Abstract
Although environmental enrichment has been shown to improve various types of memory in young and aging mice, no study has directly compared the degree to which enrichment improves memory at different ages throughout the lifespan in male mice. Therefore, the present study investigated the effects of long-term continuous enrichment in young (3 months), middle-aged (15 months), and aged (21 months) male C57BL/6 mice. Spatial reference memory was tested in the Morris water maze. Results demonstrate that 24 hr/day environmental enrichment for approximately 6 weeks significantly improved spatial memory in the Morris water maze in aged males, but not in young or middle-aged males. These data also indicate that 24 hr exposure to complex enriched housing conditions increases the magnitude of enrichment-induced improvements in memory among aged mice relative to those previously reported by this lab and others.
Keywords: Aging, Aged, Morris water maze, Reference memory, Mouse
1. Introduction
It has long been known that environmental enrichment can protect the developing rodent brain from the adverse consequences of social and cognitive isolation (see [28] for review). Enrichment treatment typically exposes animals to a combination of social, cognitive, and physical stimulation provided by cage-mates, toys, and running wheels. Enriched animals are compared to controls that are either individually housed (i.e., isolated) or housed in small groups (i.e., social). Relative to isolated and social controls, the cortices of rats reared in enriched environments evince a number of morphological alterations including increased thickness, dendritic spines and branching, and neuronal cell body size [4, 5, 13, 16]. Enrichment started in young adulthood has also been shown to affect CA1 and dentate morphology, as well as increase synaptophysin levels, in the hippocampus [7, 22]. Further, young rodents reared in enriched environments outperform social and isolated controls on numerous learning and memory tasks [15, 23, 35].
Recent work in adult and aged rodents suggests that the beneficial effects of enrichment on memory and neural function may be initiated at any point in the lifespan [2, 10, 14, 28]. For example, enrichment can enhance cortical and hippocampal plasticity in adult rats [14] and mice [20] and can protect against learning and memory deficits in adult CA1-specific NMDAR1 subunit-knockout mice [26]. In middle-aged rodents, enrichment improves learning in the Hebb-Williams maze [3] and spatial memory in the Morris water maze [11, 21, 25]. Enrichment in middle-aged rats and mice also increases forebrain weight [3], hippocampal neurogenesis [21], and hippocampal and cortical neurotrophin levels [19, 25]. Among aged rodents, enrichment improves spatial memory in the Morris water maze [10], reverses short-term memory impairments in a Symbolic Delayed Matching to Sample Task [32], and increases incidental learning and food seeking behaviors [34]. It also increases cortical thickness [6], reduces hippocampal gliosis [32], and increases neurogenesis in the dentate gyrus [31].
In spite of the fact that enrichment has been shown to improve memory in rodents of all ages in separate studies, no study has directly compared the effects of enrichment in young, middle-aged, and aged male rodents. Because different enrichment protocols can have drastically different effects on memory [2], it is important to compare the effects of enrichment in different age groups within the same study to enable more precise conclusions about the effects of this treatment throughout the lifespan. Interestingly, previous studies from our laboratory investigating effects of enrichment in middle-aged male and female [11], aged male [2], and aged female [10] mice have reported memory alterations that are dependent on age and sex, such that the beneficial effects of enrichment appear to be greatest in middle-aged females and aged males. However, these findings are confounded by the fact that these previous studies used different enrichment treatments, for different amounts of time, and tested animals on different versions of the spatial Morris water maze task. Thus, the present study was designed to investigate the effects of enrichment on spatial memory throughout the lifespan within the same study. To achieve this direct comparison, young (3 months), middle-aged (15 months), and aged (21 months) male C57BL/6 mice were enriched for four weeks prior to, and then throughout, behavioral testing. Enriched mice were housed in large cages with 24 hr/day exposure to a complex enriched environment (as in [2]). Socially-housed controls were housed in standard shoebox cages with no exposure to enriching objects. The Morris water maze was used to assess hippocampal-dependent spatial reference memory. This task was chosen because it is sensitive to both age-related memory decline [8] and to enrichment [2, 10] in aged mice. We expected that enrichment would improve memory most in aged males because existing age-related memory decline in these groups [8] would leave more room for improvement. Further, we recently reported greater effects on spatial memory in the water maze among aged male mice using a 24 hr/day enrichment protocol compared to a 3 hr/day protocol [2]. This finding was most likely due to both the increased time spent in the environments each day and the increased complexity of these environments. Therefore, we expected that the effects of enrichment observed in the present study would be more robust than those previously reported by our lab in which enrichment was administered for only 3 hrs/day [10, 17] or in less complex home-cages [11].
2. Materials and Methods
2.1. Subjects
Subjects were 85 male C57BL/6 mice obtained from the National Institutes on Aging colony at Harlan Sprague Dawley (Indianapolis, IN). Upon arrival at Yale, mice were 3 months (n = 25), 15 months (n = 30), and 21 months (n = 30) of age. Mice were housed up to 5 per shoebox cage (controls) or up to 8 in large enrichment cages in a room with a 12:12 light/dark cycle (lights on at 07:00), with all testing performed during the light phase. Mice had ad libitum access to food (Harlan 2018 18% Protein Rodent Diet) and water. Animals were handled for 5 min/day at least five times prior to behavioral testing to habituate them to being picked up by the experimenter. All procedures were approved by the Institutional Animal Care and Use Committee of Yale University, and conformed to the guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.2. Environmental Enrichment
Mice were housed in enriched or control conditions for four weeks prior to and then throughout behavioral testing for a total of approximately 6 weeks. Mice were randomly assigned to the control or enrichment conditions yielding a total of 6 treatment groups: young control (YC, n =15), middle-aged control (MC, n = 14), aged control (AC, n = 14), young enriched (YE, n = 10), middle-aged enriched (ME, n = 16) and aged enriched (AE, n = 16).
Control mice were housed in standard shoebox cages and had no exposure to toys or running wheels. Enriched mice were housed in large transparent plastic bins (Ancare, Bellmore, NY; 66 cm long × 46 cm wide × 38 cm high) that were covered with removable transparent lids with two large ventilation holes on the top (30 cm long × 18.5 cm wide). Wire feeding racks covered by ventilation lids from the standard shoebox cages were fitted into the ventilation holes on the top of the bins as in [2]. Water access was provided by a water bottle mounted on the side of the bin (Ancare, Bellmore, NY), which allowed the spout to enter 7 cm above the base of the bin. Food pellets were placed in a stainless steel food bowl on the floor of the bin. Enrichment bins were maintained in the same colony room as the standard shoebox cages. Bins were cleaned twice per week, with new enrichment objects in different configurations introduced at each cleaning. Cognitive stimulation and exercise were provided by an assortment of objects that always included 2 running wheels, 2 rodent dwellings, 4–6 miscellaneous toys, and a large plastic tube configuration with vertical climbing aspects.
2.3. Morris Water Maze
The apparatus was as previously described [2, 9]. Testing took place in a white circular tank (97 cm in diameter) filled with water (24 ± 2°C). The water was made opaque with white nontoxic tempera paint and the maze was surrounded by various extramaze cues. Four additional extramaze cues (abstract black and white designs, 30 cm2) were attached on the side of the tank equidistant from each other during spatial water maze testing. Data were collected using an HVS 2020 (HVS Image, Hampton, England) automated tracking system.
Mice were shaped in the tank one day prior to testing using a four-trial procedure in which a smaller ring (55 cm) was placed inside of the larger (97 cm) ring to decrease the total swimming area. Mice were first placed on a visible 10 × 10 cm platform (covered in red tape) for 10 s and then removed. They were then placed at three distances progressively further from the platform and allowed to swim to the platform. If the mouse did not find the platform within 60 s, then it was led to it by the experimenter. No data were collected during shaping.
2.3.1. Spatial Water Maze
Spatial memory in the water maze was assessed as described in [2] and [9]. A transparent lucite platform (10 × 10 cm) was submerged just underneath the surface of the water and remained in the same location for all trials. Six trials per day were conducted for five consecutive days. Each mouse was placed in one of four start positions, which varied for each trial. For the first 5 trials, the mouse was given 120 s to find the platform in each trial. If the mouse did not find the platform within this time, then the experimenter led the mouse to the platform where it sat for 10 s. The mouse was then returned to its home-cage for an inter-trial interval of approximately 20 min. During these first five trials, swim time (s), swim distance (cm), and swim speed (cm/s) were recorded. Lower numbers for swim time and swim distance indicated better performance and lower numbers for swim speed indicated slower swim speeds.
The sixth trial of each day was a variable-interval probe trial [24]. During this trial, the platform was collapsed and made unavailable for escape for 20, 30, or 40 s. At the conclusion of this interval, the platform was then raised and made available for escape. The total probe trial duration was 60 s, regardless of the amount of time that the platform was collapsed. Quadrant time (the percent of time that a mouse spent in the quadrant containing the collapsed platform) and platform crossings (the number of times a mouse crossed the platform location per 10 s of the interval) were recorded. Higher numbers indicated better performance for both measures.
2.3.2. Cued Water Maze
A non-spatial cued water maze task was conducted to measure potential non-mnemonic contributions to task performance (e.g. swimming ability, motivation, and visual acuity). Cued testing began two days after the completion of spatial testing and consisted of six trials over one day. During these trials, the platform was made visible with red tape and a white circular cue attached to the side of the platform (7 ½ cm in diameter). The platform was raised just above the surface of the water, and extramaze cues attached to the side of the tank were removed. The platform was moved to a different location in the tank for each trial. All other aspects of the procedure were the same as the spatial task. Swim time (s), swim distance (cm), and swim speed (cm/s) were recorded.
2.4. Data Analysis
Spatial and cued water maze measures were averaged within a group for each session (for the spatial task) or trial (for the cued task) and analyzed using one-way analysis of variance (ANOVA) with Group as the independent variable and Session or Trial as the repeated measure (SuperANOVA, Abacus Concepts, Berkeley, CA). Although an ANOVA using the single independent variable “Group” did not allow us to measure the overall effects of Age and Enrichment across the treatment groups, it was of more interest in this study to compare control and enriched groups within an age group. A Group ANOVA followed by conservative post-hoc tests allowed us to minimize Type II errors more than an Age × Enrichment ANOVA followed by multiple T-tests [18]. Therefore, Tukey-Kramer post-hocs were performed on all significant main effects of Group. An alpha level of 0.05 was used to reject the null hypothesis.
3. Results
3.1. Subjects
All mice were in good health upon arrival in the laboratory, at which point they were randomly divided into treatment groups and housed appropriately. One aged enriched male was excluded from the data analyses due to poor swimming ability.
3.2. Morris Water Maze
3.2.1. Spatial Task
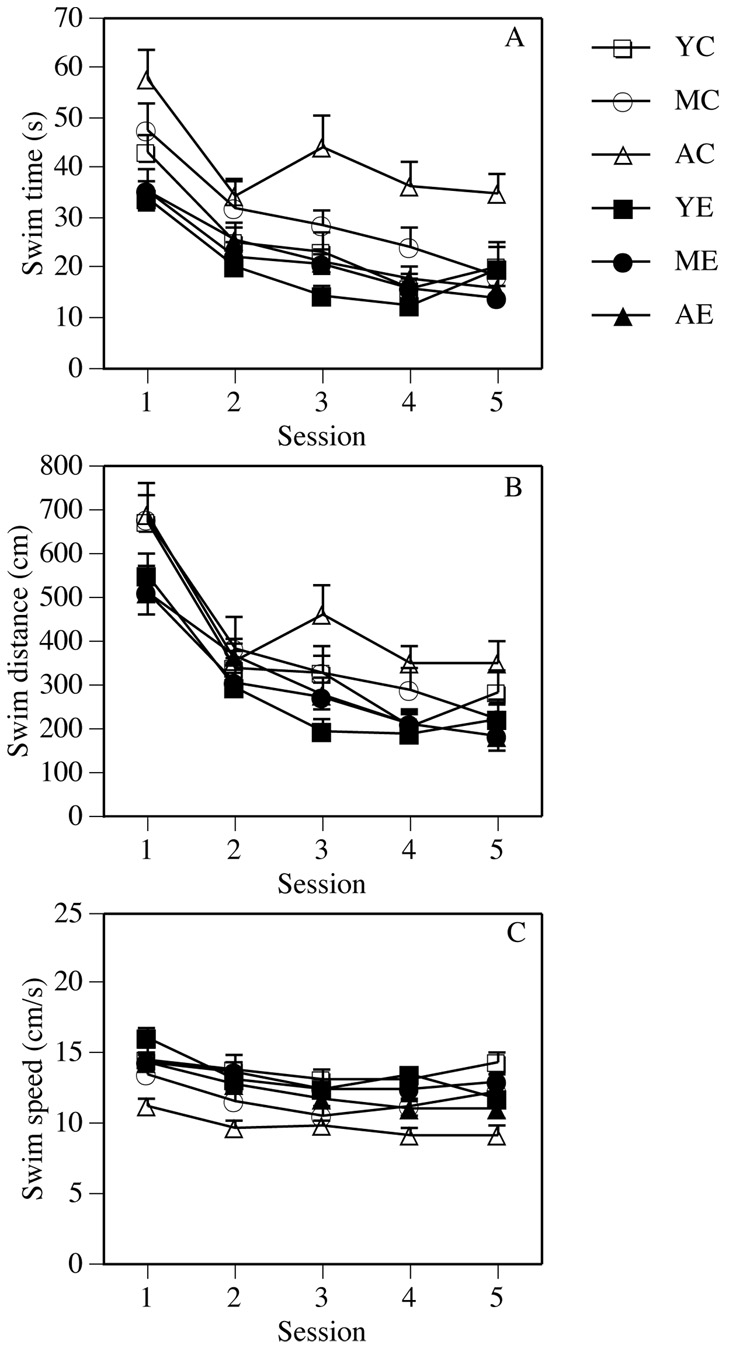
The main effect of Group was significant for swim time, swim distance, and swim speed (Fs(5,78) = 11.36, 5.34, and 8.67, respectively, Ps ≤ 0.0003). As indicated in Figure 1, enrichment generally reduced swim time (Figure 1A) and swim distance (Figure 1B), and increased swim speed (Figure 1C). Post-hoc tests indicated no difference between young control and enriched mice or between middle-aged control and enriched mice. In contrast, aged enriched mice exhibited shorter swim times and swim distances, and faster swim speeds than aged controls (Ps < 0.05). Post-hoc tests also indicated that swim time increased and swim speed decreased with age among control mice, such that aged controls had slower swim times and swim speeds than young controls (Ps < 0.05), as well as slower swim times than middle-aged controls (P < 0.05). Significant Session effects in swim time, swim distance, and swim speed demonstrated that the groups generally learned to find the platform (Fs(4,312) = 40.55, 59.22, and 25.59, respectively, Ps < 0.001, Figure 1). The Group × Session interaction was significant for swim speed (F(20,312) = 1.72, P < 0.05).
Figure 1.
Swim time (A), swim distance (B), and swim speed (C) in the spatial water maze task. Among aged males, enrichment significantly reduced swim time and swim distance, and increased swim speeds relative to aged controls. Enrichment did not affect any measure in young or middle-aged males. Each point represents the mean (± standard error of the mean (SEM)) for each group during one test session.
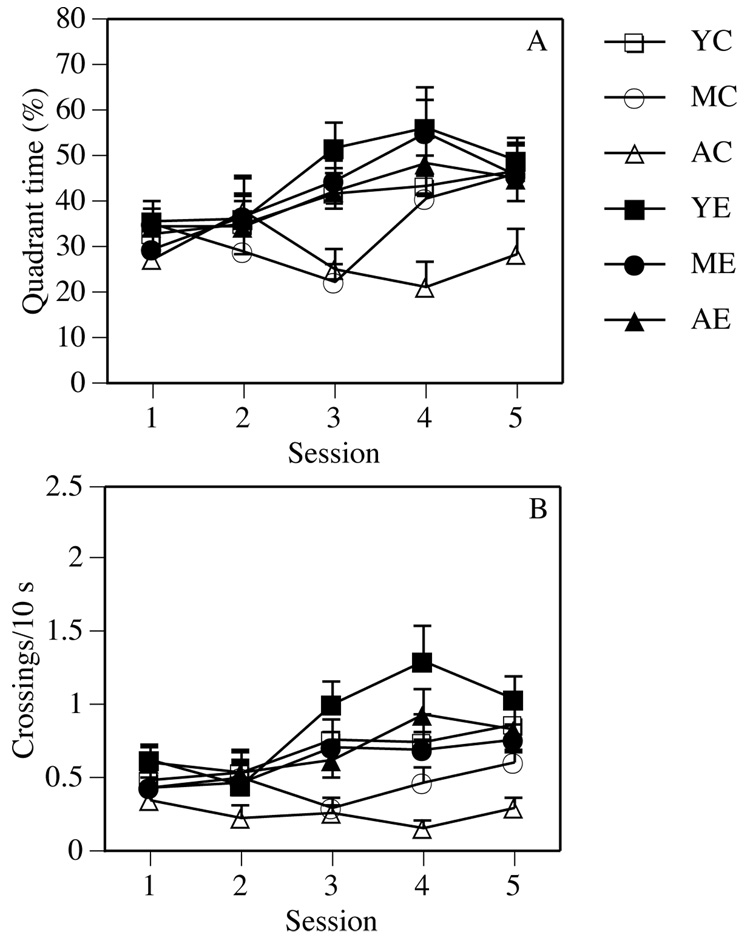
The main effects of Group were also significant for quadrant time and platform crossings during the probe trial (Fs(5,78) = 3.81 and 7.99, respectively, Ps < 0.005). Post-hoc tests revealed that enrichment significantly improved both measures among aged mice (Ps < 0.05, Figure 2). Aged controls also made significantly fewer platform crossings than young controls (P < 0.05). Enrichment had no effect on probe trial performance among young and middle-aged mice. Significant Session effects in quadrant time and platform crossings demonstrated improved performance during the course of testing (Fs(4,312) = 5.75 and 7.10, respectively, Ps < 0.001, Figure 2). No interactions were significant.
Figure 2.
During the probe trials, aged enriched males had significantly more quadrant time (A) and platform crossings (B) than aged control males. Enrichment did not affect either measure in young or middle-aged males. Each point represents the mean (± SEM) for each group during one test session.
3.2.2. Cued Task
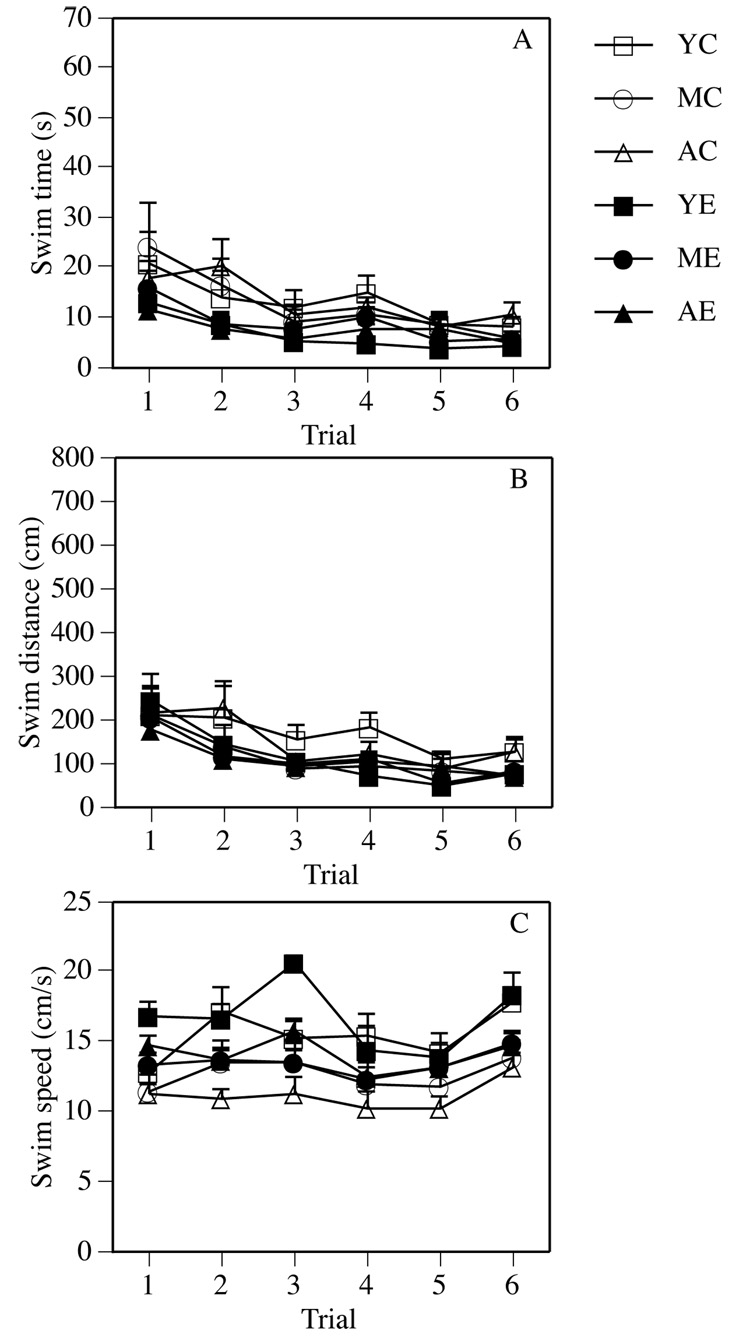
The main effect of Group in the cued task was significant for swim speed (F(5,78) = 4.60, P < 0.05), likely due to the fact that aged controls swam significantly slower than young controls (post-hoc, P < 0.05). Post-hoc tests indicated no differences in swim speed between young control and enriched mice, middle-aged control and enriched mice, or aged control and enriched mice. Cued task performance improved over the course of testing as demonstrated by significant main effects of Trial in swim time, swim distance, and swim speed, (Fs(5,390) = 13.31, 12.72, and 9.61, respectively, Ps ≤ 0.0001, Figures 3A–C). There were no significant Group × Trial interactions for any measure.
Figure 3.
There were no significant differences between control and enriched groups at any age in the cued water maze task. Each point represents the mean (± SEM) for each group during one trial.
4. Discussion
The results of the present study demonstrate that 24 hr/day complex environmental enrichment has differential effects on spatial memory in the Morris water maze among young, middle-aged, and aged male mice. Enrichment-induced enhancements in spatial task performance were limited to aged males. The lack of an effect of enrichment in young males is surprising, as these results differ from two previous studies in which enrichment in young male rats improved spatial performance in the Morris water maze [23, 30]. However, mice in the present study were housed in enriched environments from young adulthood (3 months) for only 4 weeks before testing, whereas rats in these previous reports were enriched from weaning (postnatal day 21) and for 9 to 12 weeks before testing [23, 30]. Because young control males perform very well in the water maze, it is possible that treatment needs to start at a younger age and/or occur for a longer period of time in order to enhance performance in young males. It is also possible that enriched mice in the present study did not receive enough exercise to improve memory. A previous study from our lab demonstrated that young female mice enriched with running wheels (exercise enrichment) made significantly fewer spatial working memory errors in a water escape-motivated radial arm maze relative to young female mice enriched with toys (cognitive stimulation) or an obstacle course (acrobat training)[22]. Thus, if the exercise component of enrichment is the most critical to improving memory in young mice, then young males in the present study may have required greater access to exercise in their home-cages in order for an improvement to be observed. These possibilities will need to be tested further before making definitive conclusions about the potential for enrichment to enhance memory in young males.
Enrichment also did not improve spatial memory in middle-aged males, which differs from previous reports in middle-aged male rats [25] and mice [11]. However, significant differences between these previous reports and the present study may account for our discrepant results. For instance, Pham and colleagues compared enriched rats to isolated controls, whereas the present study compared enriched mice to social controls. Previous studies have shown much greater effects of enrichment on memory relative to isolated controls than social controls [36]. Also, in our previous study, we tested mice in a 1-day version of the Morris water maze and reported enrichment effects in middle-aged males that were characterized by differences between young and middle-aged social control groups in the absence of differences between young social control and middle-aged enriched groups [11]. However, the lack of effect of enrichment in middle-aged males in the present study may be due to somewhat minimal detrimental effects of age on performance in this group. We have previously reported that 17 month-old male mice are not impaired relative to 5 month-old male mice in any measure of spatial memory in the water maze [8]. In the present study, inspection of Figure 1 and 2 reveals little difference between young and middle-aged control groups. Thus, enrichment may not have affected spatial memory in middle-aged males because there was little to improve.
In contrast to the lack of an enrichment effect in young and middle-aged males, the present study demonstrates robust beneficial effects of enrichment on memory in aged males. Enrichment consistently enhanced spatial task performance in aged males on all spatial water maze measures. These results are similar to previous reports from our lab using the same enrichment protocol [2] and to reports of enrichment-induced improvements in a spontaneous alteration task [33]. However, enrichment induced improvements in swim speed in aged males may indicate that improvements in the spatial task were due to a general enrichment-induced increase in physical fitness rather than a specific effect on memory. The fact that enrichment in aged males enhanced performance in the probe trial measures (i.e., quadrant time and platform crossings), which are minimally affected by swim speed, and did not affect any cued task measures, makes this interpretation much less likely. However, exercise alone can improve memory of a passive-avoidance task in aging males [29], so increased physical fitness may play a considerable role in the spatial memory reported in the present study. This issue should be further addressed by reducing or eliminating the exercise component of enrichment in future studies.
The magnitude of the enrichment effects in aging mice in this study are considerably greater than those observed in previous reports from our lab [10, 11]. This finding may be due to a number of reasons related to improvements in the quality of our enrichment treatment. Enriched animals in this study were exposed to enriched environments for 24 hrs/day, rather than 3 hrs/day in several of our previous reports [10, 17, 22]. Further, the 3 hr/day treatment occurred during the light phase of the light/dark cycle, whereas mice in the 24 hr/day housing had access to enrichment stimuli during the active dark phase of the cycle. Indeed, we recently reported greater spatial memory improvements in aged male mice after 24 hr/day enrichment compared to 3 hr/day enrichment [2]. Therefore, increasing the duration of exposure to enrichment treatment likely enhanced treatment effects. In addition, the enriched environments used in this study included a greater number and variety of toys and running wheels relative to previous reports [10, 11, 17]. Finally, the size of enrichment housing was larger in this study than in a previous report using 24 hr/day enrichment [11], which allowed for more animals to be housed together (thereby, increasing social interactions) and more objects to be placed in the cages. All of these factors likely played a role in enhancing the quality of enrichment in the present study, resulting in more robust effects than in our previous reports [10, 11, 17, 22].
The enrichment-induced improvements in this study may have been evident in aged mice because age-related deficits in learning and memory [8] left considerable room for improvement. Advanced age is characterized by numerous changes in the hippocampus [1, 12, 27], thus providing ample opportunity for enrichment to augment hippocampal function. For instance, previous studies have reported increased dentate gyrus neurogenesis [31], reduced hippocampal gliosis [32], and increased hippocampal synaptophysin immunoreactivity [10] in enriched senescent rats and mice. Altering any of these variables, or others, could have led to the memory improvements observed here in enriched aged males.
In conclusion, the results of the present study demonstrate for the first time within a single study that the effects of environmental enrichment on spatial reference memory can be dependent on age in male mice. Performance in the water maze in young and middle-aged males was not affected by 24 hr/day enrichment, whereas that of aged males was robustly and consistently improved. The enrichment effects observed in the present experiment were greater than those previously reported by our group, likely due to the more sophisticated enrichment protocol used in this study. As such, the complexity of the enrichment treatment should be carefully considered when planning such studies in rodents. Further, the discrepancies between our findings in young and middle-aged mice compared to previous reports illustrate the importance of comparing the same enrichment protocol and behavioral testing procedures across ages within the same study. In total, the present findings provide a more complete understanding of how environmental enrichment influences memory throughout the male lifespan. This work supports the notion that a combination of cognitive and physical stimulation can reverse age-related memory decline, even when initiated in old age.
Acknowledgements
We would like to thank Chinonyere Nzerem and Jessica Falco for assistance with behavioral testing, and Jodi Gresack, Kristin Kerr, Stephanie Fernandez, Patrick Orr, and Dr. Michael Lewis for their thoughtful comments on the manuscript. This project was sponsored by a Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (#P30AG21342) grant to KMF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barnes CA, McNaughton BL. An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behav Neurosci. 1985;99:1040–1048. doi: 10.1037//0735-7044.99.6.1040. [DOI] [PubMed] [Google Scholar]
- 2.Bennett JC, McRae PA, Levy LJ, Frick KM. Long-term continuous, but not daily, environmental enrichment reduces spatial memory decline in aged male mice. Neurobiol Learn Mem. 2006;85:139–152. doi: 10.1016/j.nlm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Cummins RA, Walsh RN, Budtz-Olsen OE, Konstantinos T, Horsfall CR. Environmentally-induced changes in the brains of elderly rats. Nature. 1973;243:516–518. doi: 10.1038/243516a0. [DOI] [PubMed] [Google Scholar]
- 4.Diamond MC, Krech D, Rosenzweig MR. The effects of an enriched environment on the histology of the rat cerebral cortex. J Comp Neurol. 1964;123:111–120. doi: 10.1002/cne.901230110. [DOI] [PubMed] [Google Scholar]
- 5.Diamond MC. Extensive cortical depth measurements and neuron size increases in the cortex of environmentally enriched rats. J Comp Neurol. 1967;131:357–364. [Google Scholar]
- 6.Diamond MC, Johnson RE, Protti AM, Ott C, Kajisa L. Plasticity in the 904-day-old male rat cerebral cortex. Exp Neurol. 1985;87:309–317. doi: 10.1016/0014-4886(85)90221-3. [DOI] [PubMed] [Google Scholar]
- 7.Faherty CJ, Kerley D, Smeyne RJ. A Golgi-Cox morphological analysis of neuronal changes induced by environmental enrichment. Brain Res Dev Brain Res. 2003;141:55–61. doi: 10.1016/s0165-3806(02)00642-9. [DOI] [PubMed] [Google Scholar]
- 8.Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- 9.Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- 10.Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging. 2003;24:615–626. doi: 10.1016/s0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- 11.Frick KM, Stearns NA, Pan JY, Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learn Mem. 2003;10:187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geinisman Y, de Toledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- 13.Globus A, Rosenzweig MR, Bennett EL, Diamond MC. Effects of differential experience on dendritic spine counts in rat cerebral cortex. J Comp Physiol Psychol. 1973;82:175–181. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- 14.Green EJ, Greenough WT, Schlumpf BE. Effects of complex or isolated environments on cortical dendrites of middle-aged rats. Brain Res. 1983;264:233–240. doi: 10.1016/0006-8993(83)90821-1. [DOI] [PubMed] [Google Scholar]
- 15.Greenough WT, Wood WE, Madden TC. Possible memory storage differences among mice reared in environments varying in complexity. Behav Biol. 1972;7:717–722. doi: 10.1016/s0091-6773(72)80078-6. [DOI] [PubMed] [Google Scholar]
- 16.Greenough WT, Volkmar FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol. 1973;40:491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- 17.Gresack JE, Frick KM. Environmental enrichment reduces the mnemonic and neural benefits of estrogen. Neuroscience. 2004;128:459–471. doi: 10.1016/j.neuroscience.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howell DC. Statistical Methods for Psychology. Fifth Edition. Pacific Grove, CA: Duxbury; 2002. pp. 397–399. [Google Scholar]
- 19.Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- 20.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 21.Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert TJ, Fernandez SM, Frick KM. Different types of environmental enrichment have discrepant effects on spatial memory and synaptophysin levels in female mice. Neurobiol Learn Mem. 2005;83:206–216. doi: 10.1016/j.nlm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, Petrosini L. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav Brain Res. 2005;163:78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Markowska AL, Long JM, Johnson CT, Olton DS. Variable-interval probe test as a tool for repeated measurements of spatial memory in the water maze. Behav Neurosci. 1993;107:627–632. doi: 10.1037//0735-7044.107.4.627. [DOI] [PubMed] [Google Scholar]
- 25.Pham TM, Ickes B, Albeck D, Soderstrom S, Granholm AC, Mohammed AH. Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience. 1999;94:279–286. doi: 10.1016/s0306-4522(99)00316-4. [DOI] [PubMed] [Google Scholar]
- 26.Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- 27.Rosenzweig ES, Rao G, McNaughton BL, Barnes CA. Role of temporal summation in age-related long-term potentiation-induction deficits. Hippocampus. 1997;7:549–558. doi: 10.1002/(SICI)1098-1063(1997)7:5<549::AID-HIPO10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 29.Samorajski T, Delaney C, Durham L, Ordy JM, Johnson JA, Dunlap WP. Effect of exercise on longevity, body weight, locomotor performance, and passive-avoidance memory of C57BL/6J mice. Neurobiol Aging. 1985;6:17–24. doi: 10.1016/0197-4580(85)90066-1. [DOI] [PubMed] [Google Scholar]
- 30.Schrijver NC, Bahr NI, Weiss IC, Wurbel H. Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning and HPA activity in adult rats. Pharmacol Biochem Behav. 2002;73:209–224. doi: 10.1016/s0091-3057(02)00790-6. [DOI] [PubMed] [Google Scholar]
- 31.Segovia G, Yague AG, Garcia-Verdugo JM, Mora F. Environmental enrichment promotes neurogenesis and changes the extracellular concentrations of glutamate and GABA in the hippocampus of aged rats. Brain Res Bull. 2006;70:8–14. doi: 10.1016/j.brainresbull.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Soffie M, Hahn K, Terao E, Eclancher F. Behavioural and glial changes in old rats following environmental enrichment. Behav Brain Res. 1999;101:37–49. doi: 10.1016/s0166-4328(98)00139-9. [DOI] [PubMed] [Google Scholar]
- 33.Van Waas M, Soffie M. Differential environmental modulations on locomotor activity, exploration and spatial behaviour in young and old rats. Physiol Behav. 1996;59:265–271. doi: 10.1016/0031-9384(95)02151-5. [DOI] [PubMed] [Google Scholar]
- 34.Warren JM, Zerweck C, Anthony A. Effects of environmental enrichment on old mice. Dev Psychobiol. 1982;15:13–18. doi: 10.1002/dev.420150104. [DOI] [PubMed] [Google Scholar]
- 35.Williams BM, Luo Y, Ward C, Redd K, Gibson R, Kuczaj SA, McCoy JG. Environmental enrichment: effects on spatial memory and hippocampal CREB immunoreactivity. Physiol Behav. 2001;73:649–658. doi: 10.1016/s0031-9384(01)00543-1. [DOI] [PubMed] [Google Scholar]
- 36.Winocur G. Environmental influences on cognitive decline in aged rats. Neurobiol Aging. 1998;19:589–597. doi: 10.1016/s0197-4580(98)00107-9. [DOI] [PubMed] [Google Scholar]