Abstract
Primiparous female rats rapidly respond to foster pups following an extended separation from pups after an initial maternal experience. This consolidation of maternal behavior has been referred to as maternal memory. The neurochemical regulation of maternal memory is not clearly understood. One neuropeptide that may mediate maternal memory is arginine vasopressin (AVP), a neuropeptide which is modulated around the time of parturition and has an established role in learning and memory processes. Thus, the present studies examine the possible involvement of AVP in the establishment of maternal memory in female rats. Pregnant rats were implanted with chronic cannulae connected to subcutaneous osmotic minipumps filled with a V1a receptor antagonist [d(CH2)5Tyr(Me)AVP, 0.1–12.5 ng/hr] or saline vehicle which were chronically infused either into the lateral ventricles or bilaterally into the medial amygdala beginning on day 18 of gestation. Both the osmotic pumps and the newborn pups were removed 24 hours following parturition. The effects of the V1a antagonist treatments on social recognition and maternal behavior were measured following parturition and maternal memory was assessed following a ten day separation from pups. Whereas none of the AVP treatments affected the initial establishment of maternal behavior postpartum, maternal memory was impaired in rats infused into the amygdala with the AVP antagonist (1.25 and 12.5 ng/hr). Social recognition was not impaired by intracerebroventricular infusion of either the 0.1 or 1.0 ng/hr dose of the V1a antagonist. The present results suggest a role for medial amygdaloid V1a receptors in the establishment of maternal memory.
Keywords: maternal behavior, AVP, neuropeptide, amygdala, social recognition
INTRODUCTION
Following an initial maternal experience, primiparous female rats rapidly respond to foster pups after an extended separation from their own pups [1–6] . This retention of maternal behavior has been referred to as maternal memory [7]. Primiparous rats possess a relatively strong maternal memory compared to nulliparous animals that are sensitized to display maternal behavior through exposure to foster pups [6, 8]. This observation suggests that a different mechanism establishes maternal memory in primiparous rats.
One neuropeptide that may be involved in the establishment of maternal memory is AVP, which is located in several brain regions implicated in social behavior, among them the amygdala, lateral septum, nucleus accumbens, bed nucleus of the stria terminalis, and supraoptic nucleus [9, 10]. Specifically, AVP mRNA levels during the peripartum period shift, which suggests that vasopressin may be involved in the regulation of parturitional events, including maternal behavior. Zingg and Lefebvre found that hypothalamic AVP mRNA levels are increased 2–3 times during late pregnancy and lactation compared to controls [11], whereas vasopressin mRNA levels increase in the supraoptic nucleus during lactation [12]. Although AVP mRNA appears to be elevated during lactation compared to control animals, immunoreactive vasopressin typically peaks the day prior to parturition in several hypothalamic nuclei [13] and then declines following parturition [14, 15]. Exogenous AVP is capable of inducing maternal behavior in rats [16], and the infusion of a V1 antagonist into the medial preoptic area suppresses the behavior [17]. Additionally, AVP stimulates paternal behavior in prairie voles [18] and meadow voles [19], and central vasopressin gene expression is elevated in both sexes in biparental prairie voles [20]. However, more work is needed to clarify the role of AVP during this reproductive state, as little is known about its possible role in maternal memory.
Recent studies indicate that maternal memory may involve the neural pathways activated around parturition [21, 22], whereas the long term retention of these behaviors suggests the involvement of a memory-related process [6]. The shifts in AVP activity around parturition, combined with its role in learning and memory processes make it a plausible candidate for the establishment of maternal memory (for review: [9]. It is known that both central [23] and peripheral [24] AVP administration can improve social recognition and memory in rodents, and the distribution of the V1a receptor subtype in primates suggests that it has a role in higher cognitive functions, such as memory [25]. Exogenous AVP injected into the septum improves social recognition, and V1 receptor antagonists interfere with social recognition [26–28]. Brattleboro rats, which are incapable of synthesizing biologically active AVP in their hypothalamic nuclei, display impaired social recognition compared to normal Long-Evans rats, and the microdialysis administration of synthetic AVP improves social recognition in both rat strains [26, 29]. Furthermore, partial AVP deficiency in heterozygous Brattleboro rats results in impaired spatial memory in both males and females [30]. While studies in mice reveal that V1a receptor knock out induces a specific and severe impairment in social recognition, as the mice performed normally on nonsocial and spatial memory tasks [31], an AVP fragment improves both long and short-term spatial memory in rats [32].
Based on these findings, in the present study we have asked whether AVP mediates social recognition and more specifically, the establishment of maternal memory. It was hypothesized that centrally administered AVP V1a antagonist would disrupt both social recognition and maternal memory in primiparous rats.
METHODS
Experiment 1
Animals in this study were maintained in accordance with the guidelines of the Committee of the Care and Use of Laboratory Animals Resources, National Research Council. Sprague-Dawley rats (Charles River, Kingston, NY) were triple-housed in a light- (on 0500–1900 h) and temperature- (21–24°C) controlled room with food and water available ad libidum. In order to generate timed pregnancies, females were housed with adult males, and the females were checked daily for sperm. The presence of sperm in the vaginal lavage was designated gestation day 1. On gestation day 18 rats were anesthetized with isoflurane and implanted with chronic guide cannulae directed into the right lateral ventricle. ALZET osmotic pumps (model 2002) were connected to the chronic guide cannulae and implanted subcutaneously in the upper back region. The pumps contained one of two doses of the V1a antagonist d(CH2)5Tyr(Me)AVP (0.1 or 1.0 ng/hr, Sigma), or saline vehicle. Beginning on gestation day 22, rats were monitored for the initiation of parturition. Sample sizes were 9–12 per treatment group.
Postpartum Maternal Behavior
On the day of parturition, subjects were given 3–4 hours of pup contact following the start of delivery to allow all pups to be born. The dams were then tested for maternal behavior towards their own pups. To initiate this test, the pups were removed for 15 minutes, and then returned to their mother. The dam’s behavior was continuously observed for 15 minutes after returning the pups. Animals that retrieved, grouped, and crouched over their pups in a nursing posture during the 15 minute interval were considered to display full maternal behavior. Only dams that displayed full maternal behavior postpartum were tested 10 days later for the retention of the behavior.
Social Recognition Test
Twenty-four hours following parturition, females were tested for social recognition, following methods adapted from the studies of Engelmann and Landgraf (1994) [31]. The testing consisted of a 5 min exposure to one juvenile (20–27 days old), followed by an exposure to both the original juvenile and a novel juvenile 30 min later. During each exposure, the total investigatory behavior of the female toward each juvenile was measured. Investigatory behavior was defined as directed actions of the female towards the juvenile rat, including anogenital sniffing, licking, hunting, pawing, and close pursuing. It was expected that the female would spend less time investigating the original juvenile and more time investigating the novel juvenile if social recognition abilities were intact.
Maternal Memory Testing
Upon completion of this initial maternal and social recognition testing 24 hours postpartum, the osmotic pumps were extracted under isoflurane anesthesia, and pups were removed for 10 days. Dams were then tested daily for maternal behavior towards 3–9 day-old foster pups as a measure of the retention of the behavior, i.e. maternal memory, using the postpartum maternal behavior testing protocol. In addition to 15 minutes of continuous observation, dams were spot-checked 30, 45, and 60 minutes after the introduction of the pups to record the locations of the dam and the pups. The pups were left with the dams following testing, and replaced with fed foster pups the following day. Testing was continued until the dams displayed full maternal behavior for two consecutive days. Maternal behavior latencies were calculated as the initial day of full maternal behavior minus one. Therefore, a test subject that exhibited full maternal behavior on days 1 and 2 was given a maternal latency of 0. After testing was completed, dams were euthanized with CO2, and placement of the cannulae was confirmed through India ink injection. Only animals with accurate ventricular cannula placements were included in the statistical analyses.
Experiment 2
The procedure for experiment 2 was identical to experiment 1 with the exceptions that the dosage range for the V1a antagonist was expanded to 0.1, 0.5, 1.0, 2.5, and 12.5 ng/hr, and the social recognition testing protocol was not included due to it potential confounding effect on maternal memory.
Experiment 3
The procedure for experiment 3 was identical to experiment 1, with the exceptions that that the V1a antagonist doses (1.25 and 12.5 ng/hr) were delivered bilaterally to the medial amygdala (AP = −3.1, ML = ±3.6, DV = −9.0 mm). Again, the social recognition protocol was not included. The medial amygdala, an olfactory relay, was chosen as a possible site of AVP action based upon its important role in the olfactory mediation of the onset of maternal responsiveness [33]. After testing was completed, dams were euthanized with CO2, and placement of the cannulae was confirmed through histological analysis. Only animals with accurate bilateral cannulae placements were included in the statistical analyses.
Statistics
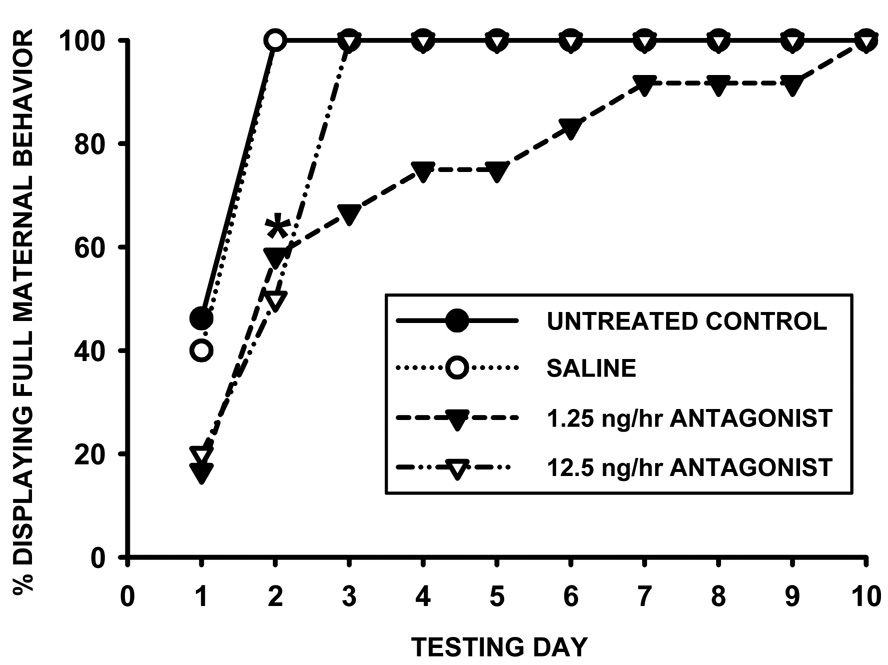
Data were analyzed with one-way ANOVA (with Tukey post hoc), as well as t-tests for additional treatment comparisons. Fisher’s Exact tests were used to compare numbers of animals maternally responding on each day of testing (Figure 5.).
Figure 5.
Percentage of females displaying full maternal behavior on each testing day following chronic V1a receptor antagonist infusion (1.25, 12.5 ng/hr) or saline control into the lateral ventricles, and a 10-day separation from pups (N = 9–12). * indicates significant differences between saline and each antagonist dose (p<0.05).
RESULTS
Experiment 1
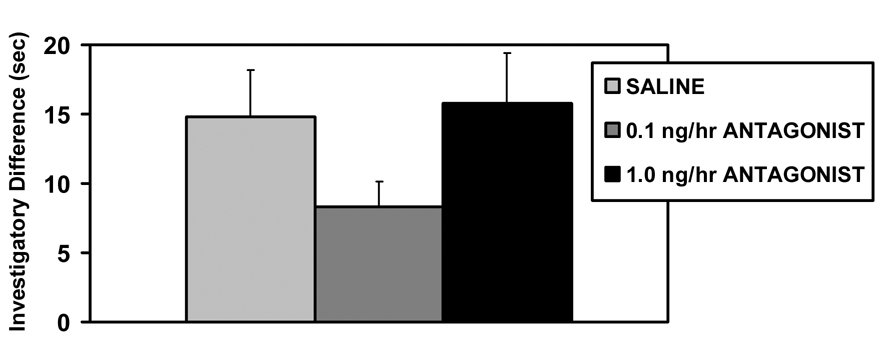
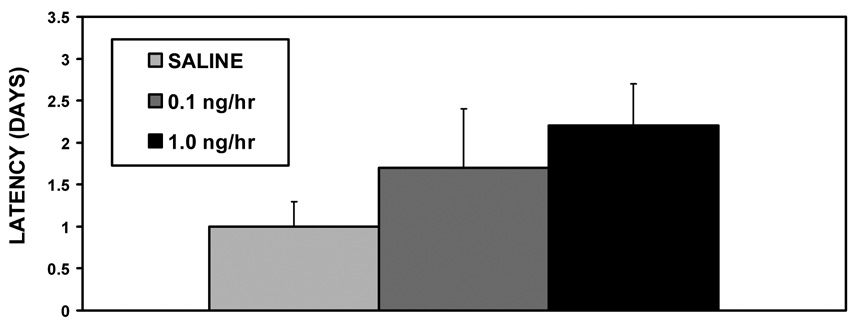
None of the V1a receptor antagonist treatments affected the initial expression of maternal behavior on the day of parturition; all the dams retrieved, grouped, and nursed their pups within 15 minutes. Peripartum intracerebroventricular (icv) infusions V1a antagonist also did not affect social recognition (F2,31=1.76, p=0.19, fig. 1) or maternal memory (F2,31=1.43, p=0.25, fig. 2). Both saline and antagonist treated dams spent more time investigating the novel juveniles (original vs. novel juvenile, t59 = −2.47, p=0.02 fig. 1).
Figure 1.
Mean (+SEM) differences between investigation time of novel and original juveniles on day 1 of lactation following chronic V1a receptor antagonist infusion (0.1, 1.0 ng/hr) or saline control into the lateral ventricles (N = 10–11).
Figure 2.
Mean (+SEM) values for maternal behavior latencies following chronic V1a receptor antagonist infusion (0.1, 1.0 ng/hr) or saline control into the lateral ventricles, and a 10-day separation from pups (N = 10–11).
Experiment 2
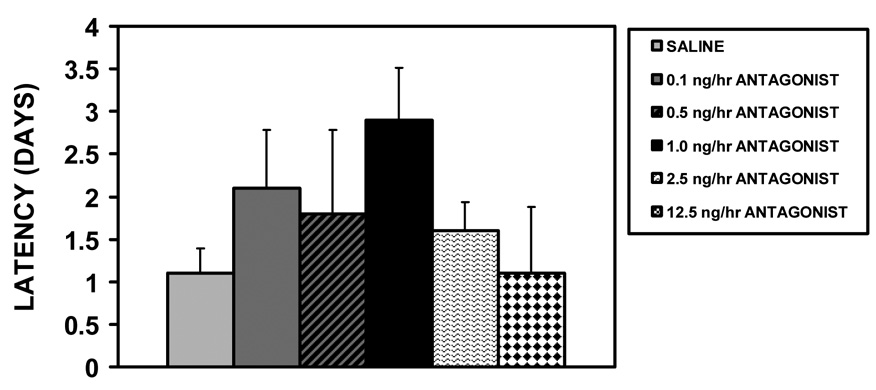
Although removing the social recognition protocol and expanding the dosage range of the AVP V1a receptor antagonist reduced variability in the maternal behavior latency data, there was not a significant effect of the V1a antagonist icv injections on initial maternal behavior at parturition (all dams exhibited full maternal behavior) or maternal memory in experiment 2 (H4=5.85, p=0.21, fig. 3).
Figure 3.
Mean (+SEM) values for maternal behavior latencies following chronic V1a receptor antagonist infusion (0.1, 0.5, 1.0, 2.5, 12.5 ng/hr) or saline control into the lateral ventricles, and a 10-day separation from pups (N = 10–12). There were no statistical differences among the icv treated groups.
Experiment 3
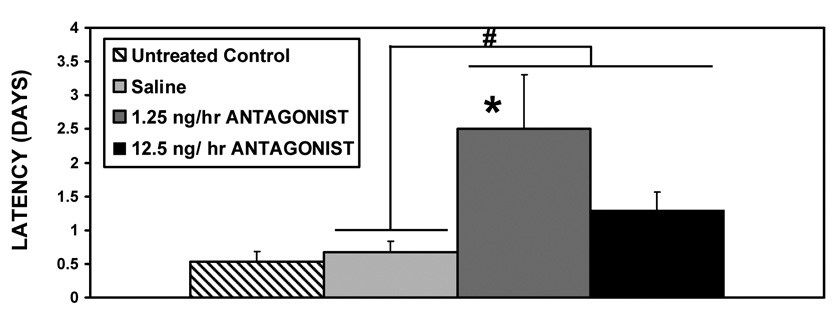
Based on the trend of increased latencies following given doses of the icv infusions in experiments 1 and 2, V1a antagonist was bilaterally administered to the medial amygdala in Experiment 3. V1a antagonist delivered bilaterally into the medial amygdala peripartum significantly increased the latencies to display full maternal behavior after the 10 day pup separation [F3,44=4.18, p=0.01 for treatment (ANOVA), with significant differences between both saline and the 1.25ng/hr dose (p=0.03), as well as between saline and both doses combined (t=111.0, p=0.03), fig. 4]. As seen in figure 5, both treatments significantly decreased the percentage of females displaying full maternal behavior on the second day of testing (Fisher’s Exact, p<0.05).
Figure 4.
Mean (+SEM) values for maternal behavior latencies following chronic bilateral V1a receptor antagonist infusion (1.25, 12.5ng/hr) or saline control into the medial amygdala, and a 10-day separation from pups (N = 9–12). * indicates a significant difference when 1.25 ng/hr antagonist treatment is compared to saline; # indicates a significant difference when combined antagonist treatments are compared to saline (p<0.05).
DISCUSSION
Peripartum chronic administration of a V1a antagonist into the medial amygdala significantly disrupted maternal memory when tested following 10 days without pup exposure. V1a antagonist treated dams took approximately 1 day longer to express full maternal behavior compared to saline treated controls. These results suggest that V1a receptors in the medial amygdala are an important component in the mechanism controlling maternal memory in primiparous females. It is known that the medial amygdala is an important component of the olfactory mechanism involved in maternal responsiveness towards pups [33]. Fos-ir levels in postpartum females with an impaired sense of smell are lower than levels in intact females [34], and pup exposure as well as non-tactile pup stimuli also increases Fos-ir in the medial and cortical amygdala in female rats [6, 35]. The medial amygdala has also been implicated in affiliation in prairie voles, where axon sparing lesions of this region decrease affiliative behavior towards both pups and females [36]. It is possible that amygdala-mediated affiliation is involved in the establishment of maternal memory.
In contrast to the effect of V1a antagonist infusions in the medial amygdala, infusions into the ventricular system did not affect maternal memory. The lack of significant effects on maternal memory in experiments 1 and 2 may be attributed to lack of site specificity of the icv injections. Furthermore, it is also possible that the social recognition protocol may have been a potential confound to the maternal memory data in experiment 1. It is interesting to note that similar doses of this AVP antagonist elicited the longest mean latencies in all three experiments (1.0 and 1.25 ng/hr), and that these doses were not the highest dose. Manipulations using AVP fragments have also found that high doses may not be as effective as lower doses [32]. It is postulated that the lack of effects of the high doses may be due to dose-dependant agonistic effects of the antagonist as noted in other AVP studies [37]. Although it does not appear that V1a effects on maternal memory are mediated through impaired social recognition, more work is needed in this area due to AVP’s established effects on this memory-mediated process in other paradigms. Based on the current data, and the observation that maternal memory in communally nesting species (including the Norway rat) may not require the traditional, individual-specific social memory tested in the social recognition test, it seems that vasopressin is necessary to consolidate the complex display of maternal behavior in response to nonspecific pup cues.
The effects on maternal memory were not dependent on V1a antagonist effects on the initial establishment of postpartum maternal behavior, as no effects were observed during testing immediately following parturition. This finding supports the hypothesis that the antagonist was acting on the mechanism controlling the establishment of maternal memory. Since the treatments were only administered around parturition, these actions must have interfered with the peripartum consolidation of maternal behavior. However, since the V1a treated females still possess shorter maternal behavior latencies compared to nulliparous females, it appears that the treated females retained some degree of maternal memory. It is likely that other factors are also involved in the retention of this behavior. Nevertheless, based on the documented changes in the AVP system around parturition [11, 13–15], and the present V1a receptor antagonist treatments, we propose that peripartum change in V1a receptor activity is one important mechanism responsible for the establishment of maternal memory.
It is noted that latency values for the untreated and saline groups were comparable to previous studies using similar protocols to test maternal memory [1, 22, 38]. In similar designs to the present experiment, most icv vehicle infused rats have maternal behavior latencies of 0–2 days (73–90% of animals respond) [21, 22]. The lack of differences between the untreated control and saline groups in the current studies indicates that the cannulation surgery itself had no discernable effects on maternal memory. Previous study has indicated that maternal memory is robust in primiparous animals, with mean response latencies of only 1.4 days even after 80 days without pups [6]. The resistance of maternal memory to manipulations such as surgery, icv infusion, and long term separation from pups, in contrast to the significant effects of V1a antagonist in the present study, suggests that the AVP system is a significant component in the consolidation of maternal behavior.
The significant differences in latencies between dams that have had their pups removed immediately and dams with as little as 30 minutes of pup exposure highlight the importance of the initial postpartum period in the consolidation of maternal behavior. If as little as 30 minutes of pup exposure is allowed, the dams have much shorter latencies to express maternal behavior compared to dams with their pups removed immediately upon birth when both groups are tested 10 days later [4]. It would be interesting to test the effects of acute antagonism of medial amygdala V1a receptors, specifically during the immediate postpartum period. A recent study suggests that pups may be especially reinforcing for primiparous females, as primiparous rats are more likely to explore an elevated plus maze if presented with pups in the open arms compared to sensitized nulliparous females. In the absence of pups, behavior in the elevated plus maze is similar in both pup-induced maternal nulliparous and primiparous dams [6]. Based on the previous work on maternal memory, it appears that the chronic antagonist treatment in the present study was primarily affecting maternal memory during the immediate postpartum period, and that the treatment interfered with the establishment of maternal memory. Since the current studies only examined the possible involvement of the medial amygdala, it is unknown whether other neural regions participate in the vasopressinergic regulation of maternal memory. It is possible that another region implicated in maternal memory, i.e. the shell of the nucleus accumbens, is involved in this process. Postpartum lesions [7], as well as the inhibition of protein synthesis with cycloheximide to the shell, but not core of the nucleus accumbens disrupts maternal memory. However, these treatments were not effective at disrupting maternal memory if administered 24 hours after pup exposure, once again supporting the theory of a sensitive period for the consolidation of maternal memory, as well as underscoring the importance of the pups in the consolidation of maternal behavior.
In summary, the present results demonstrate a role for medial amygdala V1a receptors in the establishment of maternal memory. Future studies are warranted that investigate the role of other brain regions in this process, examine the role of AVP in ongoing maternal behavior, and evaluate the ability of AVP to enhance maternal memory.
Acknowledgements
We would like to thank Elizabeth Byrnes and Phyllis Mann for assistance with the preparation of the manuscript. These studies were funded by NIH Awards F32 HD 048103 to B.C.N. and R37 HD019789 to R.S.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bridges RS. Long-term effects of pregnancy and parturition upon maternal resposiveness in the rat. Physiology and Behavior. 1975;14:245–249. doi: 10.1016/0031-9384(75)90028-1. [DOI] [PubMed] [Google Scholar]
- 2.Bridges RS. Parturition: Its role in the long term retention of maternal behavior in the rat. Physiol. and Behav. 1977;18:487–490. [Google Scholar]
- 3.Cohen J, Bridges RS. Retention of maternal behavior in nulliparous and primiparous rats. Effects of duration of previous maternal experience. J. Comp. and Physiol. Psychol. 1981;95:40–45. [Google Scholar]
- 4.Orpen GG, Fleming AS. Experience with pups sustains maternal responding in postpartum rats. Physiol. and Behav. 1987;40:47–51. doi: 10.1016/0031-9384(87)90184-3. [DOI] [PubMed] [Google Scholar]
- 5.Orpen GG, et al. Hormonal influences on the duration of postpartum maternal responsiveness in the rat. Physiol. and Behav. 1987;39:307–315. doi: 10.1016/0031-9384(87)90052-7. [DOI] [PubMed] [Google Scholar]
- 6.Scanlan VF, Byrnes EM, Bridges RS. Reproductive experience and activation of maternal memory. Behav. Neurosci. 2006;120(3):676–686. doi: 10.1037/0735-7044.120.3.676. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Fleming AS. The nucleus accumbens shell is critical for normal expression of pup-retrieval in postpartum female rats. Behavioural Brain Research. 2003;145(1–2):99–111. doi: 10.1016/s0166-4328(03)00135-9. [DOI] [PubMed] [Google Scholar]
- 8.Bridges RS, Scanlan VF. Maternal memory in adult, nulliparous rats: Effects of testing interval on the retention of maternal behavior. Developmental Psychobiology. 2005;46(1):13–18. doi: 10.1002/dev.20038. [DOI] [PubMed] [Google Scholar]
- 9.Engelmann M, et al. Behavioral consequences of intracerebral vasopressin and oxytocin: Focus on learning and memory. Neuroscience and Biobehav. Rev. 1996;20(3):341–358. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 10.Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin system in vertebrates. Brain Research Reviews. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 11.Zingg HH, Lefebvre DL. Oxytocin and vasopressin gene expression during gestation and lactation. Brain Research. 1988;464(1):1–6. doi: 10.1016/0169-328x(88)90011-3. [DOI] [PubMed] [Google Scholar]
- 12.Mezey E, Kiss JZ. Coexpression of vasopressin and oxytocin in hypothalamic supraoptic neurons of lactating rats. Endocrinology. 1991;129(4):1814–1820. doi: 10.1210/endo-129-4-1814. [DOI] [PubMed] [Google Scholar]
- 13.Caldwell JD, et al. Oxytocin and vasopressin immunoreactivity in hypothalamic and extrahypothalamic sites in late pregnant an postpartum rats. Neuroendocrinology. 1987;46(1):39–47. doi: 10.1159/000124794. [DOI] [PubMed] [Google Scholar]
- 14.Crowley RS, et al. Cytoplasmic oxytocin and vasopressin gene transcripts decline postpartum in the hypothalamus of the lactating rat. Endocrinology. 1993;133(6):2704–2710. doi: 10.1210/endo.133.6.7612074. [DOI] [PubMed] [Google Scholar]
- 15.Thomas A, Kim NB, Amico JA. Differential regulation of oxytocin and vasopressin messenger ribonucleic acid levels by gonadal steroids in postpartum rats. Brain Research. 1996;738(1):48–52. doi: 10.1016/0006-8993(96)00760-3. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen CA, et al. Oxytocin induces maternal behavior in virgin females rats. Science. 1982;216(4546):648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen CA, et al. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and preoptic areas. Behavioral Neuroscience. 1994;108(6):1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Ferris CF, Vries GJD. Role of Septal Vasopressin Innervation in Paternal Behavior in Prairie Voles (Microtus ochrogaster) Proceedings of the National Academy of Sciences. 1994;91(1):400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker KJ, Lee TM. Central vasopressin administration regulates the onset of facultative paternal behavior in Microtus pennsylvanicus (Meadow voles) Horm. Behav. 2001;39:285–294. doi: 10.1006/hbeh.2001.1655. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, et al. Hypothalamic vasopressin gene expression increases in both males and females postpartum in a biparental rodent. J. Neuroendo. 2000;12:111–120. doi: 10.1046/j.1365-2826.2000.00435.x. [DOI] [PubMed] [Google Scholar]
- 21.Byrnes EM, Rigero BA, Bridges RS. Opioid receptor antagonism during early lactation results in the increased duration of nursing bouts. Physiology and Behavior. 2000;70(1–2):211–216. doi: 10.1016/s0031-9384(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 22.Byrnes EM, Rigero BA, Bridges RS. Dopamine antagonists during parturition disrupt maternal care and the retention of maternal behavior in rats. Pharmacology, Biochemistry and Behavior. 2002;73(4):869–875. doi: 10.1016/s0091-3057(02)00941-3. [DOI] [PubMed] [Google Scholar]
- 23.Engelmann M, Landgraf R. Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiology and Behavior. 1994;55:145–149. doi: 10.1016/0031-9384(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 24.Bluthe RM, Gheusi G, Dantzer R. Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology. 1993;18(4):323–335. doi: 10.1016/0306-4530(93)90028-j. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, et al. Vasopressin and oxytocin immunoreactive neurons and fibers in the forebrain of male and female common marmosets (Callithrix jacchus) Synapse. 1997;27:14–25. doi: 10.1002/(SICI)1098-2396(199709)27:1<14::AID-SYN2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 26.Le Moal M, et al. Centrally injected arginine vasopressin (AVP) facilitates social memory in rats. Neurosci. Lett. 1987;77:353–359. doi: 10.1016/0304-3940(87)90527-1. [DOI] [PubMed] [Google Scholar]
- 27.Appenrodt E, Juszczak M, Schwarzberg H. Septal vasopressin induced preservation of social recognition in rats was abolished by pinealectomy. Behavioural Brain Research. 2002;134(1–2):67–73. doi: 10.1016/s0166-4328(01)00450-8. [DOI] [PubMed] [Google Scholar]
- 28.Dluzen DE, et al. The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides. 1998;19(6):999–1005. doi: 10.1016/s0196-9781(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 29.De Wied D, Bohus B, Van Wimersma B, B T. Memory deficit in rats with hereditary diabetes insipidus. Brain Res. 1975;85:152–156. doi: 10.1016/0006-8993(75)91022-7. [DOI] [PubMed] [Google Scholar]
- 30.Aarde SM, Jentsch JD. Haploinsufficiency of the arginine-vasopressin gene is associated with poor spatial working memory performance in rats. Hormones and Behavior. 2006;49(4):501–508. doi: 10.1016/j.yhbeh.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Bielsky IF, et al. Profound Impairment in Social Recognition and Reduction in Anxiety-Like Behavior in Vasopressin V1a Receptor Knockout Mice. Neuropsychopharmacology. 2003;29(3):483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- 32.Vawter MP, De Wied D, Van Ree JM. Vasopressin fragment (4–8), improves long-term and short-term memory in the hole board search task. Neuropeptides. 1997;31(5):489–494. doi: 10.1016/s0143-4179(97)90044-5. [DOI] [PubMed] [Google Scholar]
- 33.Fleming AS, Vaccarino F, Luebke C. Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiology & Behavior. 1980;25(5):731–743. doi: 10.1016/0031-9384(80)90377-7. [DOI] [PubMed] [Google Scholar]
- 34.Walsh CJ, et al. The effects of olfactory and somatosensory desensitization on Fos-like immunoreactivity in the brains of pup-exposed postpartum rats. Behav Neurosci. 1996;110:134–153. doi: 10.1037//0735-7044.110.1.134. [DOI] [PubMed] [Google Scholar]
- 35.Fleming AS, et al. Activation of fos-like immunoreactivity in the medial preoptic area and limbic structures by maternal and social interactions in rats. Behav. Neurosci. 1994;108:724–734. doi: 10.1037//0735-7044.108.4.724. [DOI] [PubMed] [Google Scholar]
- 36.Kirkpatrick B, et al. Axon-sparing lesions of the medial nucleus of the amygdale decrease affiliative behaviors in the prairie vole (Microtus ochrogaster): Behavioral and anatomical specificity. Behav Neurosci. 1994;108:501–513. doi: 10.1037//0735-7044.108.3.501. [DOI] [PubMed] [Google Scholar]
- 37.Ferris CF, et al. Inhibition of flank marking behavior in golden hamsters by microinjection of a vasopressin antagonist into the hypothalamus. Neurosci. Letters. 1985;55:239–243. doi: 10.1016/0304-3940(85)90027-8. [DOI] [PubMed] [Google Scholar]
- 38.Byrnes EM, Bridges RS. Endogenous opioid facilitation of maternal memory in rats. Behavioral Neuroscience. 2000;114(4):797–804. doi: 10.1037//0735-7044.114.4.797. [DOI] [PubMed] [Google Scholar]