Abstract
Many agents are active in multiple myeloma, but the majority of patients relapse. This clinical pattern suggests most cancer cells are eliminated, but cells with the clonogenic potential to mediate tumor regrowth are relatively chemoresistant. Our previous data suggested that CD138+ multiple myeloma plasma cells cannot undergo long-term proliferation but rather arise from clonogenic CD138neg B cells. We compared the relative sensitivity of these distinct cell types to clinical antimyeloma agents and found that dexamethasone, lenadilomide, bortezomib, and 4-hydroxycyclophosphamide inhibited CD138+ multiple myeloma plasma cells but had little effect on CD138neg precursors in vitro. We further characterized clonogenic multiple myeloma cells and stained cell lines using the Hoechst side population and Aldefluor assays. Each assay identified CD138neg cells suggesting that they possess high drug efflux capacity and intracellular drug detoxification activity. We also found that multiple myeloma cells expressing the memory B-cell markers CD20 and CD27 could give rise to clonogenic multiple myeloma growth in vitro and engraft immunodeficient nonobese diabetes/severe combined immunodeficient mice during both primary and secondary transplantation. Furthermore, both the side population and Aldefluor assays were capable of identifying circulating clonotypic memory B-cell populations within the peripheral blood of multiple myeloma patients. Our results suggest that circulating clonotypic B-cell populations represent multiple myeloma stem cells, and the relative drug resistance of these cells is mediated by processes that protect normal stem cells from toxic injury.
Introduction
Neoplastic plasma cells are the hallmark of multiple myeloma, and their expansion in the bone marrow and production of monoclonal immunoglobulin (Ig) are responsible for the manifestations of the disease. Both traditional cytotoxic agents and an increasing number of novel compounds can produce clinical responses (1–4). However, in spite of these therapies, multiple myeloma remains largely incurable (5). Disease relapse suggests the cells responsible for tumor regrowth are relatively drug resistant (6), but a full understanding of the cell type responsible for multiple myeloma growth remains unclear. Early studies examining a murine model of multiple myeloma suggested only a minority of cells were capable of clonogenic growth (7). Similarly, studies by Salmon and Hamburger (8) found that the cloning efficiency of primary multiple myeloma specimens was ~ 1 in 1,000 to 100,000 cells. To date, it has remained unclear whether these clonogenic cells are distinct from the plasma cells that constitute the majority of tumor cells.
Normal plasma cells are terminally differentiated and arise from the maturation of B cells. Several groups have identified B cells in the blood and bone marrow of multiple myeloma patients that express the identical idiotype and Ig gene sequences as the abnormal plasma cells (9–12). The role of these B cells in the pathogenesis of multiple myeloma has remained unclear because of a lack of functional studies, but we recently found that multiple myeloma cell lines and primary bone marrow contain small populations of clonotypic B cells that do not express the characteristic plasma cell surface antigen CD138 and are capable of clonogenic growth and differentiation into multiple myeloma plasma cells in vitro and in vivo (13). Recently, these data have been confirmed by others (14).
It is possible that a biologically distinct, drug-resistant multiple myeloma progenitor population is responsible tumor regrowth after treatment (6). To test this hypothesis, we further characterized clonogenic multiple myeloma cells and found that they were resistant to a number of clinically used antimultiple myeloma agents. Furthermore, these cells resembled normal memory B cells and displayed cellular properties characteristic of normal stem cells, suggesting cancer and normal stem cells share multiple mechanisms that promote drug resistance.
Materials and Methods
Patient specimens, cell lines, and cell culture
Blood and bone marrow were obtained from 16 patients with active multiple myeloma (8 newly diagnosed and 8 relapsed) granting informed consent as approved by the Johns Hopkins Medical Institutes Institutional Review Board. Bone marrow mononuclear cells were isolated by density centrifugation and depleted of CD138+ plasma cells and CD34+ hematopoietic progenitors using antihuman CD34 and CD138 magnetic microbeads (Miltenyi Biotec). Secondary depletion of CD138neg CD34neg cells was performed using antihuman CD20, CD27, or CD3 microbeads. Peripheral blood B cells were isolated after density centrifugation using the B-cell isolation kit (Miltenyi Biotec). For nonobese diabetes/severe combined immunodeficient (NOD/SCID) mouse experiments, CD27+ B cells were further isolated by positive magnetic selection with antihuman CD27 microbeads and two successive rounds of magnetic enrichment. Cell purity was assessed by flow cytometry and showed <5% contamination by relevant antigen expressing cells.
RPMI 8226 and National Cancer Institute (NCI)-H929 cell lines were obtained from American Type Tissue Collection. For drug studies, CD138+ or CD138neg cells (1 × 105/mL) isolated from the cell lines or CD138neg CD34neg cells from primary clinical samples (2 × 105/mL) were cultured in RPMI 1640 containing 10% fetal bovine serum alone or with dexamethasone (0.1 µmol/L; Sigma), lenalidomide (1 µmol/L; Celgene), bortezomib (10 nmol/L; Millenium Phamaceuticals), 4-hydroxycyclophosphamide (4HC; 30 µg/mL), rituximab (10 µg/mL; Genentech), or alemtuzumab (10 µg/mL; Berlex) for 48 h. Human AB serum (10%) was added to cells treated with rituximab or alemtuzumab as a source of complement (15). Cells were subsequently washed twice with medium to remove drugs then plated in methylcellulose as previously described (13). Myeloma plasma cell colonies consisting of >40 cells CD138+ plasma cells were scored using an inverted microscope 14 to 21 days after plating and assessed for clonality by flow cytometry (13).
Flow cytometric analyses
The following monoclonal antibodies were used: mouse antihuman CD138-PE, CD27-FITC, CD27-APC, CD19-APC, and either antihuman κ or λ Ig light chain-FITC or phycoerythrin antibodies (BD PharMingen). After the addition of propidium iodide (2 µg/mL) to discriminate dead cells, cells were analyzed and/or sorted with a FACSAria, FACSVantage, or MoFlo fluorescent cell sorter as previously described (13). Post sorting analysis showed >96% purity of cell populations with >98% cell viability.
For side population studies, RPMI 8226 and NCI-H929 cells (106/mL) were incubated with Hoechst 33342 (10 µg/mL; Invitrogen) for 60 min at 37°C followed by staining for 30 min at 4°C with anti-CD138. Clinical B-cell samples were stained with Hoechst 33342 (5 µg/mL) for 90 min at 37°C followed by staining for 30 min at 4°C with anti-CD27 and anti-Ig light chain antibodies. The concentration of Hoechst 33342 and incubations times were initially identified using samples that provided the highest frequency of side population cells with the lowest cytotoxicity determined by propidium iodide staining. Side population cells were analyzed on a LSR flow cytometer equipped with 424/44 nm band pass and 670 nm long pass optical filters (Omega Optical). As a control, cells were incubated as above with the addition of 50 µmol/L verapamil. Cells were stained for aldehyde dehydrogenase (ALDH) using the Aldefluor reagent (Stem Cell Technologies) and CD138, CD27, or Ig light chains according to the manufacturer’s instructions. Values are presented as mean fluorescence intensity (MFI) as previously described (16).
For cell cycle analysis, RPMI 8226 and NCI-H929 cells were fixed in 70% ethanol at 4°C for 30 min then washed and labeled with anti–CD138-FITC antibodies for 30 min. After removal of excess antibody, cells were incubated with RNase (50 µg/mL) and propidium iodide (2.5 µg/mL) for 30 min at 4°C followed by flow cytometry and DNA content analysis using the ModFit program (Verity).
NOD/SCID mice
The use of NOD/SCID mice was approved by the Johns Hopkins Medical Institutes Animal Care Committee. Six to eight-week-old mice underwent pretreatment with 300 cGy irradiation (84 cGy/min using a 137Cs γ irradiator) 12 to 16 h before dorsal tail vein injection. Mice were sacrificed when they exhibited symptoms including lethargy, anorexia, hind limb paralysis or, in the absence of symptoms, at 20 to 26 weeks. Bone marrow was harvested from the long bones and engraftment was determined by staining for human CD138, CD19, and either surface or cytoplasmic kappa or lambda Ig light chains. Cells were also stained for mouse CD4 and CD8 to ensure symptoms were not due to endogenous thymic lymphomas (17). For reengraftment studies, CD19+CD27+ cells were isolated by fluorescence-activated cell sorting and injected into secondary recipients as above.
Immunoglobulin gene rearrangement detection
DNA was extracted from plasma cells isolated bone marrow aspirates using CD138 magnetic microbeads or engrafted NOD/SCID mouse bone marrow samples (1–100 × 104 cells), using the QIAamp micro DNA isolation kit (Qiagen). DNA aliquots were subjected to PCR using primers for the Ig heavy chain gene VDJ region [FR3a, 5′-ACACGGC(C/T)(G/C)TGTATTACTGTG-3′; VLJH, 5′-TGACCAGGGT(A/G/C/T)CCTTGGCCCCAG -3′] for 30 to 40 cycles. Distilled water or control DNA encoding a known monoclonal Ig heavy chain gene rearrangement were used as negative or positive controls, respectively. PCR-amplified products were subjected to capillary electrophoresis on an ABI PRISM 3100 genetic analyzer and evaluated using the Genescan 2.1 software package (Applied Biosystems). For sequence analysis, PCR products were resolved on a 2% agarose gel, and major products were isolated and ligated into the TOPO TA cloning vector (Invitrogen) followed by DNA sequence analysis.
Results
Multiple myeloma plasma cells and precursors display differential drug sensitivities
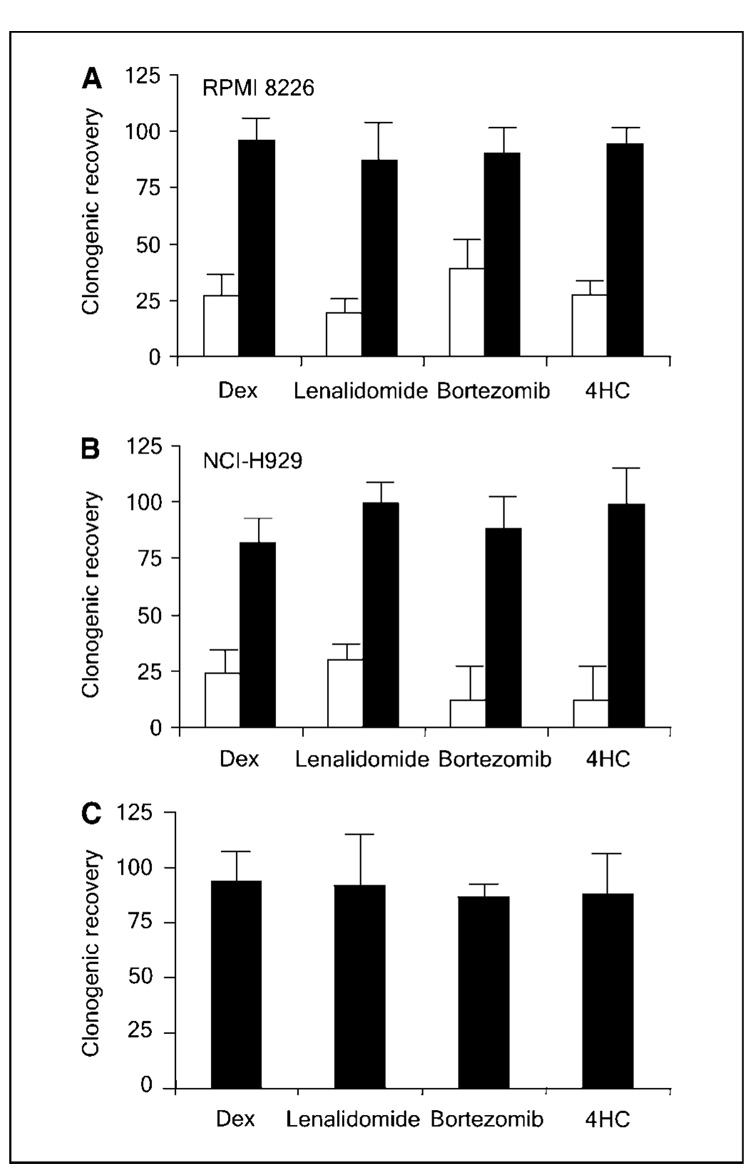
We previously showed that multiple myeloma cells derived from primary clinical bone marrow specimens and capable of in vitro clonogenic growth lack expression of the characteristic plasma cell antigen CD138 (13, 18). Some multiple myeloma cell lines seem to recapitulate the hierarchical cellular organization found within clinical specimens and contain small populations of CD138neg cells that express markers reminiscent of B cells and have relatively increased clonogenic growth potential in vitro, compared with mature CD138+ plasma cells (13, 14). To determine if multiple myeloma precursors display relative drug resistance, CD138+ plasma cells and CD138neg progenitors were isolated from the RPMI 8226 and NCI-H929 multiple myeloma cell lines and treated with four clinically active agents: the corticosteroid, dexamethasone; the thalidomide analogue, lenalidomide; the proteosome inhibitor, bortezomib; or 4HC, the active metabolite of the cytotoxic alkylator cyclophosphamide (3, 4, 19). Similar to their clinical activity, all four agents significantly inhibited the clonogenic growth of CD138+ plasma cells (Fig. 1A and B; P < 0.02 by Student’s t test for all groups compared with the untreated control). In contrast, none of the four agents significantly inhibited the clonogenic growth of CD138neg progenitors from either cell line (Fig. 1A and B; P > 0.1). Although the lack of in vitro clonogenic growth limited our ability to study mature CD138+ plasma cells derived from primary clinical samples, we studied clonogenic multiple myeloma precursors from four multiple myeloma patients (two newly diagnosed and two with relapsed disease) and found that they were not significantly affected by dexamethasone (mean, 95%; range, 77%–125%), lenalidomide (mean, 93%; range, 71%–143%), bortezomib (mean, 89%; range, 86%–107%) or 4HC (mean, 90%; range, 81%–105%; Fig. 1C; P > 0.2 for all groups compared with the untreated control). In addition, clonogenic precursors from newly diagnosed or relapsed patients were similarly resistant to each of these four agents (data not shown).
Figure 1.
Multiple myeloma cellular subsets display differential drug sensitivity. Clonogenic recovery of CD138+ (open bars) or CD138neg (black bars) cells from the multiple myeloma cell lines RPMI 8226 (A) and NCI-H929 (B) or CD138neg (C) multiple myeloma progenitors derived from four distinct clinical samples after treated with dexamethasone (Dex), lenalidomide, bortezomib, or 4HC. Values represent the mean of four experiments.
Multiple myeloma precursors display stem cell properties that mediate drug resistance
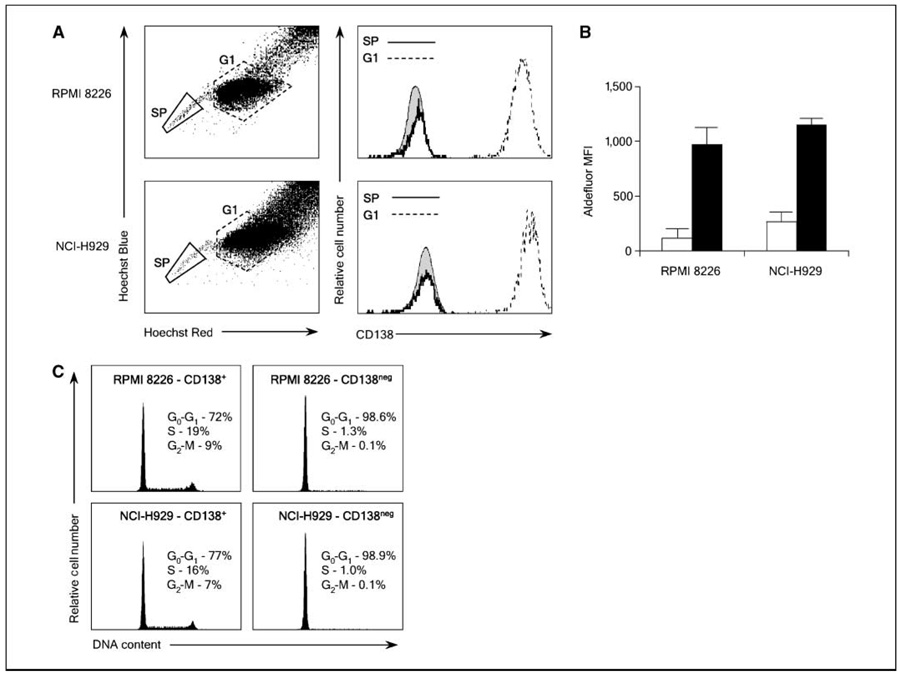
Normal tissue–restricted adult stem cells are highly resistant to toxic injury that seems to be multifactorial in nature. Furthermore, some of these processes serve as the basis for flow cytometric assays that can enrich for adult stem cells, and we examined whether these assays could distinguish cellular compartments in multiple myeloma. The ATP-binding cassette family of membrane transporters actively export xenobiotics, thereby limiting the intracellular accumulation of these compounds (20). Furthermore, efflux of the DNA binding dye Hoechst 33342 by the ABCG2/BCRP transporter is required for detection of the “side population” phenotype that is characteristic of stem cells from many tissues (21, 22). To examine whether the side population assay could identify clonogenic multiple myeloma precursors, we stained two human multiple myeloma cell lines, RPMI 8226 and NCI-H929, with Hoechst 33342 and found that each contained small populations of side population cells (0.8%–1.9% of total cells; Fig. 2A). Furthermore, costaining for CD138 showed that the side population cells were almost exclusively CD138neg (>97%) compared with the bulk of the population that was CD138+ (Fig. 2A).
Figure 2.
Multiple myeloma precursors display stem cell characteristics. A, expression of CD138 by RPMI 8226 and NCI-H929 side population (SP) or nonside population (G1) cells labeled with Hoechst 33342 and antihuman CD138. Shaded histogram, staining with an isotype control antibody. B, relative MFI of Aldefluor by RPMI 8226 and NCI-H929 CD138+ (open bars) and CD138neg (black bars) cells. Values are mean of four experiments. C, cell cycle profile of RPMI 8226 and NCI-H929 CD138+ and CD138neg cells after propidium iodide staining.
ALDH, specifically ALDH1A1, mediates the biosynthesis of all-trans-retinoic acid as well as the detoxification of a variety of compounds such as ethanol and active metabolites of cyclophosphamide (23). Normal adult stem cells typically exhibit higher relative levels of ALDH activity than their differentiated progeny, and the fluorescently labeled ALDH substrate Aldefluor can isolate stem cells from a number of adult tissues (16). Staining of the RPMI 8226 and NCI-H929 cells revealed small populations of ALDH+ cells accounting for 3.7% and 4.3% of cells, respectively (data not shown). Furthermore, costaining cells for CD138 expression showed that the CD138neg cells had significantly higher levels of ALDH activity than CD138+ plasma cells (Fig. 2B).
Cellular quiescence is exhibited by most normal adult stem cells, and this property is thought to be a major mechanism of drug resistance (24). To determine whether multiple myeloma precursors are relatively quiescent, immature CD138neg cells or CD138+ plasma cells were isolated from the RPMI 8226 and NCI-H929 cell lines and stained with propidium iodide to evaluate their cell cycle status. Similar to normal adult stem cells (25), we found that nearly all (>98%) of the CD138neg cells in both cell lines were in G0-G1, compared with only 72% or 77% of the CD138+ cells (Fig. 2C).
Clonogenic multiple myeloma cells resemble memory B cells
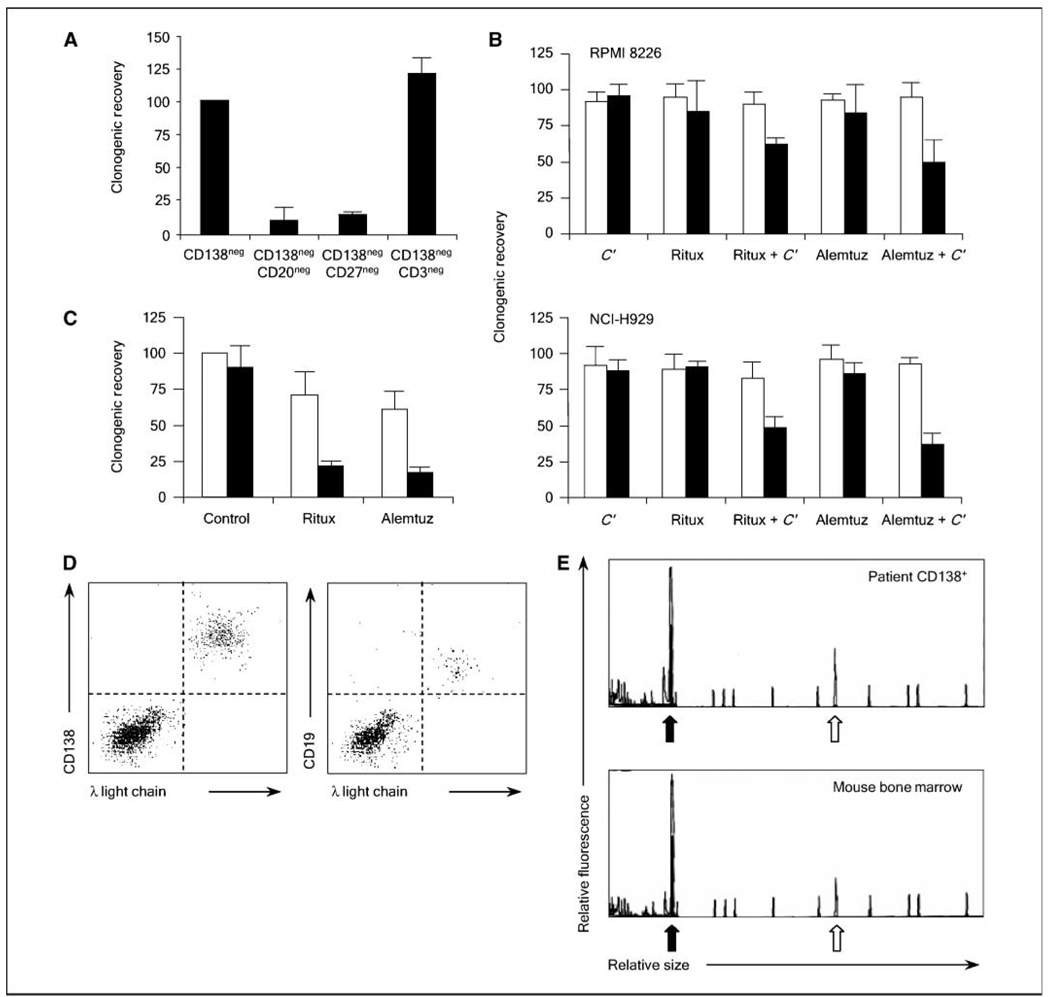
In human acute myeloid leukemia (AML) and brain tumors, cancer stem cells phenotypically resemble normal hematopoietic and neural stem cells (26, 27). These findings suggest that human cancers may arise from normal cellular compartments capable of self-renewal. Unlike normal hematopoietic and neural stem cells with the ability to generate multiple cell types, B cells that give rise to plasma cells lack multilineage potential. However, in contrast to the hematopoietic and neural systems in which self-renewal is restricted to the most primitive cellular compartments, self-renewal is maintained during multiple stages of B-cell development that permits the generation of clones producing the highest affinity antibodies as part of the adaptive immune response (28, 29). Examination of Ig gene sequences in multiple myeloma shows extensive somatic hypermutation without intraclonal variation, suggesting that multiple myeloma arises from a postgerminal center B cell (30). During the postgerminal stage of B-cell development, self-renewal is most evident in memory B cells and serves to maintain reactive B-cell clones during repeated rounds of antigen exposure (31, 32). Therefore, we hypothesized that clonogenic multiple myeloma precursors resemble memory B cells and depleted primary bone marrow specimens of cells expressing the B-cell surface antigen CD20 or the memory B-cell surface marker CD27. Compared with the starting population of CD138neg CD34neg cells, the removal of either CD20+ or CD27+ cells significantly limited clonogenic multiple myeloma growth by 88% and 83%, respectively (Fig. 3A; P < 0.001). In contrast, the removal of CD3+ T cells did not have a significant effect on the clonogenic recovery of multiple myeloma colonies (Fig. 3A; P > 0.1). Thus, the phenotype of multiple myeloma cells with in vitro clonogenic potential, CD138negCD20+CD27+, parallels normal memory B cells.
Figure 3.
Multiple myeloma progenitors resemble normal memory B cells and display properties typical of normal stem cells. A, relative colony formation by CD138neg CD34neg bone marrow mononuclear cells (CD138neg) isolated from four patients with multiple myeloma after depletion of additional cells expressing CD20, CD27, or CD3. Columns, mean; bars, SE. B, clonogenic recovery of CD138+ (open bars) or CD138neg (black bars) cells from the multiple myeloma cell lines RPMI 8226 and NCI-H929 after treatment with rituximab (Ritux), alemtuzumab (Alemtuz), and/or human complement (C’). Values represent means of 4 experiments. C, clonogenic recovery of CD138neg multiple myeloma progenitors derived from primary clinical specimens after antibody treatment with (open bars) or without (black bars) human complement. D, engraftment of NOD/SCID mice with peripheral blood memory B cells derived from patients with multiple myeloma. Flow cytometric analysis of NOD/SCID mouse bone marrow cells for expression of human CD138 and intracellular Ig λ light chain (left) or CD19 and surface Ig λ light chain (right) after injection of peripheral blood memory B cells. E, comparison of capillary electorphoretic profiles of Ig heavy chain CDR3 amplification products (black arrow) obtained by PCR of CD138+ multiple myeloma plasma cells isolated from the primary clinical bone marrow specimen or from bone marrow cells collected from a mouse injected with memory B cells from the same patient. Open arrow, a control PCR reaction product.
Surface antigen expression markedly differs between normal B cells and plasma cells; therefore, we studied whether two humanized monoclonal antibodies, rituximab and alemtuzumab, that target the B-cell antigens CD20 and CD52 could inhibit clonogenic multiple myeloma cells (33, 34). CD138neg precursors or CD138+ plasma cells were isolated from the RPMI 8226 or NCI-H929 cell lines and treated with each monoclonal antibody in combination with human complement that strongly enhances their in vitro activity through complement-dependent cytoxicity (15). Neither complement alone nor the monoclonal antibodies with or without complement affected the clonogenic growth of CD138+ plasma cells that lack the target antigens (Fig. 3B; P > 0.3 for all groups compared with the untreated control). However, both antibodies significantly inhibited the clonogenic recovery of CD138neg multiple myeloma progenitors from both cell lines when combined with complement (Fig. 3B; P < 0.01 for each combination compared with the complement alone or untreated control groups). Similarly, each combination of monoclonal B-cell antibody and complement significantly inhibited the clonogenic recovery of CD138neg multiple myeloma progenitors isolated from four primary patient specimens (Fig. 3C; P < 0.001).
Circulating clonotypic memory B cells from multiple myeloma patients engraft NOD/SCID mice
B cells sharing Ig gene sequences and idiotype specificity with multiple myeloma plasma cells have been detected in the blood and bone marrow of multiple myeloma patients (9, 10, 35). We studied the functional growth capacity of these cells and injected CD19+CD27+ B cells isolated from the peripheral blood of four patients with multiple myeloma into NOD/SCID mice. All recipient animals developed hind limb paralysis, along with detectable human CD138+ plasma cells (6.6%–15% of the total bone marrow cells) 4 to 6 months after injection (Table 1). In contrast, no engraftment was detected after the injection of 1 × 107 of the corresponding CD138+ plasma cells isolated from each multiple myeloma patient (data not shown), consistent with our previous studies (13). Furthermore, the human plasma cells were clonally related to the original multiple myeloma plasma cells by Ig light chain expression (Fig. 3D), Ig heavy chain gene CDR3 length restriction (Fig. 3E), and CDR3 DNA sequence (data not shown). Small populations of Ig light chain–restricted CD19+CD27+ cells were also detected (0.01%–0.06% of total bone marrow cells; Fig. 3D), and injection of these cells (10.8–100 × 103 cells) into secondary recipients similarly produced multiple myeloma engraftment after 4 to 6 months (Table 1).
Table 1.
Primary and secondary engraftment of NOD/SCID mice with clonotypic B cells isolated from the peripheral blood of multiple myeloma patients
| Patient | Primary engraftment | Secondary engraftment | ||||||
|---|---|---|---|---|---|---|---|---|
| Cells injected (×106) | Time (wk)* | % CD138 † | % CD19 † | Cells injected (×103) | Time (wk)* | % CD138 † | % CD19 † | |
| 1 | 1.2 | 20 | 8.7 | 0.02 | 10.8 | 22 | 12.1 | 0.06 |
| 2 | 1 | 20 | 4.3 | 0.01 | Nd | Nd | Nd | Nd |
| 3 | 4.3 | 16 | 15 | 0.06 | 100 | 26 | 8.9 | 0.05 |
| 4 | 0.8 | 25 | 6.6 | 0.02 | 14.1 | 26 | 7.2 | 0.05 |
Time after transplantation to the development of signs of multiple myeloma.
Frequency of Ig light chain–restricted cells within mouse bone marrow; nd, not done.
Clonotypic B cells in multiple myeloma exhibit stem cell properties
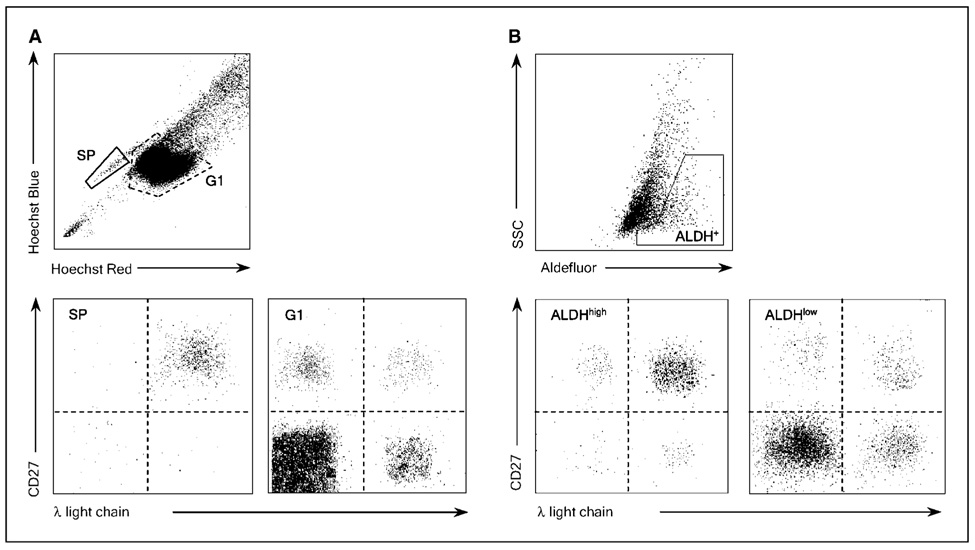
Because the side population and ALDH assays identified CD138neg precursors within multiple myeloma cell lines, we examined whether these assays could identify multiple myeloma precursors in primary clinical specimens. We stained CD19+ B cells isolated from the peripheral blood of four multiple myeloma patients with Hoechst 33342 and detected small numbers of side population cells (0.18%–0.83% of total B cells; Fig. 4A and Table 2). Further surface staining showed that the majority (89%–97%) of the side population B cells expressed CD27 and clonal surface Ig light chain restriction that matched each patient’s multiple myeloma plasma cells (Fig. 4A and Table 2). In contrast, nonside population cells contained a mixture of CD27+ memory and CD27neg naïve B cells expressing both Ig light chains (G1; Fig. 4A). We also stained these peripheral blood CD19+ B cells with Aldefluor and found small populations of ALDH+ cells. Similar to the side population B cells, most (86%–93%) of the ALDH+ B cells expressed CD27 and clonotypic surface Ig light chain (Fig. 4B and Table 2). In contrast, ALDHneg cells contained a mixture of nonclonal CD27-positive and CD27-negative cells expressing both κ and λ Ig light chains (Fig. 4B).
Figure 4.
Circulating multiple myeloma stem cells display properties typical of normal stem cells. A, expression of CD27 and surface Ig light chain expression by peripheral blood B cells with the side population or nonside population phenotype derived from a representative multiple myeloma patient. B, expression of CD27 and surface Ig light chain expression by peripheral blood B cells with high or low Aldefluor fluorescence derived from a representative multiple myeloma patient.
Table 2.
Clonal B-cell populations identified by side population and ALDH analysis
| Patient | ALDHhigh* (%) | Clonality † | SP* (%) | Clonality † |
|---|---|---|---|---|
| 1 | 1.7 | 89 | 0.59 | 94 |
| 2 | 1.1 | 93 | 0.31 | 97 |
| 3 | 2.4 | 86 | 0.83 | 89 |
| 4 | 1.0 | 92 | 0.18 | 93 |
Abbreviation: SP, side population.
Percent of total CD19+ B cells.
Percent of cells expressing CD27 and surface Ig light chain matching the original multiple myeloma clone.
Discussion
Highly clonogenic cell populations have been identified in several human cancers that are able to phenotypically recapitulate the original tumor in NOD/SCID mice (26, 27, 36–38). These cells can also be isolated from engrafted animals and retransplanted into secondary recipients; therefore, they have the capacity to produce differentiated progeny and undergo self-renewal, two defining characteristics of normal stem cells. We found that clonotypic cells isolated from multiple myeloma patients and expressing normal memory B-cell surface antigens were capable of producing multiple myeloma in NOD/SCID mice upon primary and secondary transplantation. These results suggest that multiple myeloma is organized in a hierarchical manner that parallels normal tissue development similar to AML and brain tumors in which cancer stem cells phenotypically resembling their normal counterparts give rise to differentiated progeny (26, 27).
Others have similarly reported that clonotypic B cells from clinical specimens can generate disease in NOD/SCID mice (39). In contrast, Yaccoby et al. (40) have reported that CD138+ multiple myeloma plasma cells can be successfully xenografted into SCID mice implanted with human fetal bone fragments. However, engraftment of mature plasma cells in these SCID-hu mice may primarily reflect the ability of the human bone marrow to support implanted plasma cells and/or plasmablasts, given the important role that the microenvironment plays in the survival of these cells (41). In a similar fashion, the bone fragments within SCID-hu mice have been found to support relatively mature AML blasts expressing the myeloid antigen CD33 (42), whereas only CD34+ cells lacking markers of lineage commitment engraft NOD/SCID mice (26).
Although stem cells have been identified in an increasing number of human cancers, the clinical relevance and implications of these findings remain unclear. Standard response criteria used to measure the clinical efficacy of anticancer treatments primarily reflect changes in disease bulk and activity against mature tumor cells (6). Because cancer stem cells are a relatively low frequency population in most tumor types, the true inhibition of these cells is likely to be difficult to assess early after treatment, and a prolongation of disease remission would be required to establish such activity. The initial clinical responses induced by dexamethasone, lenalidomide, bortezomib, and cyclophosphamide seen as decreased bone marrow plasmacytosis and monoclonal Ig levels in multiple myeloma likely reflect the activity of these agents against mature multiple myeloma plasma cells. However, the inability of dexamethasone or standard cytotoxic chemotherapy to produce sustained clinical remissions suggests that clonogenic cells responsible for tumor regrowth are insensitive to these agents and supports our data that multiple myeloma stem cells are not inhibited by these drugs (5). It is unknown whether lenalidomide or bortezomib can produce durable remissions in multiple myeloma because they have been only recently introduced for clinical use, but we found that clonogenic multiple myeloma progenitors are similarly resistant to these agents. It is well-known that the antitumor activity of these agents is mediated in part by modulating the interaction between myeloma plasma cells and bone marrow stromal cells (41). Therefore, it is possible that our in vitro studies failed to adequately assess the effects of these agents on the bone marrow microenvironment, but similar to other studies, we found that clonogenic multiple myeloma precursors could be isolated from the peripheral blood where these factors have little influence (39).
Our results complement a recent report describing the relative radioresistance of glioblastoma cancer stem cells compared with the differentiated cells that make up the bulk of the tumor mass (43). The stark biological differences between cancer stem cells and mature tumor cells is likely to be representative of many other malignancies as the clinical pattern of relapse after effective treatment can be seen in most human cancers. Therefore, therapeutic strategies that target the specific biology of cancer stem cells are likely required to prevent the continued production of mature tumor cells and produce sustained remissions. Monoclonal antibodies directed against B-cell surface antigens limited multiple myeloma progenitor clonogenic growth in vitro, suggesting that specific biological properties exhibited by multiple myeloma cancer stem cells may effectively serve as antitumor targets. Furthermore, we recently showed that the developmental signaling pathway Hedgehog is up-regulated in multiple myeloma stem cells and regulates cell fate decisions (44). Therefore, optimal clinical strategies may require combining agents active against multiple myeloma plasma cells to decrease tumor burden and alleviate symptoms with those that target multiple myeloma cancer stem cells to prevent tumor regrowth and relapse.
The combined use of surface phenotype with flow cytometric–based functional stem cell properties facilitates the identification of these cells within the circulation of multiple myeloma patients. Therefore, it is possible that these methods may allow the quantification of these cells to be used as a surrogate marker for clinical response during cancer stem cell–directed therapies. These results also suggest that inherent properties that distinguish normal adult stem cells from their differentiated progeny, such as quiescence and high expression of membrane-bound drug transporters and intracellular detoxifying enzymes, contribute to their relative resistance to toxic injury. The precise mechanisms responsible for the resistance of clonogenic multiple myeloma cells to dexamethasone, 4HC, lenalidomide, and bortezomib are unknown, but resistance to individual drugs usually occurs through multiple cellular processes (45). Our data suggest that these same stem cell properties contribute to the drug resistance of multiple myeloma cancer stem cells, allowing them to persist and mediate disease relapse in multiple myeloma patients initially responding to therapy.
Acknowledgments
Grant support: NIH (to W. Matsui, R.J. Jones, and R.F. Ambinder) and the American Society of Clinical Oncology (W. Matsui), as well as charitable support from the Pearse family.
We thank Joshua Kellner and Kimberly Noonan for technical support.
References
- 1.Oken MM, Harrington DP, Abramson N, Kyle RA, Knospe W, Glick JH. Comparison of melphalan and prednisone with vincristine, carmustine, melphalan, cyclophosphamide, and prednisone in the treatment of multiple myeloma: results of Eastern Cooperative Oncology Group Study E2479. Cancer. 1997;79:1561–1567. [PubMed] [Google Scholar]
- 2.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 3.Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 4.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA, Rajkumar SV. Multiple Myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 6.Huff CA, Matsui W, Smith BD, Jones RJ. The paradox of response and survival in cancer therapeutics. Blood. 2006;107:431–434. doi: 10.1182/blood-2005-06-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park CH, Bergsagel DE, McCulloch EA. Mouse myeloma tumor stem cells: a primary cell culture assay. J Natl Cancer Inst. 1971;46:411–422. [PubMed] [Google Scholar]
- 8.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 9.Pilarski LM, Jensen GS. Monoclonal circulating B cells in multiple myeloma. A continuously differentiating, possibly invasive, population as defined by expression of CD45 isoforms and adhesion molecules. Hematol Oncol Clin North Am. 1992;6:297–322. [PubMed] [Google Scholar]
- 10.Billadeau D, Ahmann G, Greipp P, Van Ness B. The bone marrow of multiple myeloma patients contains B cell populations at different stages of differentiation that are clonally related to the malignant plasma cell. J Exp Med. 1993;178:1023–1031. doi: 10.1084/jem.178.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergsagel PL, Smith AM, Szczepek A, Mant MJ, Belch AR, Pilarski LM. In multiple myeloma, clonotypic B lymphocytes are detectable among CD19+ peripheral blood cells expressing CD38, CD56, and monotypic Ig light chain. Blood. 1995;85:436–447. [PubMed] [Google Scholar]
- 12.Rasmussen T, Kastrup J, Knudsen LM, Johnsen HE. High numbers of clonal CD19+ cells in the peripheral blood of a patient with multiple myeloma. Br J Haematol. 1999;105:265–267. [PubMed] [Google Scholar]
- 13.Matsui W, Huff CA, Wang Q, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kukreja A, Hutchinson A, Dhodapkar K, et al. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med. 2006;203:1859–1865. doi: 10.1084/jem.20052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagajothi N, Matsui WH, Mukhina GL, Brodsky RA. Enhanced cytotoxicity of rituximab following genetic and biochemical disruption of glycosylphosphatidylinositol anchored proteins. Leuk Lymphoma. 2004;45:795–799. doi: 10.1080/10428190310001625700. [DOI] [PubMed] [Google Scholar]
- 16.Jones RJ, Barber JP, Vala MS, et al. Assessment of aldehyde dehydrogenase in viable cells. Blood. 1995;85:2742–2746. [PubMed] [Google Scholar]
- 17.Chiu PPL, Ivakine E, Mortin-Toth S, Danska JS. Susceptibility to lymphoid neoplasia in immunodeficient strains of nonobese diabetic mice. Cancer Res. 2002;62:5828–5834. [PubMed] [Google Scholar]
- 18.Wijdenes J, Vooijs WC, Clement C, et al. A plasmocyte selective monoclonal antibody (B-B4) recognizes syndecan-1. Br J Haematol. 1996;94:318–323. doi: 10.1046/j.1365-2141.1996.d01-1811.x. [DOI] [PubMed] [Google Scholar]
- 19.Karp JE, Humphrey RL, Burke PJ. Timed sequential chemotherapy of cytoxan-refractory multiple myeloma with cytoxan and adriamycin based on induced tumor proliferation. Blood. 1981;57:468–475. [PubMed] [Google Scholar]
- 20.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 21.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirschmann-Jax C, Foster AE, Wulf GG, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodsky RA, Jones RJ. Aplastic anemia. Lancet. 2005;365:1647–1656. doi: 10.1016/S0140-6736(05)66515-4. [DOI] [PubMed] [Google Scholar]
- 24.Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 25.Gothot A, Pyatt R, McMahel J, Rice S, Srour EF. Functional heterogeneity of human CD34(+) cells isolated in subcompartments of the G0/G1 phase of the cell cycle. Blood. 1997;90:4384–4393. [PubMed] [Google Scholar]
- 26.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 27.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 28.Hardy RR, Li YS, Allman D, Asano M, Gui M, Hayakawa K. B-cell commitment, development and selection. Immunological Reviews. 2000;175:23–32. [PubMed] [Google Scholar]
- 29.Fearon DT, Manders P, Wagner SD. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science. 2001;293:248–250. doi: 10.1126/science.1062589. [DOI] [PubMed] [Google Scholar]
- 30.Bakkus MH, Heirman C, Van Riet I, Van Camp B, Thielemans K. Evidence that multiple myeloma Ig heavy chain VDJ genes contain somatic mutations but show no intraclonal variation. Blood. 1992;80:2326–2335. [PubMed] [Google Scholar]
- 31.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 32.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 33.Maloney DG, Grillo-Lopez AJ, White CA, et al. IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188. [PubMed] [Google Scholar]
- 34.Ginaldi L, De Martinis M, Matutes E, et al. Levels of expression of CD52 in normal and leukemic B and T cells: correlation with in vivo therapeutic responses to Campath-1H. Leuk Res. 1998;22:185–191. doi: 10.1016/s0145-2126(97)00158-6. [DOI] [PubMed] [Google Scholar]
- 35.Szczepek AJ, Seeberger K, Wizniak J, Mant MJ, Belch AR, Pilarski LM. A high frequency of circulating B cells share clonotypic Ig heavy-chain VDJ rearrangements with autologous bone marrow plasma cells in multiple myeloma, as measured by single-cell and in situ reverse transcriptase- PCR. Blood. 1998;92:2844–2855. [PubMed] [Google Scholar]
- 36.Al Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 38.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilarski LM, Seeberger K, Coupland RW, et al. Leukemic B cells clonally identical to myeloma plasma cells are myelomagenic in NOD/SCID mice. Exp Hematol. 2002;30:221–228. doi: 10.1016/s0301-472x(01)00788-3. [DOI] [PubMed] [Google Scholar]
- 40.Yaccoby S, Epstein J. The proliferative potential of myeloma plasma cells manifest in the SCID-hu host. Blood. 1999;94:3576–3582. [PubMed] [Google Scholar]
- 41.Mitsiades CS, Mitsiades NS, Richardson PG, Munshi NC, Anderson KC. Multiple myeloma: a prototypic disease model for the characterization and therapeutic targeting of interactions between tumor cells and their local microenvironment. J Cell Biochem. 2007;101:950–968. doi: 10.1002/jcb.21213. [DOI] [PubMed] [Google Scholar]
- 42.Namikawa R, Ueda R, Kyoizumi S. Growth of human myeloid leukemias in the human marrow environment of SCID-hu mice. Blood. 1993;82:2526–2536. [PubMed] [Google Scholar]
- 43.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 44.Peacock CD, Wang Q, Gesell GS, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci U S A. 2007;104:4048–4053. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lehnert M. Chemotherapy resistance in breast cancer. Anticancer Res. 1998;18:2225–2226. [PubMed] [Google Scholar]