Abstract
Individuals with autism exhibit significant impairments in prosody production, yet there is a paucity of research on prosody comprehension in this population. The current study adapted a psycholinguistic paradigm to examine whether individuals with autism are able to use prosody to resolve syntactically ambiguous sentences. Participants were 21 adolescents with high-functioning autism (HFA), and 22 typically developing controls matched on age, IQ, receptive language, and gender. The HFA group was significantly less likely to use prosody to disambiguate syntax, but scored comparably to controls when syntax alone or both prosody and syntax indicated the correct response. These findings indicate that adolescents with HFA have difficulty using prosody to disambiguate syntax in comparison to typically developing controls, even when matched on chronological age, IQ, and receptive language. The implications of these findings for how individuals with autism process language are discussed.
Keywords: autism, prosody, language, comprehension, intonation, syntax, pragmatics, high-functioning
Individuals with autism have well-documented impairments in the pragmatic use of language (Young, Diehl, Morris, Hyman, & Bennetto, 2005), including marked atypicalities in expressive prosody (Tager-Flusberg, 2001). Unusual prosody, which can include atypical pitch, rhythm, or stress patterns, has been observed in both high- and low-functioning individuals with autism. These difficulties often persist even when other areas of language improve (McCann & Peppé, 2003), and can become a stigmatizing barrier to social acceptance (Shriberg et al., 2001). Despite the pervasive nature of prosodic impairment, little research has addressed its etiology (McCann & Peppé, 2003). Moreover, the existing research has focused primarily on prosodic expression, with scant attention paid to comprehension. This is an important distinction because language comprehension precedes production in early development (Hirsh-Pasek & Golinkoff, 1991). Knowing how individuals with autism process prosodic information is critical to understanding their social-communicative difficulties in many areas (e.g., understanding affect, breakdowns in conversational discourse). Research in this area may also answer questions about the etiology of communication impairments. Prosody processing has been shown to facilitate early language acquisition (Demuth & Morgan, 1996; Jusczyk, 2003), and very early deficits in prosody comprehension may lead to later impairments in prosody production or even more general pragmatic deficits.
Clinical descriptions of prosody production patterns in individuals with autism have ranged from flat or monotonous to variable, pedantic, and/or having a singsong quality (Amoroso, 1992; Baltaxe, 1984; Fay & Schuler, 1980; Goldfarb, Braunstein, & Lorge, 1956; Provonost, Wakstein, & Wakstein, 1966). Prosodic differences have been found to be significantly related to ratings of social and communicative functioning (Paul, Shriberg et al., 2005). Deficits in prosodic production are included as a diagnostic characteristic of the disorder in the Autism Diagnostic Interview, Revised (ADI-R; Rutter, Le Couteur, & Lord, 2003) and the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 1999), which are the gold-standard diagnostic tools used with this population. The importance of these clinical accounts of prosodic expression is not the type of prosody used (singsong versus monotone) but the mismatch between the words spoken, the context in which the words were spoken, and the prosody that was used by the speaker.
Despite the numerous descriptive accounts of prosodic impairments in autism, systematic research on prosodic abilities in individuals with autism is limited, especially with regard to those who have normal intellectual abilities (i.e., high-functioning autism; HFA). Participants defined as having HFA are typically described as having IQs above 80 and/or typical expressive language abilities, although there is no established definition, and studies can vary widely in their characterization of HFA. Recent studies found that approximately half of all individuals with HFA have dysfluent or impaired phrasing and inappropriate stress placement (Paul, Augustyn, Klin, & Volkmar, 2005; Shriberg et al., 2001). More specific analyses of phrasing indicate that individuals with HFA show difficulties using word or sentence stress (Fine, Bartolucci, Ginsberg, & Szatmari, 1991). Research indicates that individuals with autism who also have significant intellectual impairment (i.e., low-functioning autism; LFA) have variable ranges in the pitch and intensity of their expressive prosody (Amoroso, 1992; Baltaxe, 1984).
While there is evidence for impairment in prosodic expression, fewer studies have examined prosodic comprehension in autism. This is striking because recent research indicates that aspects of prosody perception may be impaired in individuals with autism during infancy (Kuhl, Coffey-Corina, Padden, & Dawson, 2005). The majority of rigorous investigations into prosody comprehension in autism focus on understanding mental or affective states from prosody (Hobson, 1986; Hobson, Ouston, & Lee, 1988; Kleinman, Marciano, & Ault, 2001; Rutherford, Baron-Cohen, & Wheelwright, 2002). Many of these studies found deficits in these areas and suggested that impairments in prosody comprehension were related to deficits in theory of mind (Baron-Cohen, 1995), or deficits in affective processing (Hobson, 2004).
One way to investigate if there are deficits for prosody processing in autism beyond affective/mental state understanding is to examine the ability of individuals with autism to utilize prosody to determine linguistic (syntactic/semantic) structure from clause boundaries, an approach which is commonly used in psycholinguistic studies (Kraljic & Brennan, 2005; Snedeker & Trueswell, 2003). Prosody can be used in a way that indicates the meaning of a sentence without necessarily reflecting a person’s affective or mental state. Typical adults show sensitivity to how prosody influences sentence structure (Snedeker & Trueswell, 2003). Some studies have concluded that children as old as 11–12 years still have difficulty using prosody to resolve ambiguities in word and sentence meaning (Vogel & Raimy, 2002), although Snedeker & Yuan (in press) have suggested that children as young as 5 years may be better at this than previously thought.
Several studies have begun to examine how individuals with autism use prosody to make linguistic decisions. Paul and colleagues (Paul, Augustyn et al., 2005) found that adolescents and young adults with HFA (average age = 17 years) were marginally worse at comprehending grammatical stress differences within words (e.g., “PROgress” versus “proGRESS”)1 than typically developing controls matched on chronological age. The HFA group was not, however, impaired at using prosody to determine phrase structure (e.g., [chocolate cake] [and cookies] versus [chocolate] [cake and cookies])2. Because of the relatively small sample size of the control group (N=13), however, it was likely difficult to detect medium effect sizes. Additionally, the groups were not matched on language or IQ, so it is possible that group differences were a result of an underlying language or intelligence differences rather than a prosodic deficit. Moreover, the authors noted that stimuli were produced in vivo by the experimenter and therefore they were not standardized across administrations. This is important because even small variations in prosody can affect the interpretation of a phrase structure. Finally, the practice items included training that highlighted the importance of paying attention to prosody.
Similarly, Peppé and colleagues (Peppé, McCann, Gibbon, O'Hare, & Rutherford, 2006) did not find differences in perception of phrasal chunking in children with HFA (ages 6–13 years) when compared to typically developing children (no chronological ages given) who were matched on verbal mental age. The authors used the Profiling Elements of Prosodic Systems – Children (PEPS-C; Peppé & McCann, 2003), a standardized measure of prosodic functioning, one component of which involved resolving syntactic ambiguities in phrases. Participants viewed two pictures (one with a chocolate cake and bread buns, the other with chocolate, cake, and bread buns) and chose the correct picture based on the prosody they heard ([chocolate cake] [and buns] versus [chocolate] [cake and buns]). Although the authors did not find significant group differences, both groups performed roughly at chance on the task, which may have masked group differences. Chance performance is understandable, given that the performance of typical individual in this mental age range has been inconsistent (see Snedeker & Yuan, in press, for a brief review).
Several smaller studies looked at aspects of comprehension of prosody as a determinant of linguistic structure. Fostnot & Jun (1999) examined four children with autism (IQ/language levels not specified) and found that deficits in imitating timing and chunking patterns were correlated with severity of autism. Scott, Stamm, Lee, & Dapretto (2005) showed that children with autism (IQ/language levels not specified) did not use prosody to learn words in a novel language task. In contrast, an early study by Frith (1969) of adolescents with LFA found intact use of stress to aid sentence recall. Findings from a brain imaging study suggested intact processing of linguistic-prosodic stimuli (Erwin et al., 1991).
In summary, there has been a paucity of research on prosody in autism, and particularly on prosody comprehension. It is unclear whether observed deficits in prosody processing can be explained by underlying deficits in mental state or affective processing, or whether the impairment encompasses using prosody to make linguistic decisions as well. Research on the use of prosody to determine sentence structure by individuals with autism has produced inconsistent findings across studies. Moreover, it has yet to be determined whether impairments are specific to prosody or related to deficits in general language ability. Additionally, authors of previous studies identified a need for controlled stimuli of appropriate difficulty for higher-functioning individuals (Paul, Augustyn et al., 2005; Peppé, McCann, Gibbon, O'Hare, & Rutherford, 2007).
The present study aimed adapted a psycholinguistic paradigm to investigate whether individuals with autism have difficulty using prosody to disambiguate sentence structure in order to determine sentence meaning. Deficits in using prosody in this manner would suggest that factors other than mental state or affective processing may be implicated in the etiology of observed prosodic deficits in autism. We hypothesized that individuals with autism would have difficulty using prosody to resolve syntactic ambiguities when compared to typically developing controls matched on chronological age, IQ, and general language abilities.
Methods
Participants
Participants were 21 individuals with HFA and 22 typically developing control participants. Ages in both groups ranged from 11 to 19 years. Because most research on prosody comprehension has been done with typical adults, this age range allowed for comparison to previous studies while still retaining a developmental perspective. All participants were native English speakers. Intellectual functioning was established with the Wechsler Intelligence Scale for Children, 4th Ed. (WISC-IV; Wechsler, 2003) or the Wechsler Adult Intelligence Scale, 3rd Ed. (Wechsler, 1997). Participants also received a language and hearing evaluation. Language functioning was examined via the Receptive Language Index (RLI) of the Clinical Evaluation of Language Fundamentals, 4th Ed. (CELF-IV; Semel, Wiig, & Secord, 2003). Hearing was evaluated using a Maico MA 32 Audiometer. Participants with hearing loss in either ear greater than 20 decibels within the frequency range of 1000–4000 Hz were excluded based on the American Speech and Hearing Association’s criteria for determining hearing impairment in children 5–18 years old. Individuals with autism were excluded if they had a diagnosis of a genetic syndrome (e.g., fragile X syndrome) or a definable postnatal etiology for their developmental symptoms (e.g., head trauma, tumor).
For the HFA group, diagnoses were confirmed with a combination of the Autism Diagnostic Interview-Revised (ADI-R; Rutter et al., 2003) with the caregiver, and the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1999) with the child. Only subjects who met diagnostic criteria for autism on both the ADI-R and ADOS were included. For the typically developing controls, only participants who failed to meet diagnostic criteria for any pervasive developmental disorder on both the ADI-R and ADOS were included in this group. In addition, the control group had no parent-reported behavioral disorders as indicated by a factor T-score above 70 on the Child Behavior Checklist (Achenbach & Rescorla, 2001), no clinically relevant psychiatric diagnoses on the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS; Kaufman, Birmaher, Brent, Rao, & Ryan, 1996), no history of learning disabilities, and no family history of an autism spectrum disorder in their 1st or 2nd degree relatives.
The overall means of the two groups were matched on age, Full Scale IQ, and receptive language abilities (see Table 1). Only subjects who demonstrated a CELF-4 RLI standard score and Wechsler Full Scale IQ score greater than 85 were included. Groups were also matched on gender composition. The groups were primarily Caucasian (90% in HFA group, 95% of control group).
Table 1.
Descriptive Characteristics of the Sample
| HFA Group | Control Group | F or X2 | p | |
|---|---|---|---|---|
| M (SD) | M (SD) | |||
| [range] | [range] | |||
| N | 21 | 22 | ||
| Chronological Age | 15.28 (2.39) | 15.58 (2.31) | .25 | .62 |
| [11–19] | [11–19] | |||
| Full Scale IQ | 111.81 (12.46) | 110.52 (10.23) | .16 | .69 |
| [88–131] | [94–124] | |||
| Receptive Language | 106.14 (11.62) | 104.91 (7.50) | .50 | .48 |
| [86–128] | [86–119] | |||
| Gender (M:F) | 17:4 | 17:5 | .07 | .54 |
Note: Full Scale IQ was measured by WISC-IV or WAIS-III. Receptive Language was measured by CELF-IV RLI (M=100, SD=15).
Task Design
To examine whether individuals with HFA have difficulty using prosody to make basic decisions regarding sentence structure, we adapted a syntactic ambiguity paradigm (Kraljic & Brennan, 2005; Snedeker & Trueswell, 2003). In this paradigm, some sentences have ambiguous meaning that is disambiguated by prosody, and for others, syntax is structured so that the meaning is not ambiguous:
-
1a.
[Put the dog] [in the basket on the star]
-
1b.
[Put the dog in the basket that’s on the star]
-
1c.
[Put the dog] [in the basket that’s on the star]
In all of these conditions, the meaning of the sentence is the same (i.e., put an animal inside of a container that is sitting on a shape), and the prosodic cues and/or syntactic structure signal a verb phrase (VP) attachment. In other words, they indicate that the prepositional phrase (in the basket on the star) is attached at the level of the verb (put) and interpreted as the destination for the movement of the dog. Therefore, the resulting behavior is that the dog is placed in a basket that is on a star. Alternatively:
-
2a.
[Put the dog in the basket] [on the star]
-
2b.
[Put the dog that’s in the basket on the star]
-
2c.
[Put the dog that’s in the basket] [on the star]
In these sentences, the interpretation is that a dog that is already in a basket should be placed on a star. Therefore, “in the basket” describes the location of the dog, while the destination of the movement is the star. This interpretation is based on a noun phrase (NP) attachment: the prepositional phrase (in the basket) attaches within the noun phrase and modifies the head noun (dog).
Stimulus sentences were created for three conditions: 1) where prosody disambiguated the sentence meaning (Prosody Only condition), 2) where syntax was not ambiguous but no additional prosodic cue to structure was provided (Syntax Only condition), or 3) where prosodic boundaries were given that were congruent with an unambiguous syntactic structure (Prosody + Syntax condition). The sentence-internal prosodic boundary in the Prosody Only and Prosody + Syntax conditions was characterized by a significant pause and a boundary tone (H%, as represented by the ToBI system; Silverman et al., 1992) marking the end of the phrase. In the Syntax Only condition, the entire sentence was pronounced as a single prosodic phrase, without significant pauses and with a boundary tone (L%) only at the end of the sentence. In each of the three conditions, there was one sentence construction designed to elicit a VP-attachment interpretation and one created to elicit an NP-attachment interpretation (6 constructions total). Furthermore, each of these 6 sentence constructions was recorded 4 times, each time with a different animal-container-shape combination (e.g., dog-box-star, pig-basket-blanket, cat-bowl-square, cow-cup-circle). This resulted in a total of 24 target sentences, with 8 per condition.
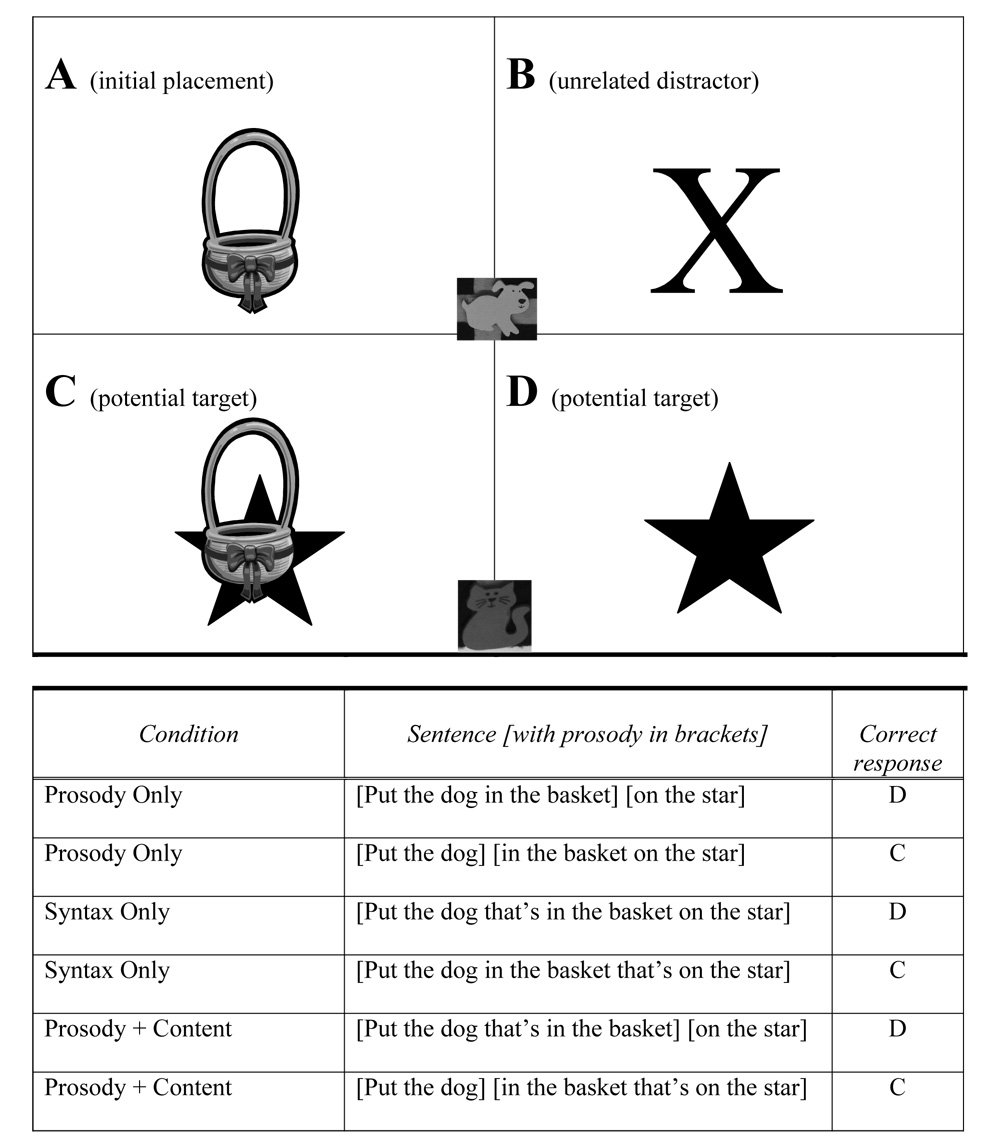
During the task, the participant was seated at a table with an array of objects easily within reach (e.g., a container, a shape, a container on top of a shape, an unrelated distracter, and two flat animals; see Figure 1). All objects were affixed to the board. In some conditions, the experimenter handed the participant a toy animal which was not affixed to the board (e.g., a dog), and in other conditions, the same toy was already positioned on the board (but not affixed). Participants were told in the instructions which objects were attached (and thus, not appropriate for moving) and which objects were not. Objects were affixed to the board so only one correct response was possible to prevent a participant from being able to put both the animal and the container on the shape, which left putting the animal on the shape as the only viable correct response. Two boards were created (dog/cat board, pig/cow board) for this task. While one board was being administered, the other board was being set up by a research assistant (outside the testing room) for the next trial. The two boards were used for 12 trials a piece.
Figure 1.
Syntactic Ambiguity Task Layout.
Note. In this task, participants were given the above instructions to move a toy animal (e.g., dog; not pictured) around the board. The cat and the dog on the board represent flat animals that were velcroed to the board and could not be moved by the participants.
Participants listened to stimuli through a CD player, a comfortable and audible level was chosen by the subjects, since none of these tasks required sound discriminations. Stimuli were prerecorded so that all participants heard the exact same stimuli. On each of the 24 trials, the participant heard 4 sentences indicating a movement or action relating to objects on the board. One of the sentences (or the original setup when the board was handed to the participant) created the Target Setup in which the participant put the animal into a container (e.g., a basket) that was on the board (e.g., “Put the dog in the basket”; see Figure 1, Box A). The participant then heard a target sentence instructing him/her to move the animal to a new position (Figure 1, Box B, C, or D). It should be noted that two smaller flat animals were affixed to the board (see Figure 1) in order to minimize the lexical bias of “Put the dog” when only one animal is present (Spivey, Tannenhaus, Eberhard, & Sedivy, 2002). The two filler sentences in each trial were designed to be unrelated to the target sentence. For example, one trial contained a mailbox as the unrelated distracter (Figure 1, Box B), and one of the fillers was “Check the mail,” while the other filler was “Count the stars on the board.”
Accuracy was measured as the percentage of correct responses overall and separately for each condition. Trials across all 3 conditions were presented together. The order of trials was randomized, and then divided into four overall orders, which were counterbalanced across groups (Order 1 = Trials 1-24; Order 2 = Trials 13-24, then Trials 1-12; Order 3 = Trials 24-1; Order 4 = Trials 12-1, then trials 24-13). The purposes of this randomization were to minimize the chance that participants would realize that prosody comprehension was the goal of the study and to lessen the influence practice effects could have on any one particular trial.
Prosody Only condition
In this condition, prosody indicated the correct response in the presence of ambiguous syntax. In other words, this condition tested the ability of participants use prosodic information with an ambiguous sentence construction to determine sentence structure. As shown in Figure 1, the following target sentences had identical lexical content and ambiguous syntactic structure, but the prosody (noted by brackets) indicated different actions: (1) [Put the dog in the basket] [on the star] indicates that Box D is the appropriate response, (2) [Put the dog] [in the basket on the star] indicates that Box C is appropriate. In the first case, the dog (which was already in the basket) was to be placed on the star. In the second case, the dog should have been placed in a different basket that was sitting on a star. It was predicted that this condition would be difficult for individuals with autism because it requires the use of a prosodic cue alone to disambiguate the sentence meaning.
Syntax Only condition
Sentences were presented with ambiguous prosodic phrasal breaks, but syntactic information indicated the correct response, thus testing a participant’s basic linguistic processing in the absence of prosodic cues. For example, in the following examples taken from Figure 1, the prosody was ambiguous and the syntax clearly indicated the appropriate action: (1) [Put the dog that’s in the basket on the star] indicated Box D, (2) [Put the dog in the basket that’s on the star] indicated Box C.
Prosody + Syntax (Congruent) condition
This condition tested the participant’s responses when syntactic structure was unambiguous, and prosodic cues were congruent with the structure. Sentences were presented with both prosodic breaks and a disambiguating syntactic construction (e.g., [Put the dog] [in the basket that’s on the star]).
Performance was compared across the three conditions. It was predicted that individuals with HFA would perform similarly to controls in the Syntax Only and Prosody + Syntax conditions because they were matched on receptive language abilities. Poor performance in the Prosody Only condition would suggest impairments in their ability to use prosody to interpret sentence structure in a strictly linguistic context (i.e., in the absence of mental state processing).
Piloting
All stimuli were piloted first with college students and then with typically developing children and adolescents (ages 9–19) to determine appropriate difficulty levels and number of trials. Overall, piloting provided support that this task was a valid measure of resolution of ambiguities. Children and undergraduates were able to utilize prosodic and syntactic cues to disambiguate sentence meaning. The Prosody Only condition was the most difficult, and was more difficult for children than adults. Finally, sentences in the Prosody Only condition (and to a lesser extent, the Syntax Only condition) were found to have a slight lexical bias (resulting from the word “put”) to a VP-attachment interpretation. This is consistent with previous research indicating that some verbs (e.g., put, hit) that necessitate a location argument (a place for an object to go) tend to have a bias toward a VP-attachment interpretation (Trueswell, Tanenhaus, & Kello, 1993).Therefore, performance in the VP-attachment sentences was likely to be better than NP-attachment sentences, so these two categories were analyzed both together and separately in the actual study to identify within- and between-group patterns of performance.
Results
Effect sizes were calculated as partial eta squared (η2partial), where appropriate, to measure the degree of association between variables. Values between .01 and .06 are considered a small effect size, between .06 and .14 are considered a medium effect size, and above .14 are considered a large effect size. In other analyses, we calculated effect size with Cohen’s d; effect sizes between .2 and .5 are considered small, between .5 and .8 medium, and above .8 large.
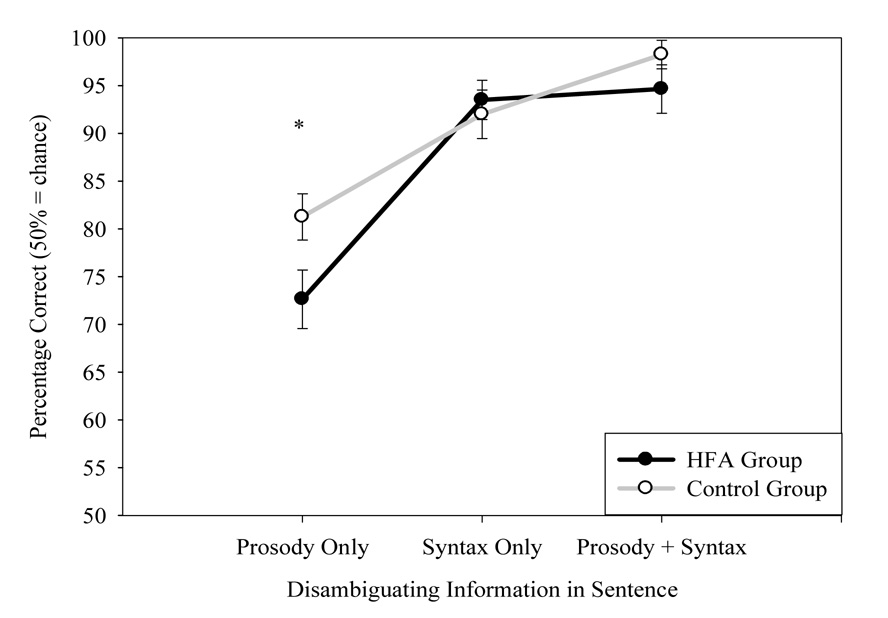
Performance on all conditions of the syntactic ambiguity task was measured by the total number of correct movements (i.e., movements to the intended target). To measure the effect of prosody and syntactic structure on sentence processing in participants with HFA and controls, a repeated measures ANOVA was performed with sentence type (Prosody Only, Syntax Only, Prosody + Syntax) as the within-subjects factor. Overall, there was no main effect of group, F(1,41)=1.27, p=.27, η2=.03. There was a main effect of sentence disambiguation condition, F(2,82)=50.50, p<.001, η2=.55. Across both groups, participants performed worse in the Prosody Only condition than the Congruent (Prosody + Syntax) condition, t(43)=8.58, p<.001, d=2.68, and the Syntax Only condition, t(43)=6.66, p<.001, d=2.08. Averaging over groups, participants were also marginally better at the Prosody + Syntax condition than the Syntax Only condition, t(43)=1.71, p=.10, d=.53. This pattern indicated that using prosody to disambiguate sentence structure was more difficult than the two conditions with unambiguous syntax, but that the presence of congruent prosody made correct sentence interpretation marginally easier even when both conditions had syntactic structure that was not ambiguous.
As predicted, there was a significant group × sentence type interaction, F(2,82)=3.42, p<.04, η2=.08. Consistent with the hypotheses, the participants with HFA were significantly worse than controls at using prosody alone to disambiguate sentence meaning, F(1,41)=4.91, p<.03, η2=.11, but performed similarly to controls when judging meaning based on syntax only, F(1,41)=.18, p=.67, η2=.004, or on congruent prosody and syntax, F(1,41)=.21, p=.65, η2=.005 (see Figure 2).
Figure 2.
Group Performances on the Syntactic Ambiguity Task
Note: Error bars represent the standard error of the mean (± SEM).
*p<.05
Attachment bias
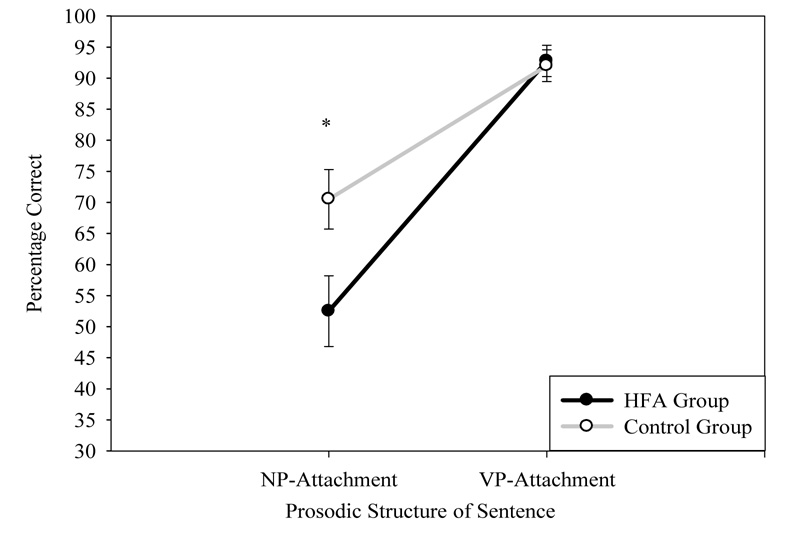
Because a bias for VP-attachment in the Prosody Only Condition was observed in piloting, data were examined to investigate whether there were group differences in attachment bias that could have affected the findings. Although this was not a planned comparison, it was important to understand this effect in order to address this issue better in future studies. Three repeated measures ANOVAs were conducted, one for each sentence condition (i.e., Prosody Only, Syntax Only, Prosody + Syntax). For all ANOVAs, group was the between-subjects factor, and attachment type (VP-attachment, NP-attachment) was the within-subjects factor. Because the analyses above already examined the main effects of group on sentence conditions, the critical tests of the following ANOVAs were the main effect of attachment type and the group × attachment type interaction. In the Prosody Only condition, there was a main effect of attachment type, with participants performing better on VP-attachment items than on NP-attachment items, F(1,41)=50.71, p<.001, η2=.55. In addition, there was a significant group × attachment type interaction, F(1,41)=4.70, p<.04 η2=.10 (see Figure 3). Participants with HFA were significantly worse at identifying NP-attachment than controls, F(1,41)=5.89, p<.02, η2=.13, but performed similarly to controls on VP-attachment trials, F(1,41)=.05, p=.82, η2=.001. In the Syntax Only condition, there was also a main effect of attachment type, with participants across both groups showing a bias for a VP-attachment, F(1,41)=4.51, p<.04, η2=.10. There was, however, no group × attachment type interaction, F(1,41)=.74, p<.40, η2=.02. In the Prosody + Syntax condition, there was no main effect of attachment type, F(1,41)=1.32, p=.26, η2=.03, or group × attachment type interaction, F(1,41)=.61, p=.44, η2=.02.
Figure 3.
Effect of Type of Prosodic Structure on Group Performance on Syntactic Ambiguity Task, Prosody Only Condition.
Note: Error bars represent ± SEM.
Age effects
Because some studies have suggested that children as old as 11 to 12 years still have difficulty using prosody to resolve ambiguities in word and sentence meaning (Vogel & Raimy, 2002), we conducted exploratory analyses to investigate differential effects age might have on the HFA and control groups. Post hoc analyses showed that performance in the Prosody Only condition was marginally correlated with age in the typical control group r(20)=.39, p<.08, and not in HFA group, r(19)=.20, p=.39, although these r’s were not significantly different using Fisher’s z’ transformations, Z=.64, p=.53. Age was not significantly correlated with performance on the Syntax Only condition in either the HFA group, r(19)=−.17, p=.45, or the control group, r(20)=.−.18, p=.42, and age was also not related to performance in the Prosody + Syntax condition in the HFA group, r(19)=.06, p=.79, or the control group, r(20)=.10, p=.66. Because there were significant group differences in performance between attachment conditions in the Prosody Only condition, we also conducted analyses on the NP-attachment and VP-attachment conditions. Correct identification of NP-attachment in the Prosody Only condition was marginally correlated with age in controls, r(20)=.39, p<.07, but not in the HFA group, r(19)=.14, p=.55, although this difference was not statistically significant, Z=.82, p=.40. Age was not correlated with performance in the VP-attachment condition for either the HFA group, r(19)=.17, p=.46, or the control group, r(20)=.10, p=.66. Thus, there is some indication that typically developing adolescents showed improvement with age in the NP-attachment condition, but it was not significantly more than was shown by the HFA group. Because these analyses were post hoc, and the findings were only marginally significant, these data should be interpreted with caution.
Discussion
This study investigated the ability of children and adolescents with HFA to use prosodic phrasing to understand the intended meaning of a spoken utterance. As predicted, the participants with HFA had more difficulty using prosody to disambiguate sentence meaning than typically developing controls matched on age, IQ, and receptive language abilities. Individuals with HFA performed similarly, however, when syntax was unambiguous, regardless of whether or not prosodic structure provided additional congruent information. These findings are striking given that the participants with HFA were adolescents with IQs in the normal to above average range who were well matched to controls on receptive language abilities. Previous studies that examined the use of prosody to resolve lexical ambiguities (Paul, Augustyn et al., 2005) or ambiguities within phrases (Peppé et al., 2006) suggested intact processing in this population. One reason that the current study identified differences in using prosody in this context is that, compared to previous studies, the current study examined the resolution of syntactic ambiguities (as opposed to lexical/phrasal ambiguities), a choice which may have increased the difficulty of the task overall. It is possible that using prosody for syntactic processing is a more complex process—and one that is learned later in development—than the resolution of lexical/phrasal ambiguities.
An unexpected finding in the present study was that individuals with HFA had difficulty interpreting an ambiguous sentence structure as having an NP-attachment structure (e.g., [Put the dog in the box] [on the star]) when prosody was the only available disambiguating information. It should be noted, however, that performance in the VP-attachment condition was at ceiling, and this may not be a valid dissociation. It is possible that individuals with HFA have particular difficulty identifying NP-attachments, although the mechanism for a deficit such as this is unclear. Alternately, it is possible that the “ambiguous” sentence structure in this task was not purely ambiguous. Research on typical adults showed that some verbs (such as the verb put used in this study) have a lexical bias toward a VP-interpretation (Snedeker & Trueswell, 2003). The normal order of arguments for put is NP PP (prepositional phrase), where the NP designates the object to be moved and the PP a location. It is more common for a locative PP (e.g., in the basket) occurring after the object noun to serve as the location argument than to attach within the NP. Therefore, a VP-attachment interpretation is more likely to be assumed, because of its relatively high frequency. Verbs such as put tend to assign a provisional grammatical analysis to the constituent as soon as they are encountered (Chambers, Tanenhaus, & Magnuson, 2004). Similarly, in our piloting, we found that VP-attachments were easier to identify than NP-attachments.
The finding that individuals with HFA had difficulty interpreting NP-attachment when presented with an incongruent lexical bias raises the possibility of alternate explanations for these findings. First, it is possible that participants with HFA are able to use prosody, but in this design they assigned a provisional syntactic structure to the sentence (because of the nature of put) before the point of disambiguation. Later in the sentence, they were unable to disengage from this interpretation once the disambiguating prosodic information was apparent. Individuals with HFA have well-documented impairments in cognitive flexibility (e.g., Ozonoff & Jensen, 1999), which may limit their ability to revise their initial interpretation, leading them to persist in their initial VP-attachment interpretation regardless of subsequent information. It is also possible that individuals with HFA exhibit a developmental delay in using prosody to overcome this lexical bias. Research has indicated that typically developing children are not able to use prosody to overcome this bias until late childhood or early adolescence (Trueswell, Sekerina, Hill, & Logrip, 1999; Vogel & Raimy, 2002). In our study, the ability to overcome this lexical bias showed marginal improvement with age in controls, and to a lesser extent in the HFA group, but the difference between groups was not significant. Future work based on a broader age range or on longitudinal data in prosody use could address this question of deviance versus delay.
It is also possible that individuals with autism have difficulty integrating prosody with information streams. Importantly, speaker’s prosody can affect a listener’s interpretation of a sentence prior to the onset of an ambiguity, demonstrating that prosodic cues not only influence sentence parsing but also can be used to predict unspoken constructions (Snedeker & Trueswell, 2004). If individuals with HFA were integrating prosodic information with speech, their provisional interpretation would have been based on the prosodic cue even with the lexical bias. Instead, individuals with HFA (and younger controls in previous studies; Trueswell et al., 1999) appeared to rely on one stream of information (sentence content) and disregarded prosodic information. Using this strategy, they would be more likely to yield to the lexical bias and assign a provisional VP-attachment interpretation. Therefore, it is possible that integration deficits could explain these findings without deficits in cognitive flexibility.
Implications for Understanding Autism, Prosody, and the Brain
The findings from this study raise several important questions that are crucial to the relationship between putative prosody processing deficits in autism, the relationship of those deficits to theories of autism, and how research in this area can provide insight into neural substrates of prosody processing. The first implication of these findings is that the prosody impairment in autism may not be specific to affective processing. Theories of autism have posited that deficits in emotion or mental state processing may be central to the disorder (Baron-Cohen, 1995; Hobson, 2004). The linguistic/affective prosody distinction has also been important for investigating how prosody is processed in the brain in typical individuals. Some results suggest that all suprasegmental aspects of speech are lateralized to the right hemisphere (Berckmoes & Vingerhoets, 2004; Eisenmajer et al., 1996), while others have argued that prosody that serves a linguistic function (e.g., lexical stress, phrasing, speech acts) involves the left hemispheric structures (Gandour et al., 2004; Wildgruber, Pihan, Ackerman, Erb, & Grodd, 2002). Our findings are consistent with the idea that the prosody system as a whole may be disrupted in autism, and that related brain structure/function abnormalities found in autism will be related to the system as a whole, not just related to processing affective prosody.
A possible explanation for our findings is that children with autism have difficulty integrating the information from prosody with other aspects of language such as sentence structure. A recent theory developed from imaging data suggests that there is a temporo-frontal pathway lateralized in the right hemisphere for prosody and a similar pathway lateralized in the left hemisphere for syntax/semantics, and that these pathways are connected via the corpus callosum. Studies have found reductions in size of the corpus callosum in autism (Hardan, Minshew, & Keshavan, 2000; Piven, Bailey, Ranson, & Arndt, 1997), as well as decreased functional connectivity between hemispheres (Egaas, Courchesne, & Saitoh, 1995; Piven et al., 1997), which may lead to reduced information integration capacity (Just, Cherkassky, Keller, & Minshew, 2004; Schultz, Romanski, & Tsatsanis, 2000). Consequently, studies of autism and typical development should examine further the role of the corpus callosum in prosody processing.
It is also possible that frontal areas involved in the integration and interpretation of information could be implicated in prosody processing deficits in autism. A recent study of irony comprehension (Wang, Lee, Sigman, & Dapretto, 2006) found increased activation in the inferior frontal gyrus (IFG) and bilateral temporal poles when prosody, sentence meaning, and/or context were incongruent. In our study, the condition in which children with autism had the most difficulty was when prosody indicated an NP-attachment interpretation, while there was an apparent lexical bias for a VP-attachment. Thus, future studies should examine the role of interpreting incongruent cues from prosody and other aspects of language or context, and also the role of the IFG in this process. Advances are needed in understanding how prosody is processed in the brain to fully understand this auditory network and how it is disrupted in individuals with autism.
Limitations and Future Directions
One design limitation of the present study is that for both control conditions—in which either syntax alone or prosody + syntax indicate meaning—performance was very high in both groups. It is thus difficult to determine whether or not deficits in these contrast conditions were masked because they were not sufficiently difficult for comparison. A related concern is that the conditions were not matched in difficulty in the control group, which increases the possibility that any group differences could be the result of a psychometric artifact. In particular, the condition for which there was a predicted difference (Prosody Only) was significantly more difficult than the other two conditions for the control group, making it more likely that we would detect differences in that condition (Chapman & Chapman, 2001; Miller, Chapman, Chapman, & Collins, 1995). Moreover, the comparison conditions did not contain ambiguity, so it could be the case that children with autism have difficulty resolving ambiguity in general, and not just using prosody for this purpose. Future studies should include other disambiguation cues, such as contextual and/or lexical cues, in isolation (without prosody) to equate comparison conditions on overall difficulty level.
It should be noted that matching the present groups on receptive language abilities may have masked deficits in prosody comprehension in HFA. First, it is likely that prosodic abilities are strongly related to overall language functioning because of their joint roles in communication. Thus, it is possible that individuals with pronounced prosody processing deficits were excluded based on related language deficits, which in turn influenced the study’s results. While language tests such as the CELF-IV do not directly measure prosody comprehension, performance on these tests is necessarily influenced by prosody comprehension. These findings should be interpreted in the context of this matching procedure, and future studies should examine carefully the relationship between prosody and general language functioning and the role this relationship plays in understanding communication deficits in autism.
Clinical Implications
Prosody plays an integral role in everyday social and academic functioning, and deficits in prosody have been shown to persist even as other areas of language development improve (McCann & Peppé, 2003). Nonetheless, it is rarely a focus of treatment, and there is a strong need for empirically validated interventions for prosody and other aspects of pragmatic communication (Wolf, Fein, & Akshoomoff, 2007). In the present study, an important skill deficit in autism appears to be one of using prosody to interpret sentence structure/meaning. Thus, intervention should focus on integrating these modalities of communication. Second, these results indicate deficits in prosody processing, which in itself is thought to facilitate early language acquisition, and may play an important role in the development of expressive prosody and language in general. Indeed, performance in the Prosody Only condition was marginally correlated with receptive language scores, r=.4, p=.08, even though we selected only children in the normal to above average range of functioning, further supporting the importance of the relationship between prosody and language. Therefore, prosody processing and production should be a focus of study in the very early development of children with autism. Early identification of language deficits, even those present before the onset of speech, may lead to earlier identification and improved treatments for the disorder. Finally, a better understanding of how individuals with autism process prosody will lead to a more accurate understanding and classification of prosody production as a diagnostic characteristic of the disorder. The present findings suggest deficits in the routine use of prosody to facilitate interpretation of other aspects of speech in autism. From a language production perspective, it is possible that differences in atypical productive prosodic patterns (e.g., monotone versus sing-songy) are not as important as the idea that there is a mismatch between prosody and speech, as evidenced by poor integration of the two information streams from a productive standpoint.
Summary
This study showed how the use of psycholinguistic paradigms can be beneficial to the understanding of language and prosody processing in individuals with autism. We adapted a syntactic ambiguity paradigm (Snedeker & Trueswell, 2003) to identify a deficit in using prosody to determine linguistic structure in autism. Results suggest that there are autism-specific deficits in the use of prosody to resolve ambiguity. Future research would continue to benefit from the adaptation of well-validated psycholinguistic paradigms to understand communication deficits in this population.
Acknowledgements
We are grateful to the children and families who participated in this research. This project was supported in part by NIH grants U54 MH066397 (Rochester STAART Center) and M01 RR00044 (General Clinical Research Center). Finally, we also thank our research assistants who assisted in the execution of the project: Mallory Bucell, Kelley Knoch, Julia Watson, and Greg Witkin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Lexical stress (i.e., syllable accent) is indicated in this paper by capitalization of the stressed syllable.
Prosodic phrasing is indicated by brackets.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Amoroso H. Disorders of vocal signaling in children. In: Papousek H, Jurgens U, Papousek M, editors. Nonverbal Vocal Communication: Comparative and developmental approaches. Cambridge: Cambridge University Press; 1992. pp. 192–204. [Google Scholar]
- Baltaxe C. Use of contrastive stress in normal, aphasic, and autistic children. Journal of Speech and Hearing Research. 1984;27:97–105. doi: 10.1044/jshr.2701.97. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Mind blindness. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Berckmoes C, Vingerhoets G. Neural foundations of emotional speech processing. Current Directions in Psychological Science. 2004;13:182–185. [Google Scholar]
- Chambers CG, Tanenhaus MK, Magnuson JS. Actions and affordances in syntactic ambiguity resolution. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30:687–696. doi: 10.1037/0278-7393.30.3.687. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Commentary on two articles concerning generalized and specific cognitive deficits. Journal of Abnormal Psychology. 2001;110:31–39. doi: 10.1037//0021-843x.110.1.31. [DOI] [PubMed] [Google Scholar]
- Demuth K, Morgan JL. Signal to Syntax: Bootstrapping from Speech to Grammar in Early Acquisition. Mahwah, NJ: Lawrence Erlbaum Associates; 1996. [Google Scholar]
- Egaas B, Courchesne E, Saitoh O. Reduced-size of corpus-callosum in autism. Archives of Neurology. 1995;52:794–801. doi: 10.1001/archneur.1995.00540320070014. [DOI] [PubMed] [Google Scholar]
- Eisenmajer R, Prior M, Leekam S, Wing L, Gould J, Welham M, et al. Comparison of clinical symptoms in autism and Asperger's disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:1523–1531. doi: 10.1097/00004583-199611000-00022. [DOI] [PubMed] [Google Scholar]
- Erwin R, Vanlancker D, Guthrie D, Schwafel J, Tanguay P, Buchwald JS. P3 responses to prosodic stimuli in adult autistic subjects. Electroencephalography and Clinical Neurophysiology. 1991;80:561–571. doi: 10.1016/0168-5597(91)90139-o. [DOI] [PubMed] [Google Scholar]
- Fay W, Schuler AL. Emerging language in autistic children. Baltimore: University Park Press; 1980. [Google Scholar]
- Fine J, Bartolucci G, Ginsberg G, Szatmari P. The use of intonation to communicate in pervasive developmental disorders. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1991;32:771–782. doi: 10.1111/j.1469-7610.1991.tb01901.x. [DOI] [PubMed] [Google Scholar]
- Frith U. Emphasis and meaning in recall in normal and autistic children. Language and Speech. 1969;12:29–38. doi: 10.1177/002383096901200103. [DOI] [PubMed] [Google Scholar]
- Gandour J, Tong YX, Wong D, Talavage T, Dzemidzic M, Xu YS, et al. Hemispheric roles in the perception of speech prosody. Neuroimage. 2004;23:344–357. doi: 10.1016/j.neuroimage.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Goldfarb W, Braunstein P, Lorge I. A study of speech patterns in a group of schizophrenic children. American Journal of Orthopsychiatry. 1956;26:544–555. doi: 10.1111/j.1939-0025.1956.tb06201.x. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Keshavan MS. Copus callosum size in autism. Neurology. 2000;55:1033–1036. doi: 10.1212/wnl.55.7.1033. [DOI] [PubMed] [Google Scholar]
- Hirsh-Pasek K, Golinkoff RM. Language comprehension: A new look at some old themes. In: Krasnegor NA, Rumbaugh DM, Schiefelbush RL, Studdert-Kennedy M, editors. Biological behavioral determinants of language development. Hillsdale, N.J.: Erlbaum; 1991. [Google Scholar]
- Hobson RP. The autistic child's appraisal of expressions of emotion - a further study. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1986;27:671–680. doi: 10.1111/j.1469-7610.1986.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Hobson RP. Autism and Emotion. In: Volkmar FR, Paul R, Klin A, Cohen DJ, editors. Handbook of Autism and Pervasive Developmental Disorders. 3rd Edition ed. Vol. 1. New York: Wiley and Sons; 2004. Diagnosis, Development, Neurobiology, and Behavior. [Google Scholar]
- Hobson RP, Ouston J, Lee A. Emotion recognition in autism - coordinating faces and voices. Psychological Medicine. 1988;18:911–923. doi: 10.1017/s0033291700009843. [DOI] [PubMed] [Google Scholar]
- Jusczyk PW. The role of speech perception capacities in early language acquisition. In: Mack MBM, editor. Mind, Brain, and Language: Multidisciplinary Perspectives. Mahwah, N.J.: Lawrence Erlbaum Associates; 2003. pp. 61–83. [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. Kiddie Schedule for Affective Disorders and Schizophrenia - Present and Lifetime Version. Pittsburgh, PA: University of Pittsburgh School of Medicine; 1996. [Google Scholar]
- Kleinman J, Marciano PL, Ault RL. Advanced theory of mind in high-functioning adults with autism. Journal of Autism and Developmental Disorders. 2001;31:29–36. doi: 10.1023/a:1005657512379. [DOI] [PubMed] [Google Scholar]
- Kraljic T, Brennan SE. Prosodic disambiguation of syntactic structure: For the speaker or for the addressee? Cognitive Psychology. 2005;50:194–231. doi: 10.1016/j.cogpsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Coffey-Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Developmental Science. 2005;8:1–12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- McCann J, Peppé S. Prosody in autism spectrum disorders: a critical review. International Journal of Language and Communication Disorders. 2003;38:325–350. doi: 10.1080/1368282031000154204. [DOI] [PubMed] [Google Scholar]
- Miller MB, Chapman JP, Chapman LJ, Collins J. Task-difficulty and cognitive deficits in Schizophrenia. Journal of Abnormal Psychology. 1995;104:251–258. doi: 10.1037//0021-843x.104.2.251. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Jensen J. Brief report: Specific executive function profiles in three neurodevelopmental disorders. Journal of Autism and Developmental Disorders. 1999;29:171–177. doi: 10.1023/a:1023052913110. [DOI] [PubMed] [Google Scholar]
- Paul R, Augustyn A, Klin A, Volkmar FR. Perception and production of prosody by speakers with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2005;35:205–220. doi: 10.1007/s10803-004-1999-1. [DOI] [PubMed] [Google Scholar]
- Paul R, Shriberg LD, McSweeny J, Cicchetti D, Klin A, Volkmar F. Brief report: Relations between prosodic performance and communication and socialization ratings in high functioning speakers with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2005;35:861–869. doi: 10.1007/s10803-005-0031-8. [DOI] [PubMed] [Google Scholar]
- Peppé S, McCann J. Assessing intonation and prosody in children with atypical language development: the PEPS-C test and the revised version. Clinical Linguistics & Phonetics. 2003;17:345–354. doi: 10.1080/0269920031000079994. [DOI] [PubMed] [Google Scholar]
- Peppé S, McCann J, Gibbon F, O'Hare A, Rutherford M. Assessing prosodic and pragmatic ability in children with high-functioning autism. Journal of Pragmatics. 2006;38:1776–1791. [Google Scholar]
- Peppé S, McCann J, Gibbon F, O'Hare A, Rutherford M. Receptive and expressive prosodic ability in children with high-functioning autism. Journal of Speech, Language, and Hearing Research. 2007;50:1015–1028. doi: 10.1044/1092-4388(2007/071). [DOI] [PubMed] [Google Scholar]
- Piven J, Bailey J, Ranson BJ, Arndt S. An MRT study of the corpus callosum in autism. American Journal of Psychiatry. 1997;154:1051–1056. doi: 10.1176/ajp.154.8.1051. [DOI] [PubMed] [Google Scholar]
- Provonost W, Wakstein M, Wakstein D. A longitudinal study of speech behaviors and language comprehension in fourteen children diagnosed as atypical or autistic. Exceptional Children. 1966;33:19–26. doi: 10.1177/001440296603300104. [DOI] [PubMed] [Google Scholar]
- Rutherford MD, Baron-Cohen S, Wheelwright S. Reading the mind in the voice: A study with normal adults and adults with Asperger syndrome and high functioning autism. Journal of Autism and Developmental Disorders. 2002;32:189–194. doi: 10.1023/a:1015497629971. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Schultz RT, Romanski L, Tsatsanis K. Neurofunctional models of Autistic disorder and Asperger's syndrome: Clues from neuroimaging. In: Klin A, Volkmar FR, Sparrow SS, editors. Asperger's Syndrome. New York: Plenum Press; 2000. pp. 179–209. [Google Scholar]
- Scott AA, Stamm K, Lee SS, Dapretto M. Implicit language learning in children with autism: An fMRI study of word segmentation; Paper presented at the International Meeting For Autism Research; Boston, MA. 2005. [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals - 4th Edition. San Antonio: The Psychological Corporation; 2003. [Google Scholar]
- Shriberg LD, Paul R, McSweeny JL, Klin A, Cohen DJ, Volkmar FR. Speech and prosody characteristics of adolescents and adults with high-functioning autism and Asperger Syndrome. Journal of Speech Language and Hearing Research. 2001;44:1097–1115. doi: 10.1044/1092-4388(2001/087). [DOI] [PubMed] [Google Scholar]
- Silverman K, Beckman M, Pitrelli J, Ostendorf M, Wightman C, Price P, et al. TOBI: A standard for labeling English prosody. Vol. 2. Banff University of Alberta; 1992. [Google Scholar]
- Snedeker J, Trueswell J. Using prosody to avoid ambiguity: Effects of speaker awareness and referential context. Journal of Memory and Language. 2003;48:103–130. [Google Scholar]
- Snedeker J, Trueswell JC. The developing constraints on parsing decisions: The role of lexical-biases and referential scenes in child and adult sentence processing. Cognitive Psychology. 2004;49:238–299. doi: 10.1016/j.cogpsych.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Snedeker J, Yuan S. Early Syntactic parsing is interactive: Rapid effects of prosodic and lexical constraints in young children (and adults) Journal of Memory and Language. doi: 10.1016/j.jml.2007.08.001. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivey MJ, Tannenhaus MK, Eberhard KM, Sedivy JC. Eye movements and spoken language comprehension: Effects of visual context on syntactic ambiguity resolution. Cognitive Psychology. 2002;45:447–481. doi: 10.1016/s0010-0285(02)00503-0. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. Understanding the language and communicative impairments in autism. International Review of Research in Mental Retardation. 2001;23:185–205. [Google Scholar]
- Trueswell JC, Sekerina I, Hill NM, Logrip ML. The kindergarten-path effect: studying on-line sentence processing in young children. Cognition. 1999;73:89–134. doi: 10.1016/s0010-0277(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Trueswell JC, Tanenhaus MK, Kello C. Verb-specific constraints in sentence processing: Separating effects of lexical preference from garden-paths. Journal of Experimental Psychology. 1993;19:528–553. doi: 10.1037//0278-7393.19.3.528. [DOI] [PubMed] [Google Scholar]
- Vogel I, Raimy E. The acquisition of compound vs. phrasal stress: the role of prosodic constituents. Journal of Child Language. 2002;29:225–250. doi: 10.1017/s0305000902005020. [DOI] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M, Dapretto M. Neural basis of irony comprehension in children with autism: the role of prosody and context. Brain. 2006;129:932–943. doi: 10.1093/brain/awl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. San Antonio: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children - 4th Edition. San Antonio: Psychological Corporation; 2003. [Google Scholar]
- Wildgruber D, Pihan H, Ackerman H, Erb M, Grodd W. Dynamic brain activation during processing of emotional intonation: Influence of acoustic parameters, emotional valence, and sex. Neuroimage. 2002;15:856–869. doi: 10.1006/nimg.2001.0998. [DOI] [PubMed] [Google Scholar]
- Wolf JM, Fein DA, Akshoomoff N. Autism spectrum disorders and social disabilities. In: Hunter SJ, Donders J, editors. Pediatric Neuropsychological Intervention. Cambridge: Cambridge University Press; 2007. pp. 151–174. [Google Scholar]
- Young EC, Diehl JJ, Morris D, Hyman SL, Bennetto L. The use of two language tests to identify pragmatic language problems in children with autism spectrum disorders. Language, Speech, and Hearing Services in Schools. 2005;36:62–72. doi: 10.1044/0161-1461(2005/006). [DOI] [PubMed] [Google Scholar]