Abstract
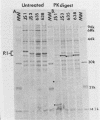
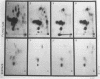
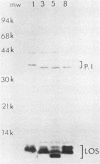
The protein IAs of serum-sensitive (FA635) and serum-resistant (FA638) transformants of Neisseria gonorrhoeae, which have identical pedigrees, have been shown to be different by the use of a monoclonal antibody and were also shown to be different by proteinase K cleavage and primary structural and surface peptide mapping. The difference in structure is within the surface-exposed region of the molecule. The only other difference observed between the two strains was a very slight difference in lipooligosaccharide silver staining in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. These data suggest that protein I alone or in combination with lipooligosaccharide may significantly contribute to serum resistance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander N. M. Oxidation and oxidative cleavage of tryptophanyl peptide bonds during iodination. Biochem Biophys Res Commun. 1973 Sep 18;54(2):614–621. doi: 10.1016/0006-291x(73)91467-8. [DOI] [PubMed] [Google Scholar]
- Barrera O., Swanson J. Proteins IA and IB exhibit different surface exposures and orientations in the outer membranes of Neisseria gonorrhoeae. Infect Immun. 1984 Jun;44(3):565–568. doi: 10.1128/iai.44.3.565-568.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G., Black W. J., Nachamkin I., Stewart P. W. Monoclonal antibody that recognizes an outer membrane antigen common to the pathogenic Neisseria species but not to most nonpathogenic Neisseria species. Infect Immun. 1984 Mar;43(3):994–999. doi: 10.1128/iai.43.3.994-999.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G., Buchanan T. M., Sparling P. F. Confirmation of association of protein I serotype of Neisseria gonorrhoeae with ability to cause disseminated infection. Infect Immun. 1983 May;40(2):816–819. doi: 10.1128/iai.40.2.816-819.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J. F., Zurlinden E., Lammel C. J., Brooks G. F. Relation of protein I and colony opacity to serum killing of Neisseria gonorrhoeae. J Infect Dis. 1982 Jan;145(1):37–44. doi: 10.1093/infdis/145.1.37. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Warren K. A., Tam M., Frank M. M. Monoclonal antibodies directed against gonococcal protein I vary in bactericidal activity. J Immunol. 1985 May;134(5):3411–3419. [PubMed] [Google Scholar]
- Judd R. C. 125I-peptide mapping of protein III isolated from four strains of Neisseria gonorrhoeae. Infect Immun. 1982 Aug;37(2):622–631. doi: 10.1128/iai.37.2.622-631.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. Evidence for N-terminal exposure of the protein IA subclass of Neisseria gonorrhoeae protein I. Infect Immun. 1986 Nov;54(2):408–414. doi: 10.1128/iai.54.2.408-414.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. Surface peptide mapping of protein I and protein III of four strains of Neisseria gonorrhoeae. Infect Immun. 1982 Aug;37(2):632–641. doi: 10.1128/iai.37.2.632-641.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Sandstrom E. G., Chen K. C., Buchanan T. M. Serology of Neisseria gonorrhoeae: coagglutination serogroups WI and WII/III correspond to different outer membrane protein I molecules. Infect Immun. 1982 Nov;38(2):462–470. doi: 10.1128/iai.38.2.462-470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Guymon L. F., Sparling P. F. Identification of a new genetic site (sac-3+) in Neisseria gonorrhoeae that affects sensitivity to normal human serum. Infect Immun. 1982 Mar;35(3):764–769. doi: 10.1128/iai.35.3.764-769.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt S. K., Jones F., Shockley T. E., Jackson J. H. Cotransformation of a serum resistance phenotype with genes for arginine biosynthesis in Neisseria gonorrhoeae. Infect Immun. 1980 Jul;29(1):287–289. doi: 10.1128/iai.29.1.287-289.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Colony opacity and protein II compositions of gonococci. Infect Immun. 1982 Jul;37(1):359–368. doi: 10.1128/iai.37.1.359-368.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]