To the Editor: The pathophysiology of anemia in older adults is incompletely understood, and a substantial proportion of anemia in this population remains unexplained.1 The factors that play a role in anemia in adults are incompletely identified. Advanced glycation end products (AGEs) are a heterogeneous group of bioactive molecules formed by the non-enzymatic glycation of proteins, lipids, and nucleic acids.2 AGEs have been widely implicated in the pathogenesis of cardiovascular and renal disease, and diabetes.2,3
Carboxymethyl-lysine (CML) is a dominant AGE that accumulates in large arteries, kidney, muscle, bone, and erythrocytes, and CML can lead to the formation of highly reactive dicarbonyl compounds that react with proteins and propagate intramolecular or intermolecular cross-link formation. CML progressively accumulates within erythrocytes during their life span in the circulation.4 AGEs reduce the deformability of erythrocytes, an effect that can be reversed by AGE inhibitors.5 AGEs on the surface of erythrocytes increase the binding of erythrocytes to blood vessel walls through interactions with the receptor for AGEs (RAGE) on the endothelial surface.6 Altered deformability of erythrocytes induced by AGEs, and erythrocyte AGE-RAGE interactions could potentially shorten the life-span of erythrocytes and contribute to anemia. A previous study described elevated serum AGEs in anemic patients with diabetes.7
We characterized serum CML and anemia in 751 adults in the Baltimore Longitudinal Study of Aging (BLSA). The BLSA is a prospective open cohort study of community-dwelling volunteers, largely from the Baltimore/Washington area.8 The BLSA has continuing approval from the Institutional Review Board (IRB) of the MedStar Research Institute, and the protocol for the present study was also approved by the IRB of the Johns Hopkins School of Medicine. Serum carboxymethyl-lysine (CML) levels were used as the index measure of serum AGEs in this study. CML is a dominant circulating AGE, the best characterized of all the AGEs, and a dominant AGE in tissue proteins.9 CML was measured using a competitive ELISA (AGE-CML ELISA, Microcoat, Penzberg, Germany).
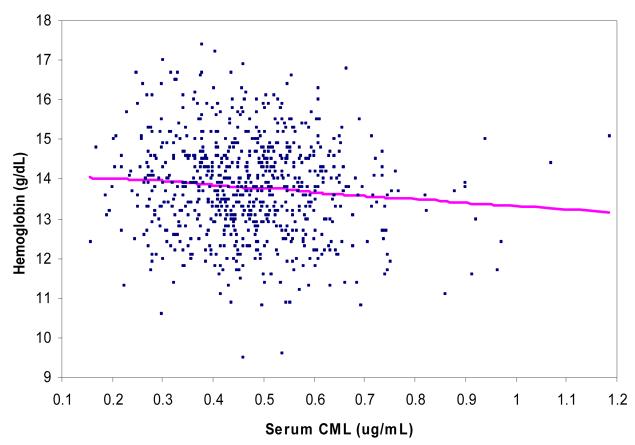
Of 751 adults, 75 (10.0%) had anemia (hemoglobin <12 g/dL for women and <13 g/dL for men). Among anemic and non-anemic subjects, serum CML concentrations were 0.50 and 0.45 μg/mL, respectively (P = 0.005). Serum CML (per 1 Standard Deviation in all models) was associated with anemia (Odds Ratio [O.R.] 1.28, 95% Confidence Interval [C.I.] 1.01-1.63, P = 0.046) in a multivariate logistic regression model adjusting for age, sex, race, coronary heart disease, heart failure, diabetes, and renal insufficiency. The relationship between serum CML and hemoglobin is shown in a scatterplot in Figure 1. Serum CML was associated with hemoglobin (beta = −0.12, standard error = 0.04, P = 0.003) in a multivariate linear regression model adjusting for the same covariates above.
Figure 1.
Scatterplot of the relationship of serum CML with hemoglobin with Lowess smoothing line.
Alternative models were explored in which all subjects with diabetes were excluded. In non-diabetic subjects, serum CML was associated with anemia (O.R. 1.33, 95% C.I. 1.03-1.72, P = 0.029) in a multivariate logistic regression model, adjusting for age, sex, race, smoking, coronary heart disease, heart failure, and renal insufficiency. Serum CML was associated with hemoglobin (beta = −0.12, SE = 0.04, P = 0.002) in a multivariate linear regression model, adjusting for the same covariates.
The present study suggests that elevated AGEs, as indicated by serum CML, are associated with anemia. To our knowledge, this is the first study to report an association between elevated AGEs and anemia in a population of community-dwelling adults. These findings are consistent with a previous report of elevated AGEs and anemia among diabetics.7 Whether elevated serum CML and anemia are causally related is not clear. As noted previously, CML alters the deformability of erythrocytes and increases interactions between erythrocytes and the endothelial surface via interactions of erythrocyte AGE with RAGE.4-6 In addition, CML forms adducts with hemoglobin,10 but whether the formation of hemoglobin-CML affects the lifespan of erythrocytes is unknown.
AGEs are a potentially modifiable risk factor, as systemic levels of AGEs are derived primarily from exogenous AGEs ingested in foods and endogenous AGEs formed in the body. Serum AGE concentrations can be reduced substantially by decreasing dietary intake of AGEs by avoiding foods that are processed at high temperatures, i.e., deep fried, grilled, and broiled.2,3 AGE-breakers or inhibitors reduce endothelial dysfunction and improve cardiovascular and renal function,2,3 but whether they affect hemoglobin is unknown. Future studies are needed to determine whether AGEs influence the fragility or lifespan of erythrocytes. AGEs could be a potential target for interventions to prevent onset as well as progression of anemia, as serum AGEs can be lowered by change in dietary pattern and pharmacological treatment.
Supplementary Material
ACKNOWLEDGMENT
Grant Support: This work was supported by National Institute on Aging Grants R01 AG027012, R01 AG029148, and the Intramural Research Program, National Institute on Aging, NIH.
Sponsor's Role: NIH sponsored the Baltimore Longitudinal Study of Aging. NIH had no role in the design, conduct, and preparation of this paper.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
REFERENCES
- 1.Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 years and older in the United States: Evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 2.Basta G, Schmidt AM, de Caterina R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Vlassara H, Striker G. Glycotoxins in the diet promote diabetes and diabetic complications. Curr Diabetes Rep. 2007;7:235–241. doi: 10.1007/s11892-007-0037-z. [DOI] [PubMed] [Google Scholar]
- 4.Ando K, Beppu M, Kikugawa K, et al. Membrane proteins of human erythrocytes are modified by advanced glycation end products during aging in the circulation. Biochem Biophys Res Comm. 1999;258:123–127. doi: 10.1006/bbrc.1999.0606. [DOI] [PubMed] [Google Scholar]
- 5.Iwata H, Ukeda H, Maruyama T, et al. Effect of carbonyl compounds on red blood cells deformability. Biochem Biophys Res Commun. 2004;321:700–706. doi: 10.1016/j.bbrc.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Wautier JL, Wautier MP, Schmidt AM, et al. Advanced glycation end products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in the vasculature: A link between surface-associated AGEs and diabetic complications. Proc Natl Acad Sci U S A. 1994;91:7742–7746. doi: 10.1073/pnas.91.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas MC, Tsalamandris C, MacIsaac R, et al. Low-molecular-weight AGEs are associated with GFR and anemia in patients with type 2 diabetes. Kidney Int. 2004;66:1167–1172. doi: 10.1111/j.1523-1755.2004.00868.x. [DOI] [PubMed] [Google Scholar]
- 8.Shock NW, Greulich RC, Andres RA, et al. Normal Human Aging: The Baltimore Longitudinal Study of Aging. U.S. Government Printing Office; Washington, D.C.: 1984. [Google Scholar]
- 9.Reddy S, Bichler J, Wells-Knecht KJ, et al. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry. 1995;34:10872–10878. doi: 10.1021/bi00034a021. [DOI] [PubMed] [Google Scholar]
- 10.Motomiya Y, Oyama N, Iwamoto H, et al. Nε-(carboxymethyl)lysine in blood from maintenance hemodialysis patients may contribute to dialysis-related amyloidosis. Kidney Int. 1998;54:1357–1366. doi: 10.1046/j.1523-1755.1998.00091.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.