Abstract
The mechanism by which the paramyxovirus hemagglutinin-neuraminidase (HN) protein couples receptor binding to activation of virus entry remains to be fully understood, but the HN stalk is thought to play an important role in the process. We have characterized ectodomain constructs of the parainfluenza virus 5 HN to understand better the underlying architecture and oligomerization properties that may influence HN functions. The PIV 5 neuraminidase (NA) domain is monomeric whereas the ectodomain forms a well-defined tetramer. The HN stalk also forms tetramers and higher order oligomers with high alpha-helical content. Together, the data indicate that the globular NA domains form weak intersubunit interactions at the end of the HN stalk tetramer, while stabilizing the stalk and overall oligomeric state of the ectodomain. Electron microscopy of the HN ectodomain reveals flexible arrangements of the NA and stalk domains, which may be important for understanding how these two HN domains impact virus entry.
Keywords: Paramyxovirus, Parainfluenza virus 5, Hemagglutinin-Neuraminidase (HN), virus entry, sialic acid, membrane fusion/HN stalk
INTRODUCTION
Paramyxoviruses are membrane-enveloped viruses that contain a negative sense single-stranded RNA genome. Infection of target cells by these viruses requires the fusion of the viral and cellular membranes, which is typically mediated by two glycoproteins on the surface of the virions: the fusion (F) glycoprotein and a receptor-binding protein, HN, H or G, depending on the specific virus (Lamb and Parks, 2007). In the case of parainfluenza virus 5 (PIV 5; formerly called SV5), the receptor binding protein is the hemagglutinin-neuraminidase (HN), which can bind to and also cleave cellular sialosides. The process of membrane fusion is directly promoted by the refolding of F, which is a class I viral fusion protein and whose pre-and post-fusion structures have been determined recently (Yin et al., 2005; Yin et al., 2006). The HN protein provides three functions, including binding sialic acid receptors during virus entry, cleaving sialic acid from cellular and viral glycoproteins during virus budding and release, and activating F and the process of membrane fusion (Lamb and Jardetzky, 2007). However, the process by which HN activates F and membrane fusion still remains poorly understood. It is thought that the binding of HN to sialic acid containing receptors on the cell membrane may alter interactions between F and HN, thereby activating conformational changes in F and initiating membrane fusion (Lamb and Parks, 2007; Yuan et al., 2005; Zaitsev et al., 2004).
HN has a short N-terminal cytoplasmic tail, a single N-terminal transmembrane (TM) domain and an ectodomain which consists of a stalk region and a globular head in which the receptor binding and neuraminidase activities reside (Hosaka and Shimizu, 1972; Hsu, Scheid, and Choppin, 1979; Thompson et al., 1988). We refer here to the entire soluble HN ectodomain (enzyme active neuraminidase [NA] head domain and the stalk region) as HN(ecto), the soluble head NA domain lacking the stalk, as HN(NA), and the soluble stalk as HN stalk. The mature HN protein is modified with N-linked carbohydrate and forms a noncovalently associated tetramer, which, depending on the virus, can be composed of two disulfide-linked dimers (McGinnes, Sergel, and Morrison, 1993; Ng, Hiebert, and Lamb, 1990; Ng, Randall, and Lamb, 1989; Thompson et al., 1988). The stability of the tetramer structure was found to affect the fusogenic activity of HN (McGinnes, Sergel, and Morrison, 1993). However, consensus residues involved in tetramer formation have not been identified definitively because they have been mapped to various locations, including the TM domain, the cytoplasmic domain, the extracellular domain, or distributed in all three domains (Parks and Lamb, 1990; Takimoto et al., 1992; Thompson et al., 1988). The recently solved crystal structures of HN from Newcastle disease virus (NDV) (Crennel et al., 2000); human parainfluenza virus 3 (hPIV3) (Lawrence et al., 2004) and PIV 5 (Yuan et al., 2005), have suggested potentially common dimeric and tetrameric arrangements for the NA domains within the tetramer. For the NDV and hPIV3 HN proteins, only the NA domains were crystallized and these exist as monomers in solution. The intact PIV 5 HN ectodomain (including the stalk region) was crystallized and shown to form a tetramer in solution (Yuan et al., 2005). However, in the PIV5 HN crystal structure the stalk region was not visible, suggesting that it was disordered in the crystal lattice relative to the lattice of NA domains. All three HN protein structures are observed to form a similar dimeric arrangement in the crystals, but in the case of the NDV HN it has been suggested that this arrangement may be dependent upon ligand binding (Takimoto et al., 2002). The arrangement of the NA domains of the PIV 5 HN head region has been characterized as a “dimer of dimers”, but none of the interactions between neuraminidase domains appears to be strong enough to mediate tetramerization in the absence of the HN stalk region. Whereas the buried surface area for each monomer in the PIV5 HN dimer is 1810 Å2 the HN dimer-of-dimers interface only involves 10 residues and buries only 657 Å2.
For some paramyxoviruses, such as the W3A strain of PIV 5 and perhaps in some strains of measles virus, there is a limited or even lack of requirement for HN/H or G for cell-cell fusion to occur (Alkhatib, Richardson, and Shen, 1990; Bagai and Lamb, 1995; Horvath and Lamb, 1992; Horvath et al., 1992; Paterson, Hiebert, and Lamb, 1985; Vialard et al., 1990). However, it has been shown that even for the relatively HN-independent W3A strain of PIV 5, fusion is still significantly enhanced by the presence of HN (Russell, Jardetzky, and Lamb, 2001). Most paramyxoviruses have a stringent need for the homotypic attachment protein to initiate fusion (Bousse et al., 1994; Cattaneo and Rose, 1993; Ebata et al., 1991; Heminaway et al., 1995; Horvath et al., 1992; Hu, Ray, and Compans, 1992; Lamb, 1993; Morrison and Portner, 1991; Sakai and Shibuta, 1989; Tanabayashi et al., 1992; Wild, Malvoisin, and Buckland, 1991; Yao, Hu, and Compans, 1997) and experimental evidence suggests that HN interacts directly with F. For PIV 5, it has been observed that the presence of HN lowers the activation energy barrier for triggering the fusion process (Russell, Jardetzky, and Lamb, 2001). Mutational analysis also suggests the existence of an F/HN complex (Stone-Hulslander and Morrison, 1997; Stone-Hulslander and Morrison, 1999). Finally, both coimmunoprecipitation and co-capping experiments indicate that HN can physically associate with F protein, consistent with this interaction being important for fusion activation (Dallocchio, Tomasi, and Bellini, 1994; Tomasi et al., 2003; Yao, Hu, and Compans, 1997) Deng et al., 1999; Ebata et 1991; Gravel and Morrison, 2003) (reviewed in Lamb and Parks, 2007).
Significant effort has been put into the mapping of a site on the HN protein that may be involved in direct interactions with the F protein and is distinct from the NA or hemagglutinin active site(s). Indeed for hPIV2, hPIV3, and NDV HN proteins, the stalk regions have been implicated in determining the specificity of HN-mediated activation of F interactions and therefore the stalk region may form critical, direct interactions with F (Deng et al., 1999; Gravel and Morrison, 2003; Porotto et al., 2003; Yuasa et al., 1995). To date there is no structural information available on the HN stalk region, despite its potentially important role in F activation and virus entry.
Here we demonstrate that the PIV 5 HN stalk region can assemble independently into a stable tetramer, thereby driving the oligomerization of the HN ectodomain. The majority of the amino acids in the HN stalk region adopt an alpha-helical conformation, as determined by circular dichroism spectroscopy, consistent with the formation of an extended 4-stranded coil-coil structure. The PIV 5 HN NA domain lacking the stalk region exists only as monomer in solution, with no evidence for oligomerization to dimers or tetramers in the micromolar concentration range. While the entire HN ectodomain forms stable tetramers due to the presence of the stalk region, quantitative analysis of the HN proteins suggests that the HN NA domains stabilize the HN stalk to thermal unfolding and that the HN(ecto) protein contains additional helical residues compared to the isolated HN NA and stalk domains. Additional evidence for the flexibility in the NA interdomain arrangements, in the linker between the HN NA and stalk domains and within the HN stalk itself has been obtained from single molecule electron microscopy. The observations suggest that interactions between the HN NA domains influence the stalk structure and are consistent with a model in which receptor binding to the HN NA domains could propagate a signal to the HN stalk to activate F protein mediated membrane fusion.
RESULTS AND DISCUSSION
Expression and purification of HN(ecto), HN(NA) and HN(stalk)
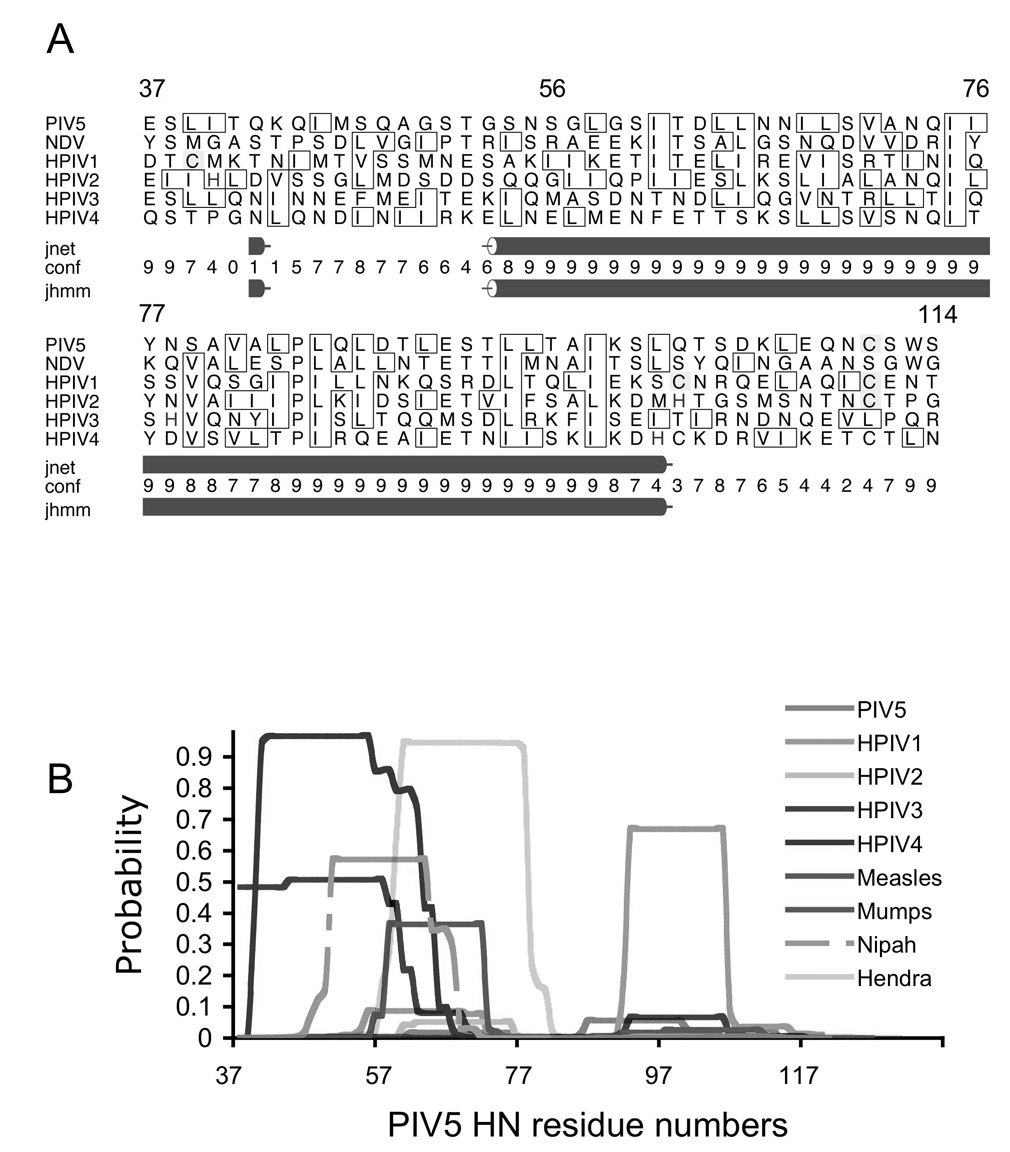
The PIV 5 HN(ecto) domain, HN(NA) domain, and the HN(stalk) region (Figure 1) were expressed and purified as described in Materials and Methods. The HN(ecto) protein and HN(stalk) constructs containing the entire sequence of the stalk region, beginning with residue 37, were typically obtained at a relatively low yield and apparently subject to proteolytic degradation (data not shown). N-terminal sequence analysis of the spontaneously degraded HN(ecto) protein showed that this fragment began with residue 56 suggesting cleavage occurs between residues 55 and 56 by unknown protease(s) during expression. Secondary structure predictions for the stalk regions of many paramyxovirus attachment proteins indicate a high propensity for helix formation beginning near this cleavage site (Figure 2). It is noteworthy that the observed cleavage site in PIV5 HN falls within a conspicuous gap in the secondary structure predictions covering approximately 10–14 residues. To improve the stability of the HN stalk-containing proteins, residues 37 to 55 were deleted from the constructs of HN(ecto) and HN(stalk). The original HN(ecto) construct beginning at residue 37 will be referred to as HN(ecto37) and the HN(ecto) starting from amino acid 56 as HN(ecto56). The HN(stalk) also begins with residue 56. Protein yields were significantly increased by this change. Comparisons of HN, H and G secondary structure predictions from multiple sequence alignments generally indicate the possibility of alpha helix formation in the paramyxovirus attachment protein stalk, although not necessarily predicting a common, contiguous alpha helical segment throughout this region. However, when HN sequences are aligned, secondary structure prediction using the Jpred server indicates a high probability of helix formation for a significant portion of the stalk (Figure 2a). The proteolytic cleavage of HN that was observed in the longer HN(ecto37) constructs occurs near the N-terminal end of predicted extended helical region (Figure 2a), indicating consistency between the predicted structure and experimental observation. We also analyzed individual sequences for their intrinsic ability to form coiled coils, using the program COILS (Figure 2). In this analysis, the PIV5 stalk region exhibits a very low coil propensity, whereas other paramyxovirus attachment proteins have highly variable predictions, both in the calculated coiled-coil probability and the specific residues implicated in coiled coil formation. Thus, although these sequence analyses suggest the possibility of alpha helix formation within the various stalk regions, with the potential for coiled-coil formation for some paramyxovirus attachment proteins, the variability in these predictions suggest potential structural differences. In particular, the PIV 5 HN stalk region did not appear from this analysis to contain a well determined coiled-coil oligomerization domain.
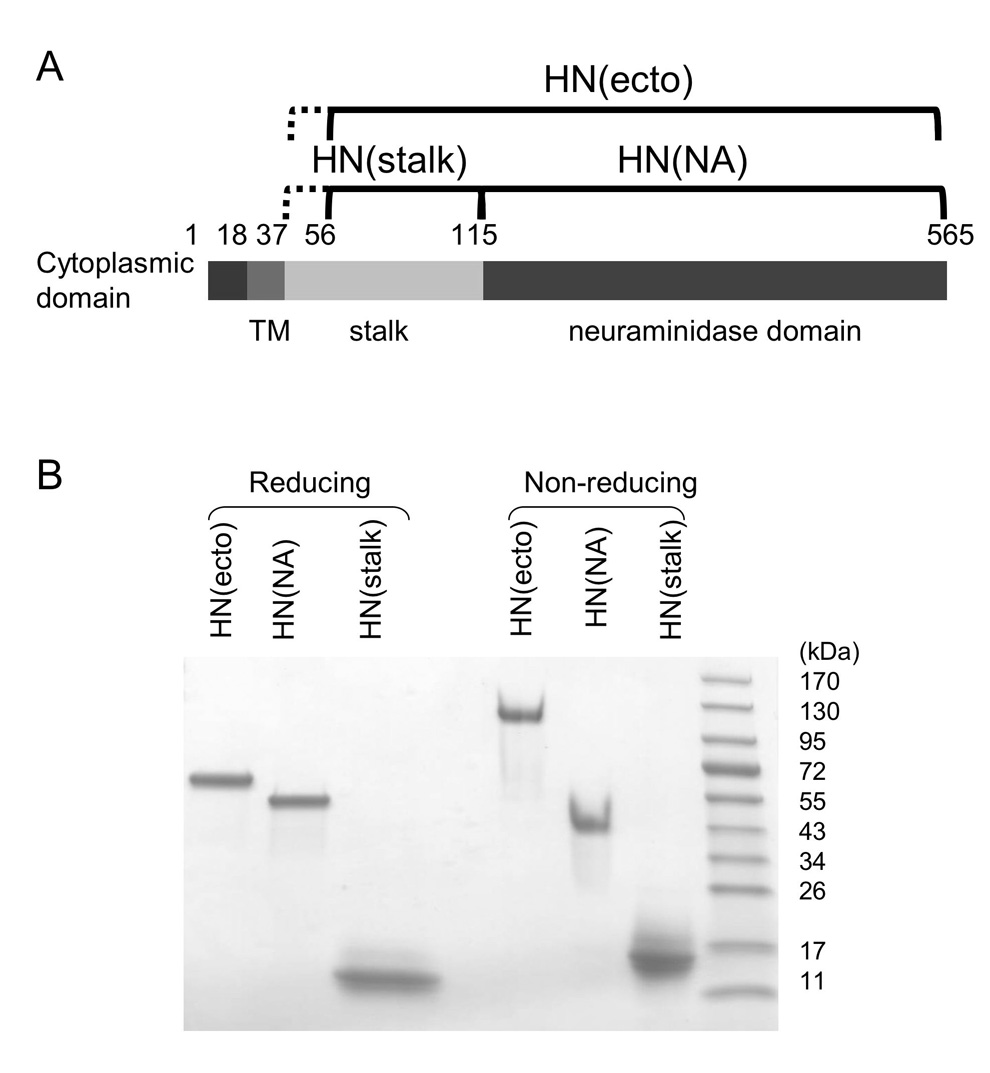
Figure 1. Expression and SDS-PAGE analysis of PIV5 HN ectodomain variants.
(A) Schematic of the PIV5 HN protein. The residues included in each of the three constructs, HN(stalk), HN(NA) and HN(ecto) are indicated above the domain diagram. (B) SDS-PAGE analysis of the purified HN(ecto), HN(NA) and HN(stalk) proteins. Samples were either reduced (left three lanes) or not reduced (right three lanes) before SDS-PAGE analysis. Molecular weight markers are indicated to the right.
Figure 2. Sequence analysis of paramyxovirus attachment protein stalk regions.
(A) Secondary structure prediction based on multiple sequence analysis generated by the online Jpred server (http://www.compbio.dundee.ac.uk/~www-jpred/; University of Dundee, UK). The rows below correspond to the following: jnet: Jnet prediction; conf: an estimation of prediction accuracy on a scale 0–9; jhmm: Jnet hmm profile prediction(Cuff and Barton, 2000; Cuff et al., 1998). PIV5 HN residue numbering is indicated above the sequence alignment. (B) Predicted coiled coil propensity for stalk regions using the program COILS (http://ch.embnet.org/software/COILS_form.html; (Lupas, Van Dyke, and Stock, 1991)). The PIV5 HN stalk region exhibits a very low coil propensity, whereas other paramyxovirus attachment proteins have highly variable predictions, both in the calculated coiled coil probability and the specific residues implicated in coiled coil formation.
Both HN(ecto) and HN(stalk) form disulfide-linked dimers
The three expressed HN domains were analyzed using SDS-PAGE to test whether the cysteine at position 111 is involved in the inter-chain disulfide formation. We have shown previously that the secreted full length HN(ecto37) protein forms disulfide-linked dimers (Yuan et al., 2005), consistent with observations made with the full length PIV 5 HN proteins and detergent solubilized native HN (Ng et al., 1989, Ng et al., 1990). Under reducing conditions, HN(ecto56) migrates with an apparent molecular weight of 65 kD that is consistent with the theoretical monomer molecular weight of ~64 kD, including estimates for carbohydrate at 4 N-linked glycosylation sites. HN(NA) migrates with an apparent MW of ~55 kD which is close to the theoretical monomer molecular weight of ~54 kD, including 3 N-linked glycosylation sites. HN(stalk) migrates at ~11 kD, which is slightly slower than the theoretical monomer molecular weight of ~8.5 kD, after addition of ~1.5 kD for a carbohydrate chain added at a potential N-linked glycosylation site at residue 110. Under non-reducing conditions, HN(NA) migrates with a molecular weight consistent with a monomeric species. In contrast to HN(NA), under non-reducing conditions, HN(ecto56) and HN(stalk) migrate on SDS-PAGE with apparent molecular weights that are consistent with covalent dimers, with sizes of ~120 kD and ~15 kD respectively (Figure 1). These results demonstrate that both HN(ecto56) and the much smaller HN(stalk) can quantitatively form an inter-chain disulfide bond. Since cysteine 111 is the only cysteine within the stalk region, this residue must be involved in the covalent linkage between chains, consistent with previous studies implicating this residue in HN dimer formation (Ng, Hiebert, and Lamb, 1990).
Analysis of HN(ecto), HN(NA) and HN(stalk) oligomerization states by gel filtration chromatography
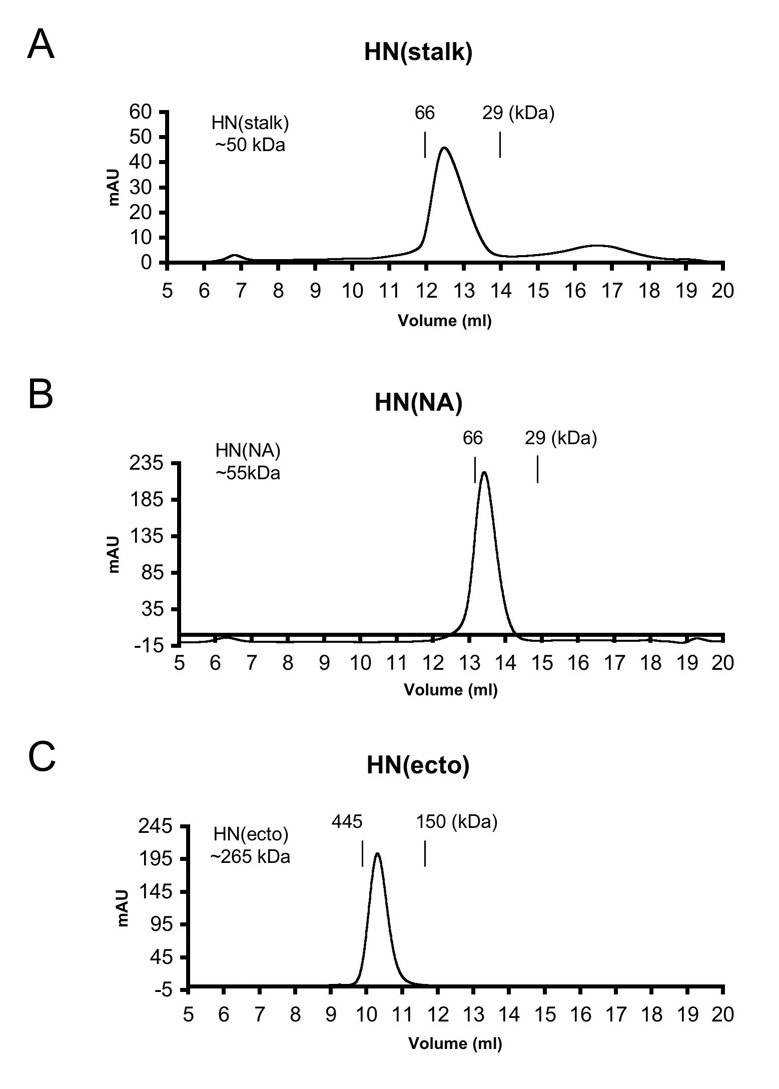
To obtain information on the oligomerization states of the HN domain in solution the purified HN(ecto37), HN(NA) and HN(stalk) proteins were analyzed by gel filtration chromatography. The HN(ecto37) protein migrates with an apparent molecular weight of ~265 kD, based on the average of 12 gel filtration runs. The calculated HN(ecto37) molecular weight is 234 kD without carbohydrate modification but it is known that 4 sites per HN monomer are glycosylated (Ng et al., 1990), and for insect cell carbohydrate chains, the additional mass per tetramer would be ~20–25 kD. The migration of the HN(ecto) protein is therefore in very good agreement with the expected MW for a tetrameric form. The apparent MW of HN(NA) was calculated to be ~55 kD, which is very close to the expected monomer size (Figure 3). Therefore, based on gel filtration analysis the HN(ecto) exists as a tetramer and HN(NA) as monomer.
Figure 3. Estimated molecular weights of HN proteins using calibrated gel filtration chromatography.
(A) HN(stalk), (B) HN(NA), and (C) HN(ecto) proteins were applied to a Superdex-200 HR 10/30 (GE) column and eluted with 50 mM Tris, pH 7.5, 250 mM NaCl. The vertical lines represent the elution positions of protein molecular weight standards used for calibration. Calculated molecular weights for the proteins are indicated in the inset to the left of the chromatograms.
Based on these observations, it appeared that the HN stalk region provides the majority of the interactions that lead to HN oligomerization and the formation of the interchain disulfide bond in HN(stalk) is consistent with this structural role. When analyzed by gel filtration chromatography, the HN(stalk) migrates as a well-defined, single peak, with an apparent molecular weight of ~50 kD (Figure 3). This observed MW is approximately 30% larger than predicted for a tetramer of the HN stalk (~38kD expected), including addition of a potential carbohydrate chain at residue 110, but the MW is consistent with the HN(stalk) forming a well defined oligomer in solution. The increased apparent MW from the gel filtration chromatography analysis could arise from a non-spherical, extended shape of the stalk, which would lead to a larger apparent Stokes radius and thus larger apparent MW.
The crystal structure of the HN(ecto37) protein showed a dimer-of-dimer interaction with a screw axis of symmetry between NA domain pairs, which could potentially allow the HN(ecto) tetramer to form higher order oligomers in solution (Yuan et al., 2005). A measurement of the affinity of the HN(ecto) oligomerization would therefore also correspond to an approximate measure the affinity of the dimer-of-dimers within a single tetramer. Thus, it was of interest to measure this affinity or place an upper boundary on its possible value. To test the possibility that HN(ecto) could form larger oligomers or dissociate from tetramers into dimers, we used initially gel filtration chromatography. The elution peak of HN(ecto37) as a tetramer was unchanged as the concentration of HN(ecto37) was varied from 30 nM to 2 µM. Thus, HN(ecto37) exists as a stable tetramer, at least within the above range of concentrations, with no evidence for higher order oligomer formation.
Analysis of HN(ecto), HN(NA) and HN(stalk) oligomerization states by analytical ultracentrifugation
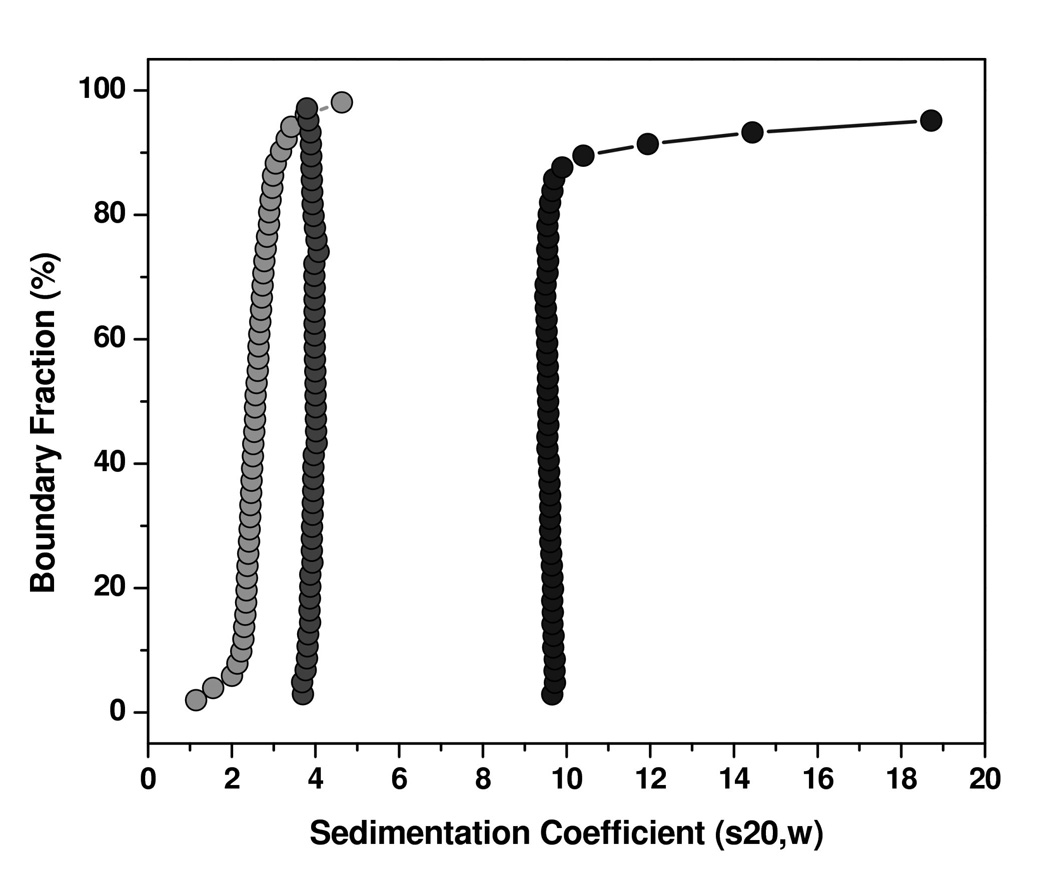
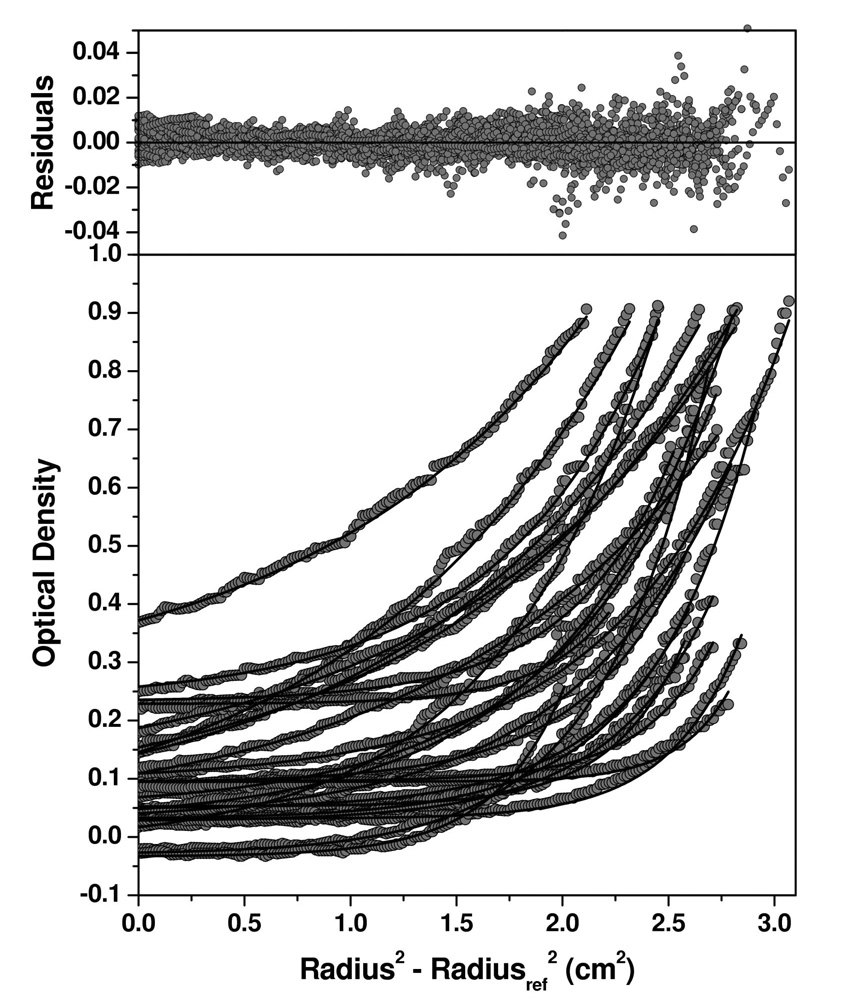
To obtain a greater insight into the oligomerization states of the HN protein constructs, analytical ultracentrifugation (AUC) studies were undertaken. The HN(ecto37), HN(stalk) and HN(NA) proteins were analyzed using sedimentation velocity experiments at multiple concentrations (ranging between 1.5 – 15 µM for HN(ecto37), 1.6–3.0 µM for HN(NA), and 6.7–29 µM for HN(stalk)). The sedimentation profiles were analyzed by the enhanced van Holde-Weischet analysis (Demeler and van Holde, 2004), which provides a model-independent sedimentation coefficient distribution for each sample, yielding the sedimentation coefficient distribution plots shown in Figure 4. HN(NA) protein sediments as a homogeneous species with a sedimentation coefficient of 3.8S, irrespective of concentration. HN(ecto37) displayed a major species sedimenting at 10S, and a minor amount of higher molecular weight aggregates which varied slightly with concentration. HN(stalk) showed a heterogeneous mixture of species, which varied with changes in the concentration between 1–5S. Based on the velocity analysis, which suggested that HN(NA) is a homogeneous species and suitable for sedimentation equilibrium analysis, we determined the molecular weight of HN(NA) by sedimentation equilibrium analysis. The results showed that HN(NA) is best fit by a single species model with a molecular weight of 50,238 Dalton (Figure 5), consistent with a monomeric species. These results were closely matched by genetic algorithm-Monte Carlo analysis (Brookes and Demeler, 2006; Brookes and Demeler, 2007; Demeler, 2005) of the velocity data, which further reported a frictional ratio of 1.2, consistent with a globular protein. For HN(ecto37), the genetic algorithm analysis reported molecular weights ranging between 150–230 kDalton, indicating possibly a dimer-tetramer equilibrium. The HN(stalk) protein was too heterogeneous to derive a meaningful molecular weight. We presume that the heterogeneity of the HN(stalk) protein is not only caused by variations in the glycosylation, but also by a mass-action effect, indicated by a shift to higher S-values as the concentration of HN(stalk) is increased (data not shown). Over the concentration range tested, the sedimentation equilibrium data indicate that HN(NA) does not dimerize. Although the HN dimer has an extensive buried surface area of contact and the arrangement is well conserved in crystal structures of the NDV, hPIV3 and PIV5 HN proteins, these data provide further evidence that the affinity of dimerization is substantially weaker than 10 µM.
Figure 4. Sedimentation velocity analysis of HN protein domains.
The enhanced van Holde-Weischet method (Demeler and van Holde, 2004) was used to obtain model-independent sedimentation coefficient distributions from the HN(stalk), HN(NA) and HN(ecto) proteins. Integral distribution plots for HN(NA) (medium grey circles) and HN(ecto) (black circles) and HN(stalk) (light grey circles) are shown. HN(NA) and HN(ecto37) proteins show sharp distributions with major species sedimenting at 3.9 S for HN(NA) and 9.6S for HN(ecto37), with HN(ecto37) showing about 10% aggregation, and HN(stalk) showing a broader distribution sedimenting between 1–5 S.
Figure 5. Sedimentation equilibrium behavior of HN(NA).
30 scans taken during the sedimentation equilibrium experiment from 6 cells containing increasing concentrations of HN(NA) protein were globally fitted to multiple models. A single ideal species model was found to best represent the experimental data. Monte Carlo analysis of this model provided a molecular weight of 50,238 +206.4/−222 Dalton (95% confidence intervals). Experimental data are shown as grey circles, the fitted model is shown with solid lines. Residuals for this model are shown in the upper panel. Fitting the data with a fixed molecular weight distribution model confirmed the finding of a single species with the same molecular weight.
Overall, the observed sedimentation coefficients, 3.8S for HN(NA) and 10S for HN(ecto37), are consistent with the gel filtration chromatography results, with HN(NA) existing as a monomer and HN(ecto) forming primarily a higher oligomeric species in solution. The sedimentation velocity experiments for HN(ecto37) were performed at concentrations ranging up to 15 µM, for which the primary 10S species is still observed. Interactions between the HN tetramers, through the NA dimer-of-dimers interaction, must also be significantly weaker than the low micromolar range.
In contrast to the well-defined solution behavior of the HN(NA) and HN(ecto) proteins, the stalk region shows more heterogenous species in AUC experiments. In sedimentation velocity experiments, HN(stalk) exhibits different behaviors depending upon the concentration. At low concentrations (~18 µM), the HN(stalk) migrates with an approximate sedimentation constant of ~2 - 2.5S. Increasing the HN(stalk) concentration to ~26 µM also increases the average S value. At the highest concentrations tested (~37 µM), severe aggregation of the HN(stalk) was observed, yielding S values ranging from 2–5S. Since the HN(stalk) protein is a covalent dimer, these data are consistent with higher order oligomerization of the dimers, but preclude a quantitative analysis of the species generated. The combined AUC data indicate that the proper oligomerization of the stalk region is maintained by the presence of the NA domains in HN(ecto), suggesting that weak interactions in the head region may still contribute to the overall stability of the HN tetramer.
Secondary structure content of HN(ecto), HN(NA) and HN(stalk)
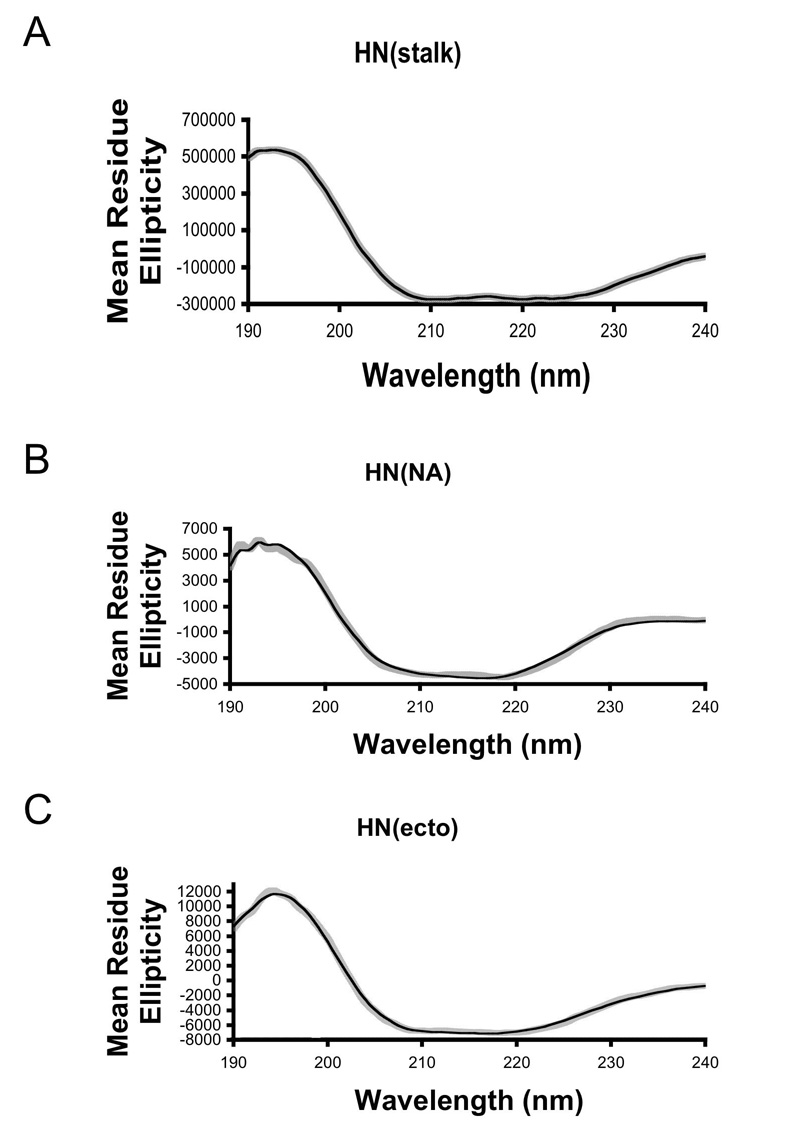
Circular dichroism spectroscopy was used to obtain information on the secondary structure content of the HN proteins. Since the absolute protein concentration influences the calculation of secondary structure content, the concentrations of the proteins were determined by UV absorbance and amino acid analysis in parallel. The data obtained by these two methods were in good agreement (Table 1). Representative CD spectra for HN(ecto56), HN(NA) and HN(stalk) are shown in Figure 6. The individual spectra were analyzed using the online DICHROWEB server, using the CDSSRT program for secondary structure assignment. The resulting fits to the CD data were of high quality and the reconstructed spectra are shown superimposed on the experimental data in Figure 6. The overall goodness-of-fit parameter is 0.018 for HN(ecto56), 0.05 for HN(NA) and 0.007 for HN(stalk) (Table 1). Therefore, the calculated secondary structures likely have good correspondence with actual values (NRMSD<0.1). The mean residue ellipticity at 220 nm is ~8,000 for HN(ecto), ~4,000 for HN(NA) and ~30,000 for HN(stalk) (Figure 6). All of the graphs show typical α-helix profiles, but with very different magnitudes. For example, the value of HN(stalk) is 7.5 fold higher than HN(NA), consistent with significantly different net α-helical content.
Table I.
CD Analysis of Helix Content of HN Proteins
| HN(stalk) |
HN(NA) |
HN(ecto) |
|
|---|---|---|---|
| Total # of amino acids | 65 | 454 | 513 |
| α helix content (X-ray; %) | n.a. | 4.9–5.3% | n.a |
| Predicted α helical content (DICHROWEB) | 0.88–0.91 | 0.05 | 0.2 |
| NRMSD (DICHROWEB) | 0.007 | 0.054 | 0.018 |
| Predicted # of α helix residues | 57.2 | 22.7 | 102.6 |
| Conc. (AAA*) (mg/ml) | 0.0144 | 0.08 | 0.0566 |
| Conc. (UV) (mg/ml) | 0.013 | 0.073 | 0.0557 |
n.a. – not available
Concentration determined by quantitative amino acid analysis (AAA).
Figure 6. Representative CD spectra of HN(ecto), HN(NA) and HN(stalk).
(A–C) The speckled grey curves represent experimental CD data from each HN protein as indicated. The black lines represent reconstructed data calculated using the CDSSTR program available through the online DICHROWEB server (http://www.cryst.bbk.ac.uk/cdweb/html/home.html; (Whitmore and Wallace, 2004)). Statistics for the quality of the fit to the experimental data (NRMSD) are collected in Table 1.
The number of residues involved in helix formation in each of the three HN proteins was also analyzed to establish if the linkage of NA and stalk domains in a single peptide chain might stabilize additional residues of the HN stalk helices. The percent of alpha-helical structure was calculated using the DICHROWEB server and the corresponding number of residues involved calculated for each sample. In the case of the HN(NA) domain, we were able to directly compare the results calculated using the CD data and the DICHROWEB server with the structure obtained by X-ray crystallography (Table 1). The percent α-helical content of HN(NA) based on the X-ray crystal structure is 4.9–5.3 %, which corresponds to between 22–24 residues of helix, depending on whether two amino acids are counted as α-helix or as an alpha turn. The estimated percent helix for the HN(NA) obtained from the analysis of the CD spectrum is remarkably similar, yielding an overall percent helix of .05, or ~23 residues (Table 1). The calculated α-helical content of HN(stalk) from CD analysis is much higher (88–91%), corresponding to ~57–59 residues forming helix, indicating that nearly the entire HN stalk region is helical in solution (residues 56–118). Interestingly, the calculated α-helical content for the HN(ecto56) protein is slightly higher than the direct sum of the HN(stalk) and HN(NA) results. For HN(ecto56), the CD analysis indicates that ~20%, or 103 amino acids, adopt an α-helical conformation, which is ~20 residues higher than the 80–82 residues expected from the separate HN(stalk) and HN(NA) analyses (Table 1). The data suggest that the helical HN stalk region could be stabilized by the NA domains or that additional residues in the NA domain form helix in the context of the entire HN ectodomain.
Thermal stability of HN(ecto), HN(NA) and HN(stalk)
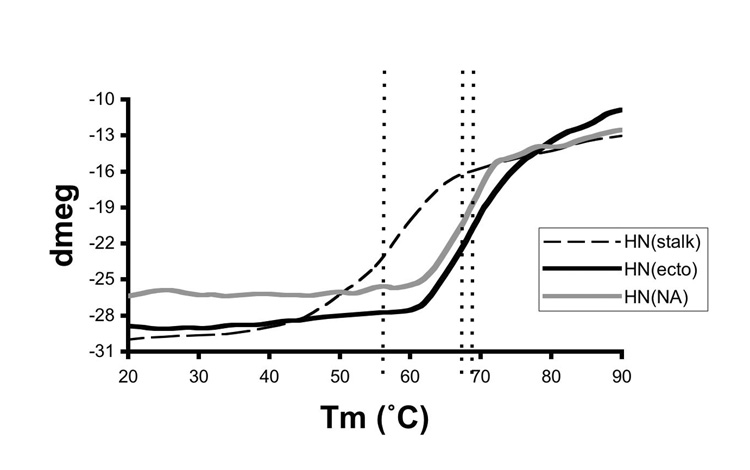
To test whether the HN stalk and NA domains act as independently folded regions within the intact HN, we used CD to monitor the thermal unfolding of the different HN constructs. The changes in CD signal at 222 nm were monitored as the temperature was increased from 20°C to 90°C for samples of HN(ecto56), HN(NA) and HN(stalk), as shown in Figure 7. The unfolding profile of HN(ecto56) reveals little or no changes up to temperatures near 60°C, at which point the CD signal starts to decrease in a cooperative transition with an apparent midpoint at near 70°C. There is no obvious separation of the HN(ecto56) transition into two separate phases, which might have been expected if the HN(NA) and HN(stalk) proteins were to exhibit very different temperature stabilities. The unfolding profiles of HN(NA) and HN(stalk) were also measured (Figure 7) and reveal transitions at lower temperatures than for HN(ecto56). For the HN(stalk), the CD signal starts to decrease near 35°C, which is much lower than the starting point of HN(ecto56) or HN(NA), with a transition midpoint near 55°C. The temperature transition midpoints of HN(NA) and HN(ecto56) are within a few degrees of each other (Figure 7). These data further indicate that the folding of the HN stalk region is stabilized by the presence of the NA domains in HN(ecto). Although the HN stalk is clearly important for stabilization of the overall HN tetramer, our observations demonstrate that the tertiary structure of the stalk is less stable as compared to the individual globular NA domains.
Figure 7. Thermal stability of HN(ecto), HN(NA) and HN(stalk).
The thermal denaturation of the HN proteins was followed by monitoring the CD intensity at a wavelength of 222 nm as a function of temperature. The dashed lines indicate the approximate midpoint of the unfolding transitions, which is ~55°C for HN(stalk), ~66°C for HN(NA) and ~69°C for HN(ecto). Melting temperature was defined as the point at which ~50% of the sample denatured. Comparison of the HN(ecto) and HN(stalk) denaturation curves reveals no evidence for the melting of the HN stalk region at lower temperatures in the entire ectodomain, suggesting that it’s helical structures is stabilized by the HN neuraminidase domains.
Electron microscopy of the HN protein
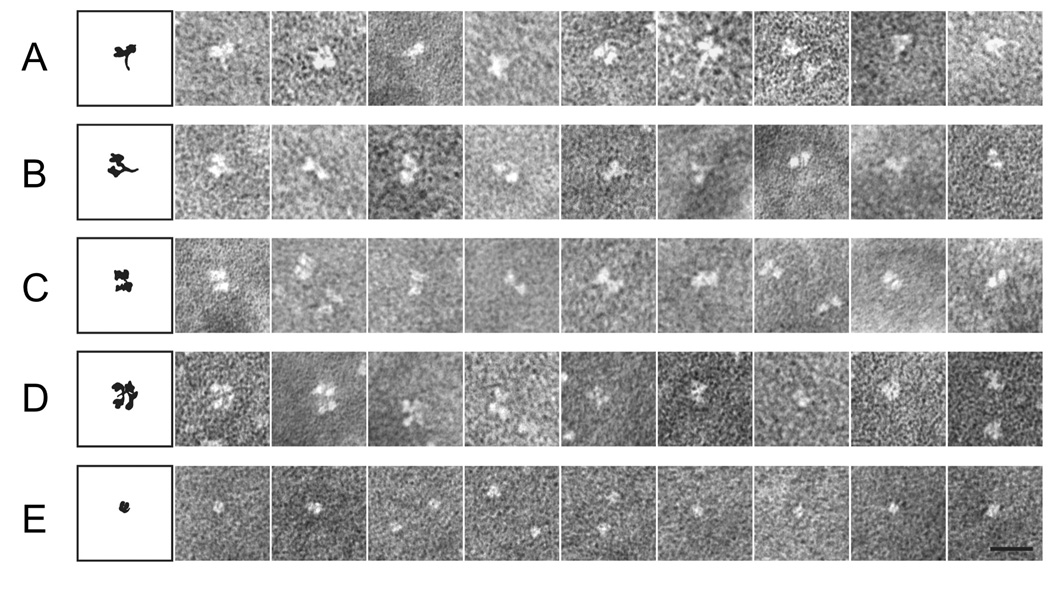
In the atomic structure of the PIV5 HN(ecto37) protein (Yuan et al., 2005), the stalk region was not visible, suggesting that it could be linked to the NA domains through a flexible region. We used electron microscopy to investigate the structure of HN(ecto37) and HN(ecto56), to determine the structural relationship between HN NA domains and also the relationship between the head and stalk regions of the protein. In EM observations of HN(ecto37) and HN(ecto56), the stalk region is clearly visible, although most images do not appear to be in a favorable “side view” of the tetramer (Figures 8 A–E). The HN(NA) and HN(stalk) proteins were also analyzed by EM. HN(NA) preparations appear as bright, single dots at varied concentrations, which likely correspond to the monomeric NA domains (Figure 8E). HN(stalk) preparations alone did not provide any informative images, with only small, bright dots observed.
Figure 8. Electron microscopy of HN(ecto) and HN(NA) proteins.
Protein samples were negatively stained and visualized as described previously (Connolly et al., 2006). (A–D) Representative images of different projection views of the HN(ecto) protein are shown to the right of a schematic diagram. The images demonstrate variability in both the interdomain packing within the globular head and in the extension of the stalk regions. In these images, the HN globular head exhibits apparently loose and variable dimer of dimer associations, with panels A and B representing “side” views of the HN ectodomain including the stalk and panels C and D representing “top” views of tetramers of the HN NA domains. The stalk regions visible in panels A and B are exhibit variable orientations relative to the NA domains and internal bending consistent with flexibility within this domain. Representative EM images of the HN(NA) protein is shown in panel (E), which appears as single globular and individual dots in the micrographs, in contrast to the florets observed for HN(ecto).
The EM observations of HN(ecto) and HN(NA) are consistent with our structural and biophysical evidence, suggesting that the protein exists in solution as a tetramer stabilized by the stalk and with weak interactions between the globular NA domains within the head region. The gallery of EM images in Figure 8 are compared with diagrams to the left representing potential orientations of the HN proteins. The HN(ecto37) crystal structure also revealed that the interaction of NA domain (dimers-of-dimers) only buried 657Å2 of surface area, suggesting that the dimer-of-dimers interaction is very loose and potentially dynamic in solution. The HN(ecto) EM images are consistent with this possibility, revealing multiple shapes and potential arrangements of the HN NA domains in different views of the tetramer, which are also potentially consistent with the movements in the head corresponding to changes in dimer to dimer arrangements. From enlarged side views of HN(ecto) EM images, we have observed variable gaps within the head region which appear to form between two dimers of the HN NA domains (Figure 8 A–D). In contrast, the HN(NA) appears to exist solely as a monomer based on the EM observations, with no evidence for dimer or tetramer formation.
In representative images of the HN(ecto) protein in which the stalk region is visible, the stalk is often bent and the bending appears to occur somewhat below the connection between the globular head region and the stalk (Figure 8 B, F), as if significant flexibility could exist within the C-terminal region of stalk itself, as well as between the head and stalk region. This flexibility is consistent with the helical secondary structure and coiled coil predictions (Figure 2), which indicate breaks between multiple helices and in PIV5 HN a weak coiled coil propensity. Nonetheless, the EM images of the HN stalk are consistent with the formation of an extended 4 stranded α-helical bundle.
CONCLUSIONS
PIV5, like other parainfluenza viruses, Sendai virus and Newcastle disease virus, utilize the HN protein to bind sialic acid receptors. Given the experimental evidence for a role of the HN stalk in mediating, at least partially, the specificity of F protein interactions that lead to virus entry, the activation mechanism must involve an event that conveys information from the receptor binding sites in the globular NA domains through to the stalk region. Our data demonstrate that the stalk region itself contains the major determinants for HN oligomerization and that it forms a predominantly helical, though flexible rod-like structure. The HN NA domains contribute to stabilizing the folding and oligomerization of the HN stalk, potentially through relatively weak interdomain interactions between NA dimers. Significant structural variability of the HN ectodomain is observable by EM in both the HN head region, with apparent repositioning of the NA domains, and in the stalk region, with internal bending motions apparent below the junctions with the head. It appears from the globular nature of the HN NA domain and from structural studies of the PIV5 HN:sialyllactose complex (Yuan et al., 2005) that receptor binding is unlikely to induce large-scale conformational changes within a single domain. We have tested a possible influence of ligand on the oligomeric and conformational states of HN(ecto) and HN(NA) using EM. In the presence of sialyllactose HN(NA) and HN(ecto56) yield similar images regardless of whether there is sialyllactose present or not, suggesting that monomeric receptor binding does not have a strong impact on the internal organization or stability of interactions between NA domains within the HN tetramer. However, independent engagement of HN NA domains with different sialosides could influence the conformation of the HN tetramer, potentially leading to the release or alteration of interactions that affect F protein conformation and thereby virus entry. The specific nature of these postulated interactions between HN and F glycoproteins remains to be established by experimental methods that can discriminate between direct and indirect influences on F glycoprotein conformational stability and refolding.
MATERIALS AND METHODS
Construction of expression vectors
DNA encoding the HN ectodomain, designated HN(ecto), and HN NA domain, designated HN(NA), was separately amplified from the PIV5 HN cDNA in the pGEM vector using PCR, concurrently introducing restriction enzyme sites (EcoRV/XhoI) for cloning. The primers employed for HN(ecto56) were 5’-GGAGGTGATGACGACGACAAGTCTGGATTAGGAAGTATCAC-3’ as the forward and 5’-ATCAGACTCGAGTTATTAGGATAGTGTCACCTGAC-3’ as the reverse primer. For HN(NA) we used 5’- TGGAGTGATATCCGCTGCAC –3’ as the forward and the same reverse primer used for HN(ecto). The HN stalk region was amplified from HN(ecto) and designated as HN(stalk). The same forward primer of HN(ecto) forward was used for HN(stalk), the reverse primer was 5’-GTGATACTTCCTAATCCAGACTTGTCGTCGTCATCACCTCC -3’. The PCR amplifications were performed using pfu turbo polymerase (Stratagene, La Jolla, CA) under the following conditions: 90°C for 2 min; 2 cycles of 94°C for 1 min, 50°C for 2 min, and 72°C for 2 min; 30 cycles of 94°C for 1 min, 65°C for 2 min, and 72°C for 2 min. The PCR products were separated by agarose gel electrophoresis and purified (Qiaquick; Qiagen, Chatsworth, CA). The fragments were double digested separately using EcoRV/XhoI restriction enzymes (Promega, Madison, WI) and subsequently cloned into a baculovirus transfer vector pBACgus-3 (Invitrogen, Carlsbad, CA) inserting into the vector SmaI/XhoI sites. The resulting constructs were verified by DNA sequencing. This cloning strategy generates extra, vector-encoded residues (Ser, Pro and Ser) at the N-terminus of residue 56 for HN(ecto), HN(stalk) and at residue 115 for HN(NA), after proteolytic removal of the N-terminal tag region. Both recombinant proteins of HN(ecto) and HN(NA) end at residue 565. HN(stalk) ends at residue 117 (Figure 1).
Insect cell expression
Baculovirus transfer vectors containing HN(ecto), HN(NA) and HN(stalk) inserts were co-transfected with Baculogold DNA into Sf9 insect cells at 2 × 106 cells/ml grown in a T-25 flask with SF 900 I serum-free medium (Gibco BRL, New York) using standard calcium phosphate conditions (Pharmingen, San Diego, CA). A low titer virus stock was prepared by infection of SF+ cells (1.5 × 106 cell/ml, in a 250 ml shaker flask) with a 1:100 dilution of the transfection supernatant. After 2 days the virus was harvested and used for additional virus amplification. A large-scale, high titer virus stock was obtained by infecting SF+ cells (1.5 ×106 cell/ml) at a 1:100 dilution in 2 L shaker flasks, followed by incubation for at least three days or until a suitable titer was obtained. The protein expression was optimized by varying the amount of virus stock used for infection, the initial cell density, the type of insect cells (SF+ or Hi5), and the harvest time post-infection (48 h, 60 h, 72 h or 90 h). The best results were obtained using the SF+ cell line for the protein expression, harvesting at 72 h, 90 h and 60 h post-infection for HN(ecto), HN(NA) and HN(stalk), respectively.
Protein preparation
Flasks containing the infected cells were supplemented with phosphate buffer at 1X final concentration (as described in the Qiagen Ni-NTA column protocol) and pelleted at 13,000 g for 30 min at 4°C. Crude supernatant containing the HN proteins were diluted 3X with 1X phosphate buffer or alternatively the volume reduced and buffer exchanged with 1X phosphate buffer using a tangential flow cartridge concentrator (Millipore, Bedford, MA) and loaded onto a pre-equilibrated Ni-chelating column. The proteins were purified as described in the manufacturer’s instruction manual (Qiagen). The affinity-purified proteins were concentrated by centrifugal ultrafiltration (Millipore) and dialyzed against enterokinase reaction buffer (25 mM Tris, pH 7.4, 50 mM NaCl) lacking calcium. The S-and His-Tag were cleaved using enterokinase with the optimized condition being room temperature for overnight. The enterokinase was removed by passing the digest mixture over an enterokinase capture column (Novagen, Madison, WI). The smaller molecular weight tag-containing fragments were separated from the cleaved HN proteins by loading onto a NI-NTA column, allowing capture of the 6His-containing tags on the column. The cleaved proteins were further purified using size-exclusion chromatography with a Pharmacia Superdex-200 column (10 ×300 mm), which was pre-equilibrated with a running buffer of 25 mM Tris, pH 7.5, 250 mM NaCl. The column was calibrated with bovine serum albumin (66 kDa), alcohol dehydrogenase (150 kDa), amylase (200 kDa), apoferritin (443 kDa), thyroglobulin (669 kDa). The eluted proteins were detected by measuring absorbance at 280 nm. Protein concentrations were routinely determined by absorbance at 280 nm using extinction coefficients of 1.469, 1.572 and 1.0 cm−1 (mg/ml)−1 for the HN(ecto), HN(NA) and HN(stalk), respectively, based on predictions obtained from the primary amino acid sequence by ExPASy. The protein identities were confirmed by molecular weight, N-terminal sequence analysis (Michigan State University) and neuraminidase activities for HN(ecto) and HN(NA) proteins. For the quantitation of secondary structure by CD, the protein concentrations were determined by measuring UV absorbance at 280 nm and by quantitative amino acid analysis carried out by the W.M. Keck Foundation Biotechnology Resource Laboratory, Yale University (Table 1).
Protein characterization
The proteins were analyzed using SDS-PAGE followed by Coomassie staining and Western blot analysis. For Western blotting, recombinant proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane by electroblotting. Blots were incubated in 5% dried skim milk in Tris-buffered saline (TBS 1 M Tris, 5 M NaCl, pH 7.5) for 1 h and subsequently washed three times with TBS for 5 min. The blots were then probed with mouse anti-His antibody (1:5000 dilution; Novagen) in TBS containing the same amount of skim milk. The blots were washed three times and the secondary antibody, goat anti-mouse IgG conjugated with alkaline phosphatase was added (Pierce, Rockford, IL). After 1 h the blots were washed three times and protein bands detected by the Enhanced Chemiluminesence System (ECL) (Amersham, Arlington Heights, IL) according to the manufacturer’s instructions.
Reducing and non-reducing SDS-PAGE was performed for the analysis of the interchain disulfide bonds involved in HN oligomerization. The HN(ecto56), HN(NA), and HN(stalk) proteins were loaded onto a 4% to 20% gradient SDS-PAGE (Bio-Rad), by adding or omitting reducing agent (β-mecaptoethanol) in the loading buffer.
Analytical Ultracentrifugation Analysis (AUC)
Analytical ultracentrifugation experiments were conducted using a Beckman Optima XLA centrifuge available in the Northwestern University Keck Biophysics Facility and using a Beckman Optima XLI centrifuge at the Center for Analytical Ultracentrifugation of Macromolecular Assemblies (CAUMA) at The University of Texas Health Science Center at San Antonio. All data were analyzed with UltraScan version 9.5 (Demeler, 2005). Protein samples were prepared in 20 mM phosphate buffer, pH 7.4, 50 mM NaCl. HN(NA) was measured at 20°C and 50 krpm, HN(stalk) was measured at 20°C and 60 krpm, and HN(ecto37) was measured at 4°C and 40 krpm. Sedimentation velocity data were collected until the sample was pelleted. The entire solution column was analyzed by direct boundary fitting using the 2-dimensional spectrum analysis with simultaneous time-invariant noise removal (Brookes, Boppana, and Demeler, 2006). Noise-corrected data were further analyzed either with the enhanced van Holde – Weischet (Demeler, 2005; Demeler and van Holde, 2004) analysis or genetic algorithm/Monte Carlo analysis (Brookes, Boppana, and Demeler, 2006). Sedimentation equilibrium experiments of HN(NA) were performed at six concentrations of HN(NA) (0.3, 0.5 and 0.7 O.D. units at 230 nM and 280 nM). A total of 30 scans were taken when equilibrium was reached at rotor speeds of 15, 20, 25, 30 and 35 krpm. The resulting data were globally fitted to multiple models, the most appropriate model was chosen based on fitting statistics and visual inspection of the residual pattern.
Electron Microscopy (EM)
HN(ecto), HN(NA) or HN(stalk) were diluted to approximately 5 ng/µl and absorbed onto freshly glow discharged, carbon coated 300 mesh copper grids. The samples were stained with 2% sodium phosphotungstate (pH 6.6, or pH 8.0 for sialyllactose binding) and examined in a JEOL 1230 transmission electron microscope (JEOL, Peabody, MA) operating at 100 kV and as described previously (Connelly et al., 2006).
Circular Dichroism (CD)
CD measurements were performed on a Jasco model 715 spectropolarimeter (Easton, MD). CD spectra were acquired in a rectangular quartz cell (Jasco) with a path length of 2.0 mm. Data were collected every 0.1 nm and typically five spectra were averaged. The band width was set at 2.0 nm and the sensitivity at 10 mdeg, and the response time was 2 s. Baseline scans of buffer (20 mM phosphate, pH 7.6, 50 mM NaF) were subtracted from the experimental reading. For temperature studies, temperature was controlled with an external water bath (Model RTE-111, Neslab, Portsmouth, NH). Thermal melts of the proteins were performed in the cuvette by heating the samples at 5°C intervals at a rate of 1 C/min from 20°C to 90°C while monitoring at 222 nm. Results are expressed in machine units (dmeg) and plotted versus temperatures. The estimates of α-helical content were done using the CDSSTR program by submitting CD data through the online DICHROWEB server. Melting temperatures were defined as the point at which ~50% of the sample denatured. Measurements were performed in at least 3 separate experiments yielding comparable results. Concentrations of the proteins were determined by quantitative amino acid analysis and by UV absorbance (See above)
ACKNOWLEDGEMENTS
We thank past and present members of the Jardetzky and Lamb Laboratories. This research was supported in part by NIH research grants to T.S.J. (GM-61050) and R.A.L. (AI-23173). R.A.L. is an Investigator of the Howard Hughes Medical Institute. We thankfully acknowledge the use of instruments in the Keck Biophysics Facility at Northwestern University [http://www.biochem.northwestern.edu/Keck/keckmain.html]. We would also like to acknowledge support for the development of the UltraScan software by NIH 1 R01 RR022200-01A1 (B.D.), the Robert J. Kleberg Jr. and Helen C. Kleberg Foundation (B.D.), NSF TGMCB070038 (B.D.), and CAUMA facility support through NCI Grant P30 CA054174 and UTHSCSA ERC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkhatib G, et al. Intracellular processing, glycosylation, and cell-surface expression of the measles virus fusion protein (F) encoded by a recombinant adenovirus. Virology. 1990;175(1):262–270. doi: 10.1016/0042-6822(90)90207-8. [DOI] [PubMed] [Google Scholar]
- Bagai S, Lamb RA. Quantitative measurement of paramyxovirus fusion: Differences in requirements of glycoproteins between simian virus 5 and human parainfluenza virus 3 or Newcastle disease virus. J. Virol. 1995;69:6712–6719. doi: 10.1128/jvi.69.11.6712-6719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousse T, et al. Regions on the hemagglutinin-neuraminidase proteins of human parainfluenza virus type-1 and Sendai virus important for membrane fusion. Virology. 1994;204(2):506–514. doi: 10.1006/viro.1994.1564. [DOI] [PubMed] [Google Scholar]
- Brookes E, Boppana RV, Demeler B. Computing Large Sparse Multivariate Optimization Problems with an Application in Biophysics. 2006 Supercomputing '06 ACM 0-7695-2700-0/06. [Google Scholar]
- Brookes E, Demeler B. Genetic Algorithm Optimization for obtaining accurate Molecular Weight Distributions from Sedimentation Velocity Experiments. In: Wandrey C, Cölfen H, editors. Analytical Ultracentrifugation VIII. Vol. 131. Springer; 2006. pp. 78–82. [Google Scholar]
- Brookes E, Demeler B. Parsimonious Regularization using Genetic Algorithms Applied to the Analysis of Analytical Ultracentrifugation Experiments. 2007 GECCO Proceedings ACM 978-1-59593-697-4/07/0007. [Google Scholar]
- Cattaneo R, Rose JK. Cell fusion by the envelope glycoproteins of persistent measles viruses which caused lethal human brain disease. J Virol. 1993;67(3):1493–1502. doi: 10.1128/jvi.67.3.1493-1502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SA, et al. Refolding of a paramyxovirus F protein from prefusion to postfusion conformations observed by liposome binding and electron microscopy. Proc Natl Acad Sci U S A. 2006;103(47):17903–17908. doi: 10.1073/pnas.0608678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff JA, Barton GJ. Application of multiple sequence alignment profiles to improve protein secondary structure prediction. Proteins. 2000;40(3):502–511. doi: 10.1002/1097-0134(20000815)40:3<502::aid-prot170>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Cuff JA, et al. JPred: a consensus secondary structure prediction server. Bioinformatics. 1998;14(10):892–893. doi: 10.1093/bioinformatics/14.10.892. [DOI] [PubMed] [Google Scholar]
- Dallocchio F, et al. Inhibition of Sendai virus hemagglutinin neuraminidase by the fusion protein. Biochem Biophys Res Commun. 1994;201(2):988–993. doi: 10.1006/bbrc.1994.1799. [DOI] [PubMed] [Google Scholar]
- Demeler B. A Comprehensive Data Analysis Software Package for Analytical Ultracentrifugation Experiments. Modern Analytical Ultracentrifugation: Techniques and Methods. In: Scott DJ, Harding SE, Rowe AJ, editors. Analytical Ultracentrifugation: Techniques and Methods. Royal Society of Chemistry; 2005. pp. 210–229. [Google Scholar]
- Demeler B, van Holde KE. Sedimentation velocity analysis of highly heterogeneous systems. Anal Biochem. 2004;335(2):279–288. doi: 10.1016/j.ab.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Deng R, et al. Mutations in the Newcastle disease virus hemagglutinin-neuraminidase protein that interfere with its ability to interact with the homologous F protein in the promotion of fusion. Virology. 1999;253(1):43–54. doi: 10.1006/viro.1998.9501. [DOI] [PubMed] [Google Scholar]
- Ebata SN, et al. The fusion and hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus 3 are both required for fusion. Virology. 1991;183(1):437–441. doi: 10.1016/0042-6822(91)90162-5. [DOI] [PubMed] [Google Scholar]
- Gravel KA, Morrison TG. Interacting domains of the HN and F proteins of newcastle disease virus. J Virol. 2003;77(20):11040–11049. doi: 10.1128/JVI.77.20.11040-11049.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heminaway BR, et al. Role of basic residues in the proteolytic activation of Sendai virus fusion glycoprotein. Virus Res. 1995;36(1):15–35. doi: 10.1016/0168-1702(94)00102-i. [DOI] [PubMed] [Google Scholar]
- Horvath CM, Lamb RA. Studies on the fusion peptide of a paramyxovirus fusion glycoprotein: roles of conserved residues in cell fusion. J. Virol. 1992;66:2443–2455. doi: 10.1128/jvi.66.4.2443-2455.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath CM, et al. Biological activity of paramyxovirus fusion proteins: factors influencing formation of syncytia. J. Virol. 1992;66:4564–4569. doi: 10.1128/jvi.66.7.4564-4569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka Y, Shimizu YK. Artificial assembly of envelope particles of HVJ (Sendai virus). II. Lipid components for formation of the active hemolysin. Virology. 1972;49(3):640–646. doi: 10.1016/0042-6822(72)90520-x. [DOI] [PubMed] [Google Scholar]
- Hsu MC, et al. Reconstitution of membranes with individual paramyxovirus glycoproteins and phospholipid in cholate solution. Virology. 1979;95(2):476–491. doi: 10.1016/0042-6822(79)90502-6. [DOI] [PubMed] [Google Scholar]
- Hu XL, et al. Functional interactions between the fusion protein and hemagglutinin-neuraminidase of human parainfluenza viruses. J Virol. 1992;66(3):1528–1534. doi: 10.1128/jvi.66.3.1528-1534.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RA. Paramyxovirus fusion: a hypothesis for changes. Virology. 1993;197:1–11. doi: 10.1006/viro.1993.1561. [DOI] [PubMed] [Google Scholar]
- Lamb RA, Jardetzky TS. Structural basis of viral invasion: lessons from paramyxovirus F. Curr Opin Struct Biol. 2007;17(4):427–436. doi: 10.1016/j.sbi.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RA, Parks GD. Paramyxoviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Fifth ed. Vol. 1. Wolters Kluwer | Lippincott WIlliams & Wilkins; 2007. pp. 1449–1496. 2 vols. [Google Scholar]
- Lupas A, et al. Predicting coiled coils from protein sequences. Science. 1991;252(5010):1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- McGinnes L, et al. Mutations in the transmembrane domain of the HN protein of Newcastle disease virus affect the structure and activity of the protein. Virology. 1993;196(1):101–110. doi: 10.1006/viro.1993.1458. [DOI] [PubMed] [Google Scholar]
- Morrison T, Portner A. The Paramyxoviruses. New York: Plenum Press; 1991. Structure, function, and intracellular processing of the glycoproteins of paramyxoviridae; pp. 347–382. [Google Scholar]
- Nakamura T, et al. Antibody-targeted cell fusion. Nat Biotechnol. 2004;22(3):331–336. doi: 10.1038/nbt942. [DOI] [PubMed] [Google Scholar]
- Ng DT, et al. Different roles of individual N-linked oligosaccharide chains in folding, assembly, and transport of the simian virus 5 hemagglutinin-neuraminidase. Mol Cell Biol. 1990;10(5):1989–2001. doi: 10.1128/mcb.10.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DT, et al. Intracellular maturation and transport of the SV5 type II glycoprotein hemagglutinin-neuraminidase: specific and transient association with GRP78-BiP in the endoplasmic reticulum and extensive internalization from the cell surface. J Cell Biol. 1989;109(6 Pt 2):3273–3289. doi: 10.1083/jcb.109.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks GD, Lamb RA. Folding and oligomerization properties of a soluble and secreted form of the paramyxovirus hemagglutinin-neuraminidase glycoprotein. Virology. 1990;178(2):498–508. doi: 10.1016/0042-6822(90)90347-t. [DOI] [PubMed] [Google Scholar]
- Paterson RG, et al. Expression at the cell surface of biologically active fusion and hemagglutinin-neuraminidase proteins of the paramyxovirus simian virus 5 from cloned cDNA. Proc. Natl. Acad. Sci. USA. 1985;82:7520–7524. doi: 10.1073/pnas.82.22.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porotto M, et al. Triggering of human parainfluenza virus 3 fusion protein (F) by the hemagglutinin-neuraminidase (HN) protein: an HN mutation diminishes the rate of F activation and fusion. J Virol. 2003;77(6):3647–3654. doi: 10.1128/JVI.77.6.3647-3654.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell CJ, et al. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. Embo J. 2001;20(15):4024–4034. doi: 10.1093/emboj/20.15.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y, Shibuta H. Syncytium formation by recombinant vaccinia viruses carrying bovine parainfluenza 3 virus envelope protein genes. J Virol. 1989;63(9):3661–3668. doi: 10.1128/jvi.63.9.3661-3668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone-Hulslander J, Morrison TG. Detection of an interaction between the HN and F proteins in Newcastle disease virus-infected cells. J Virol. 1997;71(9):6287–6295. doi: 10.1128/jvi.71.9.6287-6295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone-Hulslander J, Morrison TG. Mutational analysis of heptad repeats in the membrane-proximal region of Newcastle disease virus HN protein. J Virol. 1999;73(5):3630–3637. doi: 10.1128/jvi.73.5.3630-3637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto T, et al. Crystallization of biologically active hemagglutinin-neuraminidase glycoprotein dimers proteolytically cleaved from human parainfluenza virus type 1. J Virol. 1992;66(12):7597–7600. doi: 10.1128/jvi.66.12.7597-7600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto T, et al. Role of the hemagglutinin-neuraminidase protein in the mechanism of paramyxovirus-cell membrane fusion. J Virol. 2002;76(24):13028–13033. doi: 10.1128/JVI.76.24.13028-13033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabayashi K, et al. Expression of mumps virus glycoproteins in mammalian cells from cloned cDNAs: both F and HN proteins are required for cell fusion. Virology. 1992;187(2):801–804. doi: 10.1016/0042-6822(92)90482-5. [DOI] [PubMed] [Google Scholar]
- Thompson SD, et al. Isolation of a biologically active soluble form of the hemagglutinin-neuraminidase protein of Sendai virus. J Virol. 1988;62(12):4653–4660. doi: 10.1128/jvi.62.12.4653-4660.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi M, et al. Peptides derived from the heptad repeat region near the C-terminal of Sendai virus F protein bind the hemagglutinin-neuraminidase ectodomain. FEBS Lett. 2003;536(1–3):56–60. doi: 10.1016/s0014-5793(03)00010-3. [DOI] [PubMed] [Google Scholar]
- Vialard J, et al. Synthesis of the membrane fusion and hemagglutinin proteins of measles virus, using a novel baculovirus vector containing the beta-galactosidase gene. J Virol. 1990;64(1):37–50. doi: 10.1128/jvi.64.1.37-50.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore L, Wallace BA. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004;32(Web Server issue):W668–W673. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild TF, et al. Measles virus: both the haemagglutinin and fusion glycoproteins are required for fusion. J Gen Virol. 1991;72(Pt 2):439–442. doi: 10.1099/0022-1317-72-2-439. [DOI] [PubMed] [Google Scholar]
- Yao Q, et al. Association of the parainfluenza virus fusion and hemagglutinin-neuraminidase glycoproteins on cell surfaces. J Virol. 1997;71(1):650–656. doi: 10.1128/jvi.71.1.650-656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HS, et al. Structure of the uncleaved ectodomain of the paramyxovirus (hPIV3) fusion protein. Proc Natl Acad Sci U S A. 2005;102(26):9288–9293. doi: 10.1073/pnas.0503989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HS, et al. Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature. 2006;439(7072):38–44. doi: 10.1038/nature04322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, et al. Structural studies of the parainfluenza virus 5 hemagglutinin-neuraminidase tetramer in complex with its receptor, sialyllactose. Structure (Camb) 2005;13(5):803–815. doi: 10.1016/j.str.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Yuasa T, et al. A cell fusion-inhibiting monoclonal antibody binds to the presumed stalk domain of the human parainfluenza type 2 virus hemagglutinin-neuraminidase protein. Virology. 1995;206(2):1117–1125. doi: 10.1006/viro.1995.1035. [DOI] [PubMed] [Google Scholar]
- Zaitsev V, et al. Second sialic acid binding site in Newcastle disease virus hemagglutinin-neuraminidase: implications for fusion. J Virol. 2004;78(7):3733–3741. doi: 10.1128/JVI.78.7.3733-3741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]