruses containing antisense inserts or viruses expressing GFP or P35 continued to proliferate similar to mock-infected cells, although by 4-5 days p.i., infected cells were slightly fewer in number than mock-infected cells, consistent with moderate cytopathic effect induced by SINV infection in these cells (Fig. 2A and B). Consistent with the higher level of foreign protein expression from TE viruses than MRE viruses (Fig. 1C), TE/Mx and TE/Rpr viruses induced cell death faster than MRE/Mx and MRE/Rpr. Cell blebbing and apoptotic bodies were first observed in TE/Mx- and TE/Rpr-infected cells at 12 hpi, while MRE/Mx- and MRE/Rpr-infected cells began blebbing at 18 hpi (data not shown). By 48 hpi, nearly all the cells infected by SINV expressing Mx or Rpr had died, while their counterpart antisense-virus-infected cells continued to proliferate (Fig. 2A-C). We also tested the viability of cells infected with the viruses expressing epitope-tagged IAP antagonists. TE/Mx-HA induced cell death in C6/36 cells, but TE/Rpr-HA, MRE/Mx-HA, and MRE/Rpr-HA did not, possibly due to the epitope tag interfering with protein function (data not shown).
Figure 2.
Recombinant SINVs expressing Mx or Rpr cause lytic infection in C6/36 cells. (A and B) C6/36 cells were mock-infected or infected with the indicated viruses (m.o.i. = 0.01) and cell viability was determined by MTT assay. Data are shown as mean ± SEM of four to six independent experiments. The treatments differed significantly by two way ANOVA (p < 0.0001). (C) Morphology of infected cells. C6/36 cells were infected with the indicated SINVs (m.o.i. = 0.01) and photographed (magnification, 400X) at 48 hpi.
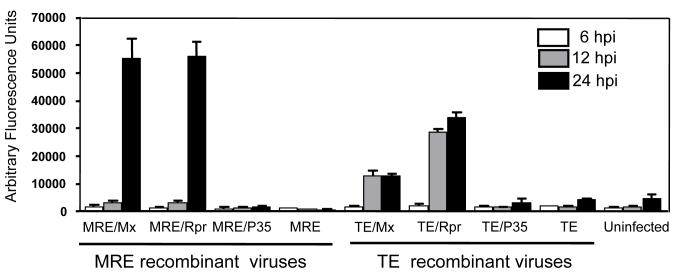
To determine whether the death caused by recombinant SINVs expressing Mx or Rpr in C6/36 cells was due to apoptosis, we examined several parameters, including caspase activation (Fig. 3). At 6 hpi, all of the infected cells exhibited caspase activity similar to that of mock-infected cells. However, at 12 hpi, TE/Mx and TE/Rpr infection caused increased levels of caspase activity, in contrast to MRE/Mx and MRE/Rpr infection, which remained fairly low (Fig. 3). By 24 hpi, MRE/Mx- and MRE/Rpr-infected C6/36 cells exhibited extensive cell blebbing and dramatically increased caspase activity. TE/Mx- and TE/Rpr- infected C6/36 cells appeared to exhibit less caspase activity at 24 hpi compared with MRE/Mx- and MRE/Rpr-infected cells, but presumably this was because many of the TE/Mx- and TE/Rpr-infected cells had already completed apoptosis and undergone secondary necrosis by this time, resulting in leakage of intracellular proteins into the culture supernatant.
Figure 3.
Activation of caspases by recombinant SINVs expressing Mx or Rpr. C6/36 cells were either mock-infected or infected with the indicated SINVs (m.o.i. = 0.01). At 6, 12, and 24 hpi, cell lysates were prepared and caspase activity was determined using Ac-DEVD-AFC as a substrate. Data are shown as mean ± SEM of three experiments. The treatments differed significantly as judged by one way ANOVA (p < 0.0001).
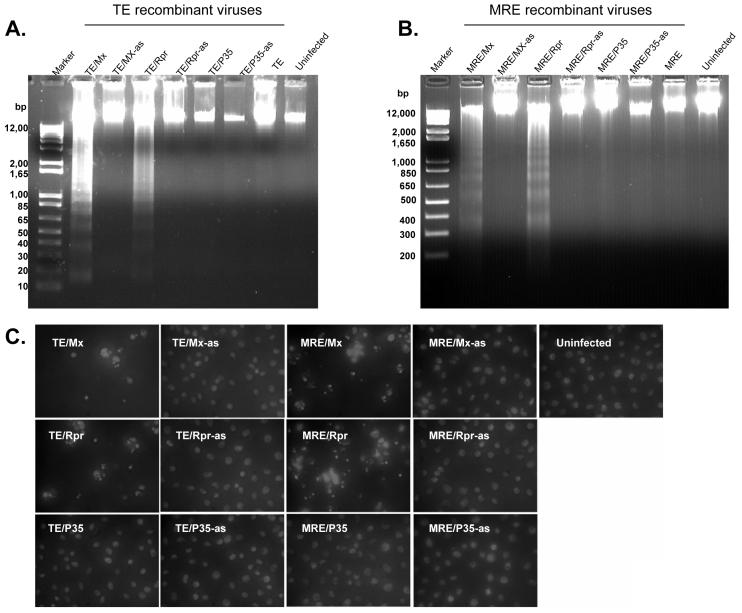
Chromatin degradation, nuclear condensation and nuclear fragmentation are also hallmarks of apoptosis. At 24 hpi, we examined the DNA from infected C6/36 cells by agarose gel electrophoresis . MRE/Mx-, MRE/Rpr-, TE/Mx- and TE/Rpr-infected cells exhibited genomic DNA fragmentation into oligonucleosomal ladders characteristic of apoptotic cells (Fig. 4A and B). In contrast, the genomic DNA from cells infected by anti-sense viruses or viruses expressing P35 was intact, similar to that of mock-infected cells (Fig. 4A and B). We also observed genomic DNA condensation and nuclear fragmentation in C6/36 cells infected with MRE/Mx, MRE/Rpr, TE/Mx and TE/Rpr at 24 hpi, in contrast to the uniform nuclear staining observed in C6/36 cells infected with anti-sense viruses and mock-infected cells (Fig. 4C).
Figure 4.
SINVs expressing Mx or Rpr cause DNA fragmentation indicative of apoptosis. (A and B) C6/36 cells were mock-infected or infected with the indicated SINVs (m.o.i. = 1). At 24 hpi, cells were lysed and DNA was analyzed by agarose gel electrophoresis and ethidium bromide staining. (C) C6/36 cells were infected with the indicated SINVs (m.o.i. = 0.01) for 48 h, stained with Hoechst 33258, and photographed (magnification, 400X).
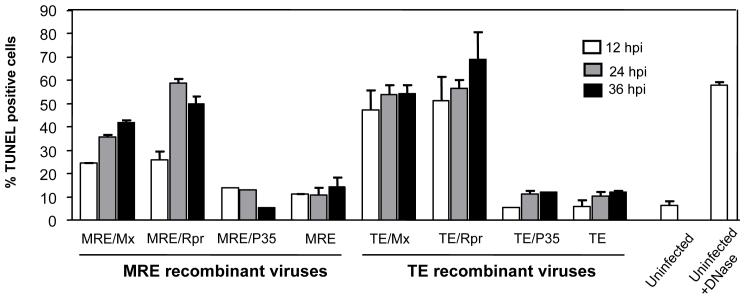
We quantified apoptotic cells using terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) and flow cytometry (Fig. 5). As expected, TE/Mx- and TE/Rpr-infected cells became TUNEL-positive more quickly than MRE/Mx- and MRE/Rpr-infected cells. At 12 hpi, 47% of TE/Mx- and 51% of TE/Rpr-infected cells were TUNEL-positive, while the proportions of MRE/Mx- and MRE/Rpr-infected cells that were TUNEL-positive were 24.5% and 25.9% respectively. At 24 and 36 hpi, the proportion of TUNEL-positive MRE/Mx- and MRE/Rpr-infected cells increased to a range of 40-50 %, while 55-65 % of TE/Mx- and TE/Rpr-infected cells were TUNEL-positive. In contrast, approximately 10 % of MRE/P35-, MRE-, TE/P35-, and TE-infected cells were TUNEL-positive at each time point, which was similar to the background staining seen in mock-infected cells.
Figure 5.
Apoptosis caused by SINVs expressing Mx or Rpr as assayed by TUNEL-staining. C6/36 cells were infected with the indicated SINVs (m.o.i. = 0.1) and harvested at 12, 24, and 36 hpi. The cells were subjected to TUNEL assay and analyzed by flow cytometry. A sample of mock-infected cells was treated with DNase as a positive control for DNA fragmentation. Data are shown as mean ± SEM of four experiments. The treatments differed significantly as judged by one way ANOVA (p < 0.0001).
P35 expression by SINV protects C6/36 cells from apoptotic stress
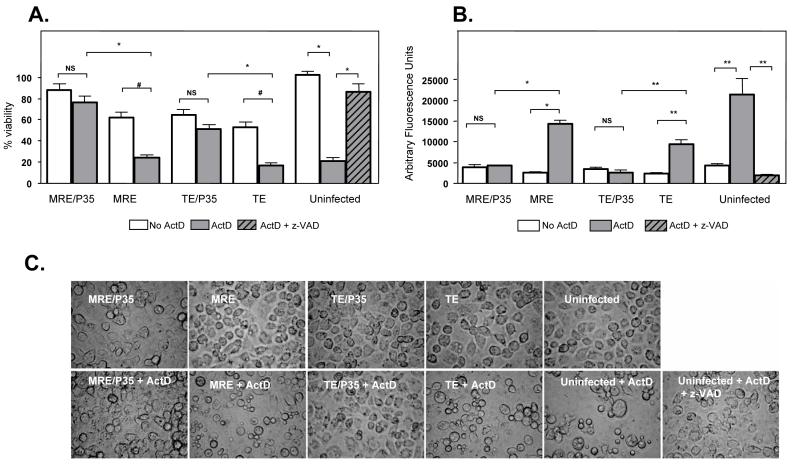
To test whether SINV-mediated expression of the caspase inhibitor P35 can protect C6/36 cells from apoptotic stress, we analyzed the viability of infected cells after treatment with actinomycin D (ActD), which induces apoptosis in many insect cell lines. After 24 h treatment with ActD we observed that over 90 % of C6/36 cells were apoptotic as judged by their morphology (Fig. 6C). By MTT assay, we found that mock-infected, ActD-treated cells were only around 20 % viable compared to untreated cells (Fig. 6A), while the relative viability of MRE- and TE-infected cells decreased from 60 % to 20 % after ActD treatment (Fig. 6A). However, the viability of MRE/P35- and TE/P35-infected cells was only slightly reduced after ActD treatment (Fig. 6A). The death induced by ActD was inhibited by the caspase inhibitor z-VAD-FMK (85 % viability compared to 20 % in cells treated with ActD alone) (Fig. 6A).
Figure 6.
P35 expression by SINV protects C6/36 cells from ActD-induced cell death. (A) C6/36 cells were mock-infected or infected with the indicated SINVs (m.o.i. = 1), and at 24 hpi treated with ActD or ActD + z-VAD-FMK. MTT assay was performed 24h after ActD treatment. Data are shown as mean ± SEM of three experiments (NS, non-significant, *p < 0.0001, #p = 0.0002 by Student’s t test). (B) C6/36 cells were mock-treated or infected with SINVs (m.o.i. = 1), and at 24 hpi treated with ActD or ActD + z-VAD-FMK. Cell lysates were prepared 18h after ActD treatment, and caspase activity was determined using Ac-DEVD-AFC as a substrate. Data are shown as mean ± SEM of four experiments (NS, non-significant, *p < 0.0001, **p < 0.005 by Student’s t test). (C) C6/36 cells were infected with the indicated SINVs (m.o.i. = 1), and at 24 hpi treated with ActD. Cells were photographed (magnification, 400X) 24 h after ActD treatment.
Without ActD treatment, MRE-, MRE/P35-, TE-, and TE/P35-infected cells had similar levels of caspase activity as mock-treated cells (Fig. 6B). After ActD treatment, MRE- or TE-infected cells showed an increase in caspase activity, but MRE/P35- or TE/P35-infected cells had no change in caspase activity compared to non-ActD-treated cells, indicating caspase inhibition by P35 (Fig. 6B).
Replication of recombinant SINVs
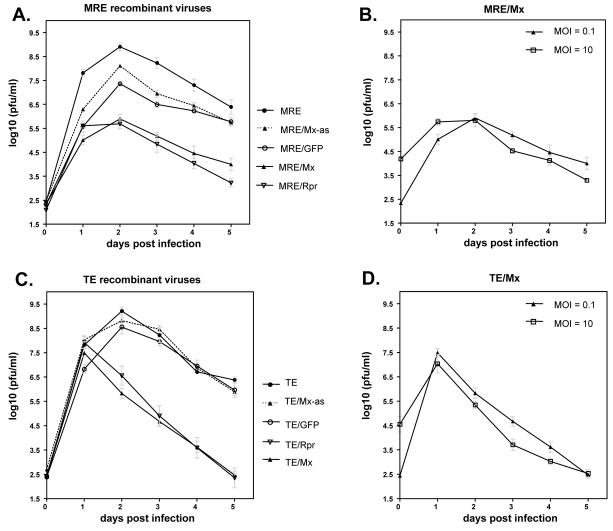
To assess the effect of apoptosis on SINV replication, virus growth curves were performed. To measure the production of virus during each 24 h period following infection (non-cumulative assay), the cells were washed three times with PBS at each time point after removal of virus-containing culture supernatant. Mx- and Rpr-expressing recombinant viruses caused lytic replication in C6/36 cells, and as expected, the amount of virus production significantly decreased after the death of C6/36 cells. The viral titers of both MRE/Rpr and MRE/Mx viruses peaked at 2 dpi (Fig. 7A), while TE/Rpr and TE/Mx -infected C6/36 cells exhibited the highest level of virus at 1 dpi (Fig. 7C), consistent with the viability results (Fig. 2A and B). Viruses containing any inserts, including antisense inserts or GFP, tended to produce lower levels of virus than the empty vectors MRE or TE, presumably due to their increased genome size (Pierro et al., 2003). In addition, the recombinant TE viruses produced around a 10-fold higher amount of virus than the corresponding recombinant MRE viruses, although the TE and MRE empty vectors produced roughly equivalent titers. This was not unexpected, given that the TE strain is adapted to replication in cell culture (Olson et al., 2000, Pierro et al., 2003). Infection with high versus low m.o.i. did not significantly affect the final viral titers (Fig. 7B and D).
Figure 7.
Non-cumulative virus growth curves in C6/36 cells. Cells were infected with the indicated viruses at m.o.i. of 0.1 (A and C), and 0.1 or 10 (B and D). The cells were washed three times with PBS after harvesting each time point. Data are shown as mean ± SEM of four experiments. The treatments in panels A and C differed significantly by two way ANOVA (p < 0.0001) while differences between treatments in panels B and D were not significant.
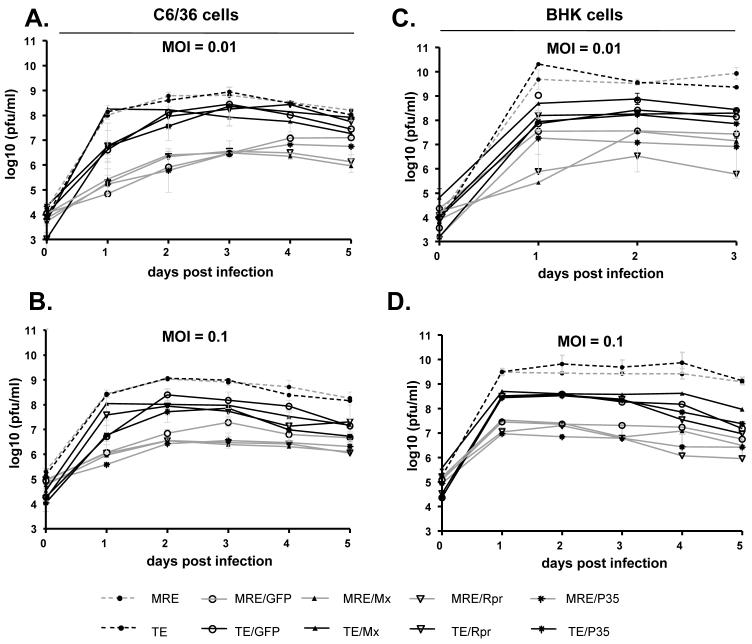
We also examined cumulative virus replication by removing a small amount of culture medium at each time point without replacing the medium or washing the cells. Generally, the production of each virus reached a plateau at 2 dpi in C6/36 cells (Fig. 8A and B) and 1 dpi in BHK-21 cells (Fig. 8C and D), and a high level of virus remained in the culture supernatant for the rest of the experiment. Similar to the above results, the MRE and TE recombinant viruses containing inserts produced approximately 10-fold less progeny virus than viruses without inserts (Fig. 8). All of the viruses caused cell death in BHK-21 cells within 1 day, including the viruses expressing P35. In C6/36 cells, the viruses expressing Mx or Rpr caused extensive apoptosis within 1-2 days, while the rest of the viruses caused only moderate cytopathic effect. Despite this difference, the recombinant viruses in each type of parental clone (TE or MRE) had similar growth patterns in both cell lines. While MRE/Mx, MRE/Rpr, TE/Mx, and TE/Rpr-infected C6/36 cells were almost all dead after 2 days, cells infected with the other viruses remained alive; however, the level of virus in the medium did not increase significantly over the next 3 days for any of the viruses (Fig. 8A and B).
Figure 8.
Cumulative virus growth curves in C6/36 and BHK-21 cells. C6/36 cells (A and B) or BHK-21 cells (C and D) were infected with the indicated viruses at an M.O.I. of 0.01 (A and C) or 0.1 (B and D). Supernatants were collected at the indicated times pi without replacing the medium or washing the cells. Data are shown as mean ± SEM of three to five experiments. The treatments in panels A-D were significantly different by two way ANOVA (p < 0.0001).
DISCUSSION
We are using SINV as a model to study the effect of inducing or inhibiting apoptosis on the ability of mosquito cells to permit arbovirus replication. Arboviruses usually do not induce apoptosis in mosquito cell lines; however, there are reports of cytopathic effects resembling apoptosis in arbovirus-infected mosquitoes, leading to the question of whether apoptosis could be an anti-viral response in certain tissues or in some arbovirus-mosquito combinations. The effects of apoptosis on arbovirus replication have not been previously investigated. In this study we have characterized the effects of expressing apoptotic regulatory genes on cell viability and virus replication in the mosquito cell line C6/36.
The genetic factors that govern susceptibility to arbovirus infection in mosquitoes are poorly understood. One pathway that increasingly appears to be important in regulating the level of virus replication in mosquitoes is RNA interference (RNAi) (Campbell et al., 2008, Keene et al., 2004, Sanchez-Vargas et al., 2004). Besides RNAi, there are other pathways that are also likely to be involved in mosquito anti-viral immunity, but at this time little evidence exists in this area. Transcript levels of members of the Toll and JNK pathways, as well as several serpin genes, were shown to be altered following SINV infection of Ae. aegypti (Sanders et al., 2005), suggesting that known innate immune pathways may be stimulated by virus infection in mosquitoes. In addition, reducing or activating Toll pathway signaling has effects on dengue virus replication in Ae. Aegypti (Xi et al., 2008). Finally, heat shock protein cognate 70B of Anopheles gambiae is upregulated by o’nyong-nyong virus infection, and that silencing of this gene results in higher levels of o’nyong-nyong replication in An. gambiae mosquitoes (Sim et al., 2007).
Apoptosis is another attractive candidate anti-viral response in mosquitoes, given its importance in other virus-host systems (Clem, 2007, Hay & Kannourakis, 2002). It has been postulated that there are at least three barriers to successful infection and dissemination of arboviruses in mosquitoes: the midgut infection barrier (the ability to establish infection and replicate in midgut epithelium), the midgut escape barrier (the ability to penetrate the midgut and establish replication in other tissues), the salivary gland infection barrier (the ability to infect salivary glands), and the salivary gland escape barrier (the ability to enter the salivary gland lumen) (Black et al., 2002). A successful apoptotic response in the midgut or salivary gland could thus limit the ability of a virus to replicate and be disseminated.
In this study, we expressed the IAP antagonists Mx and Rpr and the caspase inhibitor P35 to either purposely induce or inhibit apoptosis during SINV infection. While SINV normally causes non-lytic, persistent infection in mosquito cell lines, expression of Mx or Rpr from SINV caused apoptosis in C6/36 cells, as determined by cell morphology, caspase activity, and DNA fragmentation. Expression of P35, on the other hand, inhibited apoptosis induced by ActD treatment This result, together with the fact that P35 is a broad-spectrum caspase inhibitor which inhibits apoptosis in a wide variety of situations (Clem, 2007), suggests that this virus could be used to test the effect of inhibiting apoptosis on vector competence in mosquitoes. The viruses expressing P35 still induced apoptosis in BHK cells, despite expressing P35. The reason for this is unclear, but it may be because SINV induces apoptosis rapidly in BHK cells, perhaps before sufficient amounts of P35 can be expressed from the subgenomic promoter. In a previous report, SINV-mediated expression of another caspase inhibitor, CrmA, inhibited apoptosis in BHK cells (Nava et al., 1998), but different strains of SINV and BHK cells were used.
The two SINV expression systems used in this study, MRE and TE, differ from each other in their ability to replicate in cultured cells, and in their ability to infect mosquitoes following a blood meal. TE is derived from a laboratory strain of SINV that is well adapted to replication in cultured cells. As a consequence, we observed higher levels of foreign gene expression in C6/36 cells with TE-based viruses, and we also saw that TE viruses expressing Mx or Rpr caused apoptosis faster than their MRE-based counterparts. Higher levels of virus replication were also observed for the TE-based viruses than for the MRE-based viruses when a foreign gene insert was present in the genome, although MRE without any additional insert replicated at equivalent levels to TE in either BHK or C6/36 cells. MRE, on the other hand, is derived from a field isolate of SINV, and has higher oral infectivity in mosquitoes than TE (Foy et al., 2004). It will thus be interesting to determine how purposely inducing or inhibiting apoptosis affects the infectivity and dissemination of these viruses in mosquitoes following infection via a blood meal.
Neither induction nor inhibition of apoptosis had significant effects on the initial burst of replication of SINV in C6/36 cells. This may be in part due to the expression of these foreign genes from the viral subgenomic promoter, which is not expressed until after the viral genome has been replicated. In mammalian cells, SINV also replicates to high titers in spite of the apoptosis that is typically associated with infection, and blocking apoptosis does not have a significant effect on the levels of replication (Nava et al., 1998). However, cells that were infected by viruses expressing Mx or Rpr died after the initial burst of replication, and thus were not able to maintain high levels of virus replication over time. In an infected mosquito, this could be an important factor in determining vector competence. Sustained virus replication is presumably required for virus escape from the midgut and dissemination to other tissues, including the salivary glands. Therefore, if infected cells die after producing a burst of initial virus replication, virus dissemination may be adversely affected. In addition, other mechanisms may operate in vivo to limit virus replication. For example, early and rapid recognition of apoptotic cells by phagocytic cells (hemocytes) could result in enhanced clearance of infected cells and destruction of newly formed virus before it is able to bud from the infected cell. Thus, apoptosis could have a negative effect on the ability of SINV to productively infect and be transmitted by mosquitoes.
It is generally thought that arbovirus infection has little or no negative consequences for mosquito vectors in terms of cytopathology or decreased fecundity or life span. However, there have been reports of cytopathic effects in mosquitoes infected with arboviruses (including West Nile virus and several alphaviruses) including observations of apoptosis occurring in midgut or salivary gland (Bowers et al., 2003, Girard et al., 2005, Mims et al., 1966, Weaver et al., 1992, Weaver et al., 1988), as well as negative effects on mosquito life span (Cooper et al., 2000). It is likely that, if apoptosis has a negative effect on vector competence, there would be little apoptosis observed in successful virus-vector combinations. In these situations, the virus may either actively inhibit apoptosis or avoid inducing apoptosis altogether. Thus, apoptosis may be more likely to occur in mosquitoes which do not have the ability to vector a particular virus, and which can mount a successful anti-viral response. To date the role of apoptosis in determining viral vector competence in mosquitoes has not been studied experimentally. The recombinant viruses characterized in this study will be useful tools to study the effects of apoptosis on determining the outcome of arbovirus infection in vivo in mosquito vectors.
ACKNOWLEDGEMENTS
We thank Bart Bryant (Kansas State University) for help in constructing some of the constructs used in this study. We are grateful to Lei Zhou (University of Florida) for providing Aedes aegypti Michelob-x plasmid. We thank Tammy Koopman (Kansas State University) for assistance with flow cytometry. This work was supported by NIH grants R21AI67642 (to R.J.C.), R01AI32543 (to C.D.B) and R01AI46435 (to K.E.O.), by P20 RR107686 from the National Center for Research Resources (NCRR), P20 RR16475 from the BRIN Program of the NCRR, by the Terry C. Johnson Center for Basic Cancer Research, and by the Kansas Agricultural Experiment Station. This is contribution number 08-87-J from the Kansas Agricultural Experiment Station.
Footnotes
Publisher's Disclaimer: This is an author manuscript that has been accepted for publication in Journal of General Virology, copyright Society for General Microbiology, but has not been copy-edited, formatted or proofed. Cite this article as appearing in Journal of General Virology. This version of the manuscript may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 17, Title 17, US Code), without permission from the copyright owner, Society for General Microbiology. The Society for General Microbiology disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by any other parties. The final copy-edited, published article, which is the version of record, can be found at http://vir.sgmjournals.org, and is freely available without a subscription.
REFERENCES
- Black W. C. t., Bennett KE, Gorrochotegui-Escalante N, Barillas-Mury CV, Fernandez-Salas I, de Lourdes Munoz M, Farfan-Ale JA, Olson KE, Beaty BJ. Flavivirus susceptibility in Aedes aegypti. Arch Med Res. 2002;33:379–88. doi: 10.1016/s0188-4409(02)00373-9. [DOI] [PubMed] [Google Scholar]
- Bowers DF, Coleman CG, Brown DT. Sindbis virus-associated pathology in Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2003;40:698–705. doi: 10.1603/0022-2585-40.5.698. [DOI] [PubMed] [Google Scholar]
- Campbell CL, Keene KM, Brackney DE, Olson KE, Blair CD, Wilusz J, Foy BD. Aedes aegypti uses RNA interference in defense against Sindbis virus infection. BMC Microbiol. 2008;8:47. doi: 10.1186/1471-2180-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RJ. Baculoviruses and apoptosis: a diversity of genes and responses. Curr Drug Targets. 2007;8:1069–74. doi: 10.2174/138945007782151405. [DOI] [PubMed] [Google Scholar]
- Clem RJ, Miller LK. Control of programmed cell death by the baculovirus genes p35 and iap. Mol Cell Biol. 1994;14:5212–22. doi: 10.1128/mcb.14.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper LA, Sina BJ, Turell MJ, Scott TW. Effects of initial dose on Eastern equine encephalomyelitis virus dependent mortality in intrathoracically inoculated Culiseta melanura (Diptera: Culicidae) J.Med Entomol. 2000;37:815–819. doi: 10.1603/0022-2585-37.6.815. [DOI] [PubMed] [Google Scholar]
- Fisher AJ, Cruz W, Zoog SJ, Schneider CL, Friesen PD. Crystal structure of baculovirus P35: role of a novel reactive site loop in apoptotic caspase inhibition. EMBO J. 1999;18:2031–9. doi: 10.1093/emboj/18.8.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy BD, Myles KM, Pierro DJ, Sanchez-Vargas I, Uhlirova M, Jindra M, Beaty BJ, Olson KE. Development of a new Sindbis virus transducing system and its characterization in three Culicine mosquitoes and two Lepidopteran species. Insect Mol. Biol. 2004;13:89–100. doi: 10.1111/j.1365-2583.2004.00464.x. [DOI] [PubMed] [Google Scholar]
- Girard YA, Popov V, Wen J, Han V, Higgs S. Ultrastructural study of West Nile virus pathogenesis in Culex pipiens quinquefasciatus (Diptera: Culicidae) J Med Entomol. 2005;42:429–44. doi: 10.1093/jmedent/42.3.429. [DOI] [PubMed] [Google Scholar]
- Girard YA, Schneider BS, McGee CE, Wen J, Han VC, Popov V, Mason PW, Higgs S. Salivary gland morphology and virus transmission during long-term cytopathologic West Nile virus infection in Culex mosquitoes. Am. J. Trop. Med. Hyg. 2007;76:118–28. [PubMed] [Google Scholar]
- Hahn CS, Hahn YS, Braciale TJ, Rice CM. Infectious Sindbis virus transient expression vectors for studying antigen processing and presentation. Proc Natl Acad Sci U S A. 1992;89:2679–83. doi: 10.1073/pnas.89.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay S, Kannourakis G. A time to kill: viral manipulation of the cell death program. J Gen Virol. 2002;83:1547–64. doi: 10.1099/0022-1317-83-7-1547. [DOI] [PubMed] [Google Scholar]
- Hill CA, Kafatos FC, Stansfield SK, Collins FH. Arthropod-borne diseases: vector control in the genomics era. Nat. Rev. Microbiol. 2005;3:262–8. doi: 10.1038/nrmicro1101. [DOI] [PubMed] [Google Scholar]
- Karpf AR, Brown DT. Comparison of Sindbis virus-induced pathology in mosquito and vertebrate cell cultures. Virology. 1998;240:193–201. doi: 10.1006/viro.1997.8914. [DOI] [PubMed] [Google Scholar]
- Keene KM, Foy BD, Sanchez-Vargas I, Beaty BJ, Blair CD, Olson KE. RNA interference acts as a natural antiviral response to O’nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc Natl Acad Sci U S A. 2004;101:17240–5. doi: 10.1073/pnas.0406983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Huang Q, Isaacs JT, Reed JC, Griffin DE, Hardwick JM. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993;361:739–42. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- Lewis J, Wesselingh SL, Griffin DE, Hardwick JM. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996;70:1828–35. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims CA, Day MF, Marshall ID. Cytopathic effect of Semliki Forest virus in the mosquito Aedes aegypti. Am J Trop Med Hyg. 1966;15:775–84. doi: 10.4269/ajtmh.1966.15.775. [DOI] [PubMed] [Google Scholar]
- Myles KM, Pierro DJ, Olson KE. Comparison of the transmission potential of two genetically distinct Sindbis viruses after oral infection of Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 2004;41:95–106. doi: 10.1603/0022-2585-41.1.95. [DOI] [PubMed] [Google Scholar]
- Nava VE, Rosen A, Veliuona MA, Clem RJ, Levine B, Hardwick JM. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J. Virol. 1998;72:452–9. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly DR, Miller LK, Luckow VA. Oxford University Press; U S A: 1994. Baculovirus Expression Vectors: A Laboratory Manual; pp. 983–8. [Google Scholar]
- Olson KE, Myles KM, Seabaugh RC, Higgs S, Carlson JO, Beaty BJ. Development of a Sindbis virus expression system that efficiently expresses green fluorescent protein in midguts of Aedes aegypti following per os infection. Insect Mol. Biol. 2000;9:57–65. doi: 10.1046/j.1365-2583.2000.00162.x. [DOI] [PubMed] [Google Scholar]
- Pierro DJ, Myles KM, Foy BD, Beaty BJ, Olson KE. Development of an orally infectious Sindbis virus transducing system that efficiently disseminates and expresses green fluorescent protein in Aedes aegypti. Insect Mol. Biol. 2003;12:107–16. doi: 10.1046/j.1365-2583.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- Pierro DJ, Powers EL, Olson KE. Genetic determinants of Sindbis virus strain TR339 affecting midgut infection in the mosquito Aedes aegypti. J. Gen. Virol. 2007;88:1545–54. doi: 10.1099/vir.0.82577-0. [DOI] [PubMed] [Google Scholar]
- Pronk GJ, Ramer K, Amiri P, Williams LT. Requirement of an ICE-like protease for induction of apoptosis and ceramide generation by REAPER. Science. 1996;271:808–10. doi: 10.1126/science.271.5250.808. [DOI] [PubMed] [Google Scholar]
- Raju R, Huang HV. Analysis of Sindbis virus promoter recognition in vivo, using novel vectors with two subgenomic mRNA promoters. J Virol. 1991;65:2501–10. doi: 10.1128/jvi.65.5.2501-2510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vargas I, Travanty EA, Keene KM, Franz AW, Beaty BJ, Blair CD, Olson KE. RNA interference, arthropod-borne viruses, and mosquitoes. Virus Res. 2004;102:65–74. doi: 10.1016/j.virusres.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Sanders HR, Foy BD, Evans AM, Ross LS, Beaty BJ, Olson KE, Gill SS. Sindbis virus induces transport processes and alters expression of innate immunity pathway genes in the midgut of the disease vector, Aedes aegypti. Insect Biochem Mol Biol. 2005;35:1293–307. doi: 10.1016/j.ibmb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Sim C, Hong YS, Tsetsarkin KA, Vanlandingham DL, Higgs S, Collins FH. Anopheles gambiae heat shock protein cognate 70B impedes o’nyong-nyong virus replication. BMC Genomics. 2007;8:231. doi: 10.1186/1471-2164-8-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan R, Scott TW. Apoptosis in mosquito midgut epithelia associated with West Nile virus infection. Apoptosis. 2006;11:1643–51. doi: 10.1007/s10495-006-8783-y. [DOI] [PubMed] [Google Scholar]
- Weaver SC, Lorenz LH, Scott TW. Pathologic changes in the midgut of Culex tarsalis following infection with Western equine encephalomyelitis virus. Am. J. Trop. Med. Hyg. 1992;47:691–701. doi: 10.4269/ajtmh.1992.47.691. [DOI] [PubMed] [Google Scholar]
- Weaver SC, Scott TW, Lorenz LH, Lerdthusnee K, Romoser WS. Togavirus-associated pathologic changes in the midgut of a natural mosquito vector. J. Virol. 1988;62:2083–90. doi: 10.1128/jvi.62.6.2083-2090.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Cirilli M, Huang Y, Rich RL, Myszka DG, Wu H. Covalent inhibition revealed by the crystal structure of the caspase-8/p35 complex. Nature. 2001;410:494–7. doi: 10.1038/35068604. [DOI] [PubMed] [Google Scholar]
- Zhou L, Jiang G, Chan G, Santos CP, Severson DW, Xiao L. Michelob_x is the missing inhibitor of apoptosis protein antagonist in mosquito genomes. EMBO Rep. 2005;6:769–74. doi: 10.1038/sj.embor.7400473. [DOI] [PMC free article] [PubMed] [Google Scholar]