Abstract
Unaltered free base release in d(CGCGCG)2 exposed to X rays at 4 K or room temperature was measured by HPLC. Samples were prepared either as films hydrated to a level of Γ= 2.5 mol water/mol nucleotide or as polycrystalline with Γ ∼ 7.5 mol water/mol nucleotide. X irradiation of films at 4 K, followed by annealing to room temperature, resulted in yields for cytosine and guanine of G(Cyt) = 0.036 ± 0.001 μmol/J and G(Gua) = 0.090 ± 0.002 μmol/J. Irradiation of films at room temperature gave similar yields. The yields for polycrystalline d(CGCGCG)2 X-irradiated at room temperature were G(Cyt) = 0.035 ± 0.005 μmol/J and G(Gua) = 0.077 ± 0.023 μmol/J. The total free base release yield, G(fbr), was 0.124 ± 0.008 μmol/J for films and 0.112 ± 0.028 μmol/J for polycrystalline samples. G(fbr) is believed to be a good estimate of total strand break yield. The yields of total free radicals trapped [G(Σfr)] by the d(CGCGCG)2 films at 4 K were measured by EPR. The measured value, G(Σfr) = 0.450 ± 0.005 μmol/J, was used to calculate the yield of trappable sugar radicals, giving Gsugar(fr) = 0.04-0.07 μmol/J. We found that (1) guanine release exceeded cytosine release by more than twofold, (2) Gsugar(fr) cannot account for more than half of the free base release, and (3) G(fbr), G(Cyt) and G(Gua) were independent of the sample temperature during irradiation. Finding (1) suggests that base and or sequence influences sugar damage, and finding (2) is consistent with our working hypothesis that an important pathway to strand break formation entails two one-electron oxidations at the same sugar site.
INTRODUCTION
The work presented here is part of an ongoing effort to determine the mechanisms by which ionizing radiation damages the backbone of DNA via the direct effect. Although the direct effect is a minor pathway in dilute solutions, it contributes significantly to the damage of nuclear DNA because of the inherent radical scavenging capacity of the nuclear matrix. The direct effect in vivo has been estimated to contribute 40-50% of the DNA damage (1, 2). Further amplifying the importance of direct-type DNA damage is enhanced clustering due to the relative lack of mobility of the free radical precursors produced by direct ionization of DNA (3). In spite of their importance, the reaction pathways leading to direct-type damage are poorly understood. The term “direct-type” damage refers to the damage produced by direct ionization of the DNA plus damage of the same type that is produced by formation of holes (sites of one-electron loss) and excess electrons (sites of one-electron gain) by ionization of the DNA solvation shell and subsequent transfer to the DNA itself.
There is a fairly tight correlation between the release of unaltered base and total strand breaks for DNA in dilute aqueous solution (4). Release of unaltered base by either the direct or indirect effect of ionizing radiation involves oxidation of carbon-centered radicals formed by deprotonation of a deoxyribose radical cation or by hydrogen abstraction by OH radicals from one of the five deoxyribose carbons (1, 4-6). The release of modified base is a minor product in γ-irradiated lyophilized DNA, (Swarts et al., personal communication). Other processes such as release of base propenal (7, 8), a product of C5′-radical chemistry, is minor (9). Of the five carbon-centered radicals, only C1′ does not give rise to a prompt strand break. The C1′ radical, under anoxic conditions, gives free base and deoxyribonolactone, leaving the backbone intact. Upon addition of a suitable catalyst and heating, deoxyribonolactone degrades to yield 5-methylene furanone and a strand break (10). Thus the correlation between release of unaltered free base and deoxyribose damage is nearly 1:1 and, when heat-labile sites are included, there exists a close correspondence between deoxyribose damage and total strand breaks.
The yield of total radicals trapped, G(Σfr), in DNA irradiated and observed at low temperature has been measured by EPR spectroscopy. Based on the fraction of G(Σfr) attributable to radicals trapped on the sugar moiety, Gsugar(fr), has been determined both by component analysis (11) and by dose-response characteristics (12). Purkayastha et al. found that Gsugar(fr) is significantly less than Gtotal (ssb) measured in pEC and pUC18 plasmid DNA films (13). Both the existence and the magnitude of the shortfall were unexpected. About half of the total strand breaks are derived from precursors other than trappable sugar radicals.
In the present study, we focused on the oligodeoxynucleotide, d(CGCGCG)2. This duplex was chosen in part because samples could be prepared in the form of crystals as well as films and in part to focus on the CG bases alone. For this Z-form DNA, we tested whether a given type of base, Cyt compared to Gua, influenced the probability of damage to the deoxyribose attached (or adjacent) to it, i.e., compared the yield of released Cyt, G(Cyt), with the yield of released Gua, G(Gua). In addition, the yield of total free base release [G(frb) = G(Gua) + G(Cyt)] was compared to the yield of trapped sugar radicals, Gsugar(fr), to determine whether Gsugar(fr) was sufficient to account for G(fbr). Last, free base release obtained by X irradiation at room temperature is compared with free base release obtained by X irradiation at 4 K followed by annealing to room temperature. The findings reported herein are consistent with our previous hypothesis (13), in which it was suggested that double oxidation of the deoxyribose plays an important role in the direct damage of DNA.
MATERIALS AND METHODS
Reverse-phase desalted synthetic oligodeoxynucleotides of 5′-CpGpCpGpCpG-3′ were obtained from Ransom Hill Bioscience and used without further purification. OmniPur Nuclease-free water was from EM Science, Merck. DNA bases used as reference for HPLC experiments were from Sigma. All other chemicals and solvents are Baker Analyzed and were used as received from Mallinckrodt Baker Inc.
Film and Crystal Preparation
Amorphous films of d(CGCGCG)2 were prepared from a solution of the oligodeoxynucleotide in nuclease-free water. Appropriate aliquots of the oligodeoxynucleotide solution were allowed to sit on a Teflon petri dish inside a jar containing saturated NaOH solution, which maintains a relative humidity of 8% (14). Similarly, films were also prepared using suprasil quartz capillary method as reported earlier by Purkayastha et al. (13). Under these conditions, the dry oligodeoxynucleotide films were assumed to contain 2.5 mol water per mol nucleotide. The dried films were subsequently weighed by using a Cahn C-30 Microbalance (±1 μg) until a constant weight was reached. The DNA mass fraction in the films was 70% ± 7% as determined from OD measurements at 256 nm. All preparations were done at room temperature.
Crystals of d(CGCGCG)2 were grown according to the protocol reported by Wang et al. (15). For the films of d(CGCGCG)2, the structure at Γ= 2.5 was not determined. The conformation of d(CGCGCG)2 in the films was assumed to be Z-form based primarily on the excellent agreement between DNA structures determined by X-ray crystallography and by NMR of dilute aqueous solutions. This assumption is consistent with the observed similarities in unaltered base release in films compared with crystalline samples.
Irradiation
X rays were generated by a Varian/Eimac OEG-76H tungsten-target tube operated at 70 keV and 20 mA. The X-ray beam was filtered by a 40-μm-thick aluminum foil plus the wall of the respective sample containers. The samples for EPR study were X-irradiated at 4 K in Suprasil or Charles Supper quartz tubes (wall thickness of ∼0.15-0.1 mm) with a dose rate of 0.4-0.75 kGy/min under helium gas. The samples for HPLC were irradiated at room temperature under air inside plastic vials with a wall thickness of ∼0.5 mm and capped tightly with siliconized “O” rings; the dose rate was 2.2 kGy/min.
EPR Measurement
The sample temperature was maintained at 4 K using a Janis Dewar EPR accessory (16) and samples were exposed to a dose of 0-6 kGy. After exposure, EPR spectra were recorded as first derivatives with a Q-band (35 GHz) Varian E-12 EPR spectrometer operating with a microwave attenuation of 50 db. Free radical concentrations were determined by comparing the intensity of the EPR signal against that of a ruby standard mounted inside the EPR cavity at 4 K. The procedure for determining the free radical yields, G(Σfr), has been described by Purkayastha et al. (12).
HPLC Measurement
After room temperature irradiation, DNA films were stored at room temperature for 6-8 h, then dissolved, at a mass ratio of 1:1, in nuclease-free water. These solutions were stored at 253 K. The samples irradiated at 4 K were first warmed to room temperature and then immediately dissolved by the same protocol. Aliquots from the sample solutions, internal standard solutions of uracil, and sodium acetate buffer were combined and brought to a final concentration of 10 μM and 50 mM, respectively.
This solution (pH 6.8) was then fed to a Water Alliance™ HPLC system equipped with a 2690 solvent delivery system, Waters 996 PDA detector and an auto sampler. The unaltered free base was separated on a Phenomenex Columbus C-18 reverse-phase column (4.6 × 250 mm, 5 μ, 110 D pore size) at 303 K using 40 mM ammonium acetate (pH 6.8) as a mobile phase and by applying a linear gradient of 0.9-10% of acetonitrile over 25 min. The unaltered free bases were detected by their absorbance at 254 nm and were quantified by comparison with uracil as an internal standard using the equation
,where Cbase is the concentration in moles of the released individual bases, Curacil is the concentration in moles of uracil, Abase is the area under the curve of the eluted individual bases, Auracil is the area under the curve of uracil, εuracil is the molar extinction coefficient of uracil at 254 nm, and εbase is the molar extinction coefficient of the individual bases at 254 nm. The εbase and εuracil determined for the standard solutions using a Varian Cary UV-VIS spectrophotometer agreed with those reported by Voet et al. (17).
Calculation of DNA Target Mass and G Values
Given the measured mass of the film (or polycrystalline sample) and the concentration of DNA in the aqueous solution prepared from that sample (see above), the fraction of the film (or polycrystalline sample) mass attributable to DNA was determined. In calculating the chemical yields, G, it is important to define the target mass, i.e., the energy absorbing mass relevant to production of the measured product. Often just the DNA molecule is used as the target mass; however, under circumstances where the degree of hydration and the identity of the counter ion are known, it is advantageous to use DNA plus its solvation shell as the target mass (18). The G values presented here are based on a target mass that, in the case of films, consists of d(CpGpCpGpCpG) + 5 × NH+4 + 5 × 2.5 × H2O, where the oligomer is assumed to bind one counter ion and 2.5 waters for each of the five phosphates. The remainder of the film mass is assumed to be excess salt and as such is assumed not to be part of target. In the crystalline samples, the crystal structure is known (15) and the hydration level has been calculated (19). Consequently, 100% of the crystalline mass is the target mass; i.e., Γ∼ 7.5 H2O/nucleotide, and there is no excess salt.
RESULTS
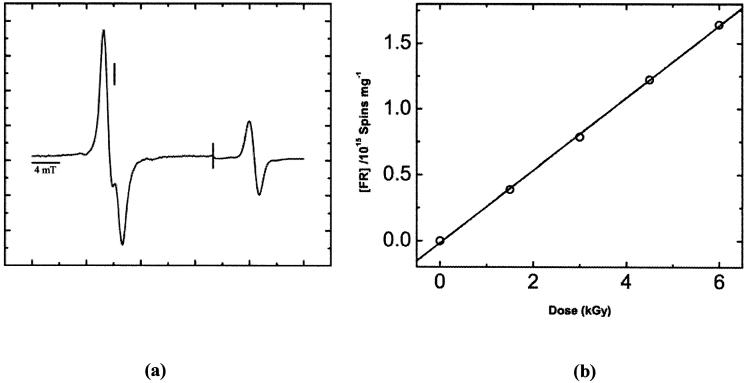
The concentration of radicals trapped in films of d(CGCGCG)2 was measured by EPR at 4 K after X irradiation at 4 K. A typical EPR spectrum is shown in Fig. 1a. From the linear increase in radical concentration at low dose, shown in Fig. 1b, the chemical yield of total free radicals trapped, G(Σfr), was determined. For d(CGCGCG)2 films, G(Σfr) was found to be 0.450 ± 0.005 μmol/J, using DNA plus its solvation shell as the target mass. From G(Σfr), the yield of radicals trapped by the deoxyribose moiety, Gsugar(fr), was calculated from the ratio, Gsugar(fr) = (0.08 to 0.16) × G(Σfr) (12, 20), which in this case gives (0.04 to 0.07) μmol/J. The values of Gsugar(fr) are tabulated in Table 1.
FIG. 1.
Panel a: First-derivative, Q-band, EPR spectrum of d(CGCGCG)2 films at Γ= 2.5, X-irradiated with a dose of 6 kGy and recorded at 4 K. The spectral width was 40 mT. The scan was paused after recording the DNA signal, then the field sweep center was increased by 20 mT and the signal gain adjusted, then the sweep was continued to record the ruby reference signal. The vertical line corresponds to the position of g = 2.0022. The microwave power attenuation was 50 dB, delivering 0.3 nW to the cavity. Panel b: Free radical dose-response data showing the linear fit used to calculate the chemical yield, G(Σfr).
TABLE 1.
Radiation Chemical Yields for Free Base Release in d(CGCGCG)2 and Comparative Yields for Trapped Radicals
| Sample form | Γ | Temperature (K) | Yields in μmol/J |
G(Gua)/G(Cyt)b | |||||
|---|---|---|---|---|---|---|---|---|---|
| G(Σfr) | Gsugar(fr)a | G(Cyt) | G(Gua) | G(fbr) | ΔG | ||||
| Film | 2.5 | 4 | 0.450 ± 0.005 | 0.04-0.07 | 0.036 ± 0.001 | 0.090 ± 0.002 | 0.126 ± 0.003 | 0.09-0.06 | 2.5 ± 0.1 |
| Room temperature | nd | 0.037 ± 0.002 | 0.085 ± 0.006 | 0.124 ± 0.008 | 2.3 ± 0.2 | ||||
| Crystal | 7.5e | 4 | 0.57c ± 0.05 | 0.06-0.09 | nd | nd | 0.11d ± 0.02 | ||
| Room temperature | nd | 0.035 ± 0.005 | 0.077 ± 0.023 | 0.112 ± 0.028 | 0.05-0.02 | 2.2 ± 0.7 | |||
Note. nd, not determined.
Values calculated using 8-16% of G(Σfr) as reported in refs. (12) and (20); those percentages in ref. (12) that were based on crystals containing heavy cations (Ba++ and Co++) were excluded.
Calculated using standard statistical procedure: for (A ± a)/(B ± b) = (D ± d), where A/B = D, then d = D * [(a/A)2 + (b/B)2]1/2.
Ref. (22), published value reduced by 20% due to a correction in the ruby standard calibration.
G(ssb) reported by (21), which is assumed to slightly under estimate G(fbr).
Ref. (19).
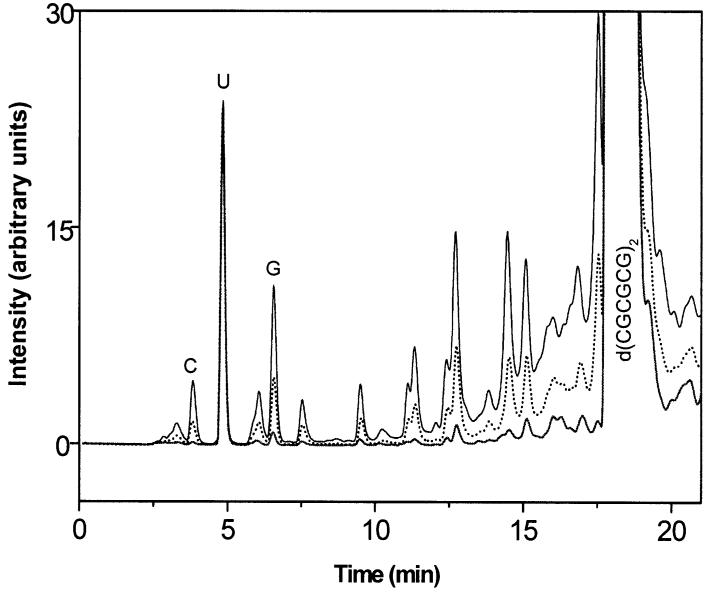
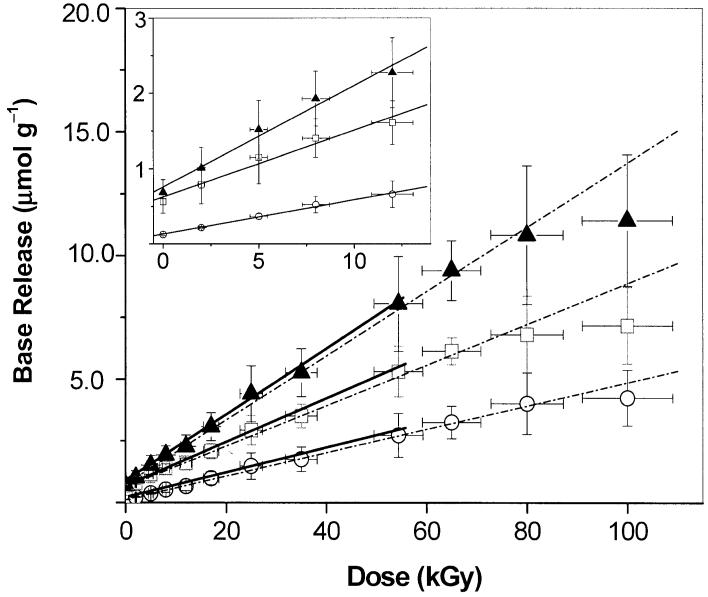
The yields of cytosine, G(Cyt), and guanine, G(Gua), were determined by HPLC. A chromatogram for d(CGCGCG)2 films X-irradiated at room temperature is shown in Fig. 2. The peaks of cytosine, guanine and uracil (as internal standard) were well resolved and the recoveries of individual bases were high (≥99%). Recovery efficiency was determined by spiking unirradiated d(CGCGCG)2 with known quantities of free base. As seen from the HPLC chromatogram, the peaks corresponding to Cyt and Gua, with retention times of 3.8 min and 6.8 min, respectively, increase with dose. Dose-response curves for the release of Cyt, Gua, and Cyt + Gua are shown in Fig. 3. The yields, G(Cyt) and G(Gua), were determined from a linear fit in the <60-kGy dose range. There was no dose saturation up to ∼80 kGy. As can be seen from the inset in Fig. 3, the low-dose points conform to the linear fit, and the small background of free guanine does not significantly distort the fit.
FIG. 2.
HPLC chromatograms of d(CGCGCG)2 films, monitored at 254 nm shown for three different X-ray doses: 0 kGy (solid), 30 kGy (dashed) and 100 kGy (solid). C = cytosine, G = guanine, and U = uracil; the latter was used as internal standard.
FIG. 3.
Dose-response curves for the release of cytosine (○), guanine (□) and total free base (▲) from X-irradiated d(CGCGCG)2 film. The broken lines shows the linear fit from 0-100 kGy and the solid lines show the linear fit to the data <60 kGy. Inset shows the linear fit to data <15 kGy. Each data point represents the weighted average for three measurements (some of the data points used to obtain the average were obtained by interpolation between the two nearest doses).
d(CGCGCG)2 films X-irradiated at 4 K and subsequently warmed to room temperature gave G(Cyt) = 0.036 ± 0.001 μmol/J and G(Gua) = 0.090 ± 0.002 μmol/J while films X-irradiated at room temperature gave G(Cyt) = 0.037 ± 0.002 μmol/J and G(Gua) = 0.085 ± 0.006 μmol/J. Whether the films were prepared on Teflon or in quartz tubes, the results were the same. For polycrystalline d(CGCGCG)2 X-irradiated at room temperature, G(Cyt) = 0.035 ± 0.005 μmol/J and G(Gua) = 0.077 ± 0.023 μmol/J. The yield for total free base release, G(fbr), was calculated from the linear fit to data points obtained by adding together the concentrations of released Cyt and Gua. These values are summarized in Table 1.
The yields were relatively insensitive to the postirradiation holding time for the films, as well as warming the films to 90°C for 15 min, after dissolution. The influence of solvent conditions, such as with and without buffer were checked and gave no significant difference. Other variables, such as aerobic compared to anaerobic, have not yet been rigorously tested, in part because we expect such influences to be relatively small. The observation that free base release is linear, and that the difference between Gua and Cyt persists, from low to very high doses is indicative of chemistry that occurs predominantly in the solid state (see below).
DISCUSSION
There are three important findings reported here. First, unaltered base release and therefore direct radiation damage of the deoxyribose bound to that base is strongly dependent on the nature of the base itself and/or the base sequence. To our knowledge, this is the first report of such a dependence. Second, the yield of free base release exceeds the yield of deoxyribose trapped radicals. We call the difference, ΔG = G(fbr) - Gsugar(fr), the shortfall; it is closely related to the shortfall, Gtotal(ssb) - Gsugar(fr), reported previously (13). Third, these results are independent of the sample temperature selected for radiation exposure.
Shortfall between Free Base Release and DeoxyriboseTrapped Radicals
The yield of the total unaltered base release, G(fbr) = 0.124 ± 0.008 μmol/J, reported in Table 1 for films, is in agreement with the estimate of G(ssb), 0.11 ± 0.02 μmol/J, obtained by measuring sugar-phosphate end products in crystalline d(CGCGCG)2 (21). Because the latter measure neglects cleavage products containing phosphate-sugar remnants, G(fbr) > Gtotal(ssb) is expected, and this difference may serve as a rough estimate of the phosphate-sugar remnant yield. These results are consistent with the conclusion by others that the release of free unaltered base is a good quantitative measure of damaged deoxyribose in duplex DNA (4, 6, 21).
The dose-response curves for free base release share an important attribute with the dose-response curves for deoxyribose trapped radicals. For both end points, dose saturation does not set in until unusually high doses, in excess of 80 kGy. This unusual resistance to dose saturation had also been observed by Swarts et al. (5) in their study of herring sperm DNA. Shared resistance to dose saturation underscores the point that trappable deoxyribose radicals are precursors to both strand breaks and free base release. It does not follow, however, that all strand breaks and free base release are produced by a reaction pathway that proceeds via a trappable deoxyribose radical.
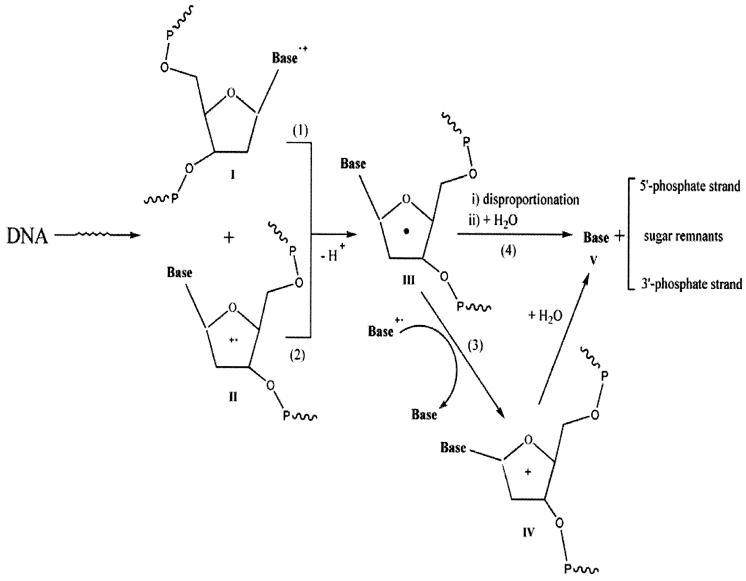
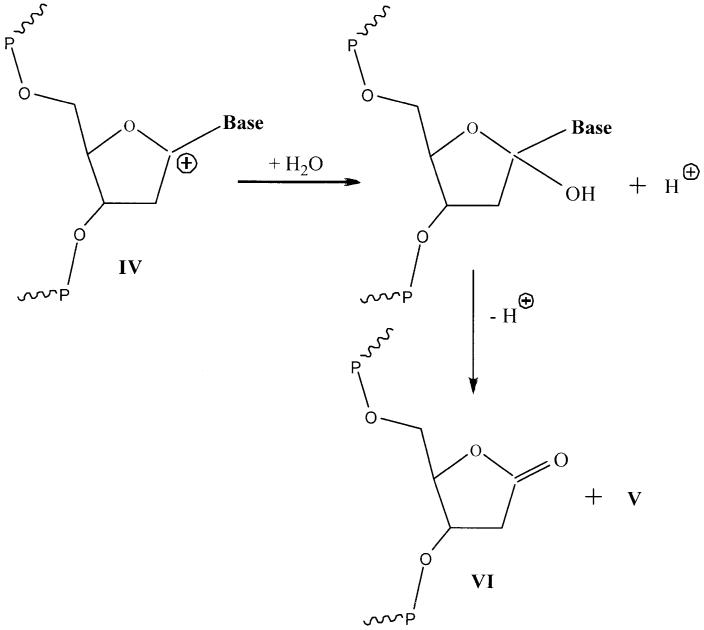
A comparison of Gsugar(fr) with G(fbr) in Table 1 reveals that G(fbr) > Gsugar(fr), a shortfall in the yield of trapped radical damage relative to that of free base release. This is quite similar to the observed inequality Gtotal(ssb) > Gsugar(fr) discovered in plasmid DNA films, where a novel mechanism was proposed by Purkayastha et al. (13). This mechanism explains the shortfall by suggesting the existence of sugar damage that is diamagnetic and therefore is not observed by EPR. The species suggested for this diamagnetic damage is a carbocation formed by two one-electron oxidations as shown in Schemes 1 and 2.
SCHEME 1.
Mechanism of sugar damage leading to unaltered base release. A specific example of formation of V from IV is given in Scheme 2.
SCHEME 2.
For the case where IV is the C1′ carbocation, the reaction with water leads to 2′-deoxyribonolactone plus free base.
We note that the free radical yield, G(Σfr), determined for the d(CGCGCG)2 films at Γ= 2.5 (0.450 ± 0.005 μmol/J) is smaller than that for the d(CGCGCG)2 crystals at Γ∼7.5 (0.57 ± 0.05 μmol/J) determined by Debije et al. (22). This difference can be attributed to the different packing in the films compared to the lattice packing in the crystals, as observed in hydrated DNA films (23, 24) and crystalline oligodeoxynucleotides (19).
Base-Dependent Sugar Damage
As shown in Table 1, the yield of guanine, G(Gua), was 2.2 to 2.5 times higher than the yield of cytosine, G(Cyt), independent of X-irradiation temperature and sample state. This observation is different from that made in a study of lyophilized salmon sperm DNA (B-form DNA) irradiated under anaerobic conditions, where the yields of the four bases were very similar (5). At Γ= 2.5 mol water/mol nucleotide, the yields (in μmol/J and based on DNA as the target mass) were G(Cyt) = 0.021 ± 0.001, G(Gua) = 0.0120 ± 0.0004, G(Thy) = 0.019 ± 0.001, and G(Ade) = 0.021 ± 0.001. Roginskaya et al. (25), using calf thymus DNA, measured yields (in μmol/J and based on the DNA film weight as the target mass) of G(Cyt) = 0.041 ± 0.002, G(Gua) = 0.037 ± 0.001, G(Thy) = 0.042 ± 0.002, and G(Ade) = 0.042 ± 0.002. It appears, therefore, that in high-molecular-weight B-form DNA at Γ= 2.5 mol water/mol nucleotide, sugar damage is relatively independent of the attached base. Also, the yields of the four bases were very similar in crystalline oligodeoxynucleotides (B-form) X-irradiated at 4 K (26). Based on the values presented in Table 1, the Z-form of d(CGCGCG)2 behaves differently than these B-form DNA samples. Consequently, at least for Z-form DNA, damage of a specific sugar is influenced by the base attached to that sugar or by the sequence context of that sugar.
There are a number of possible explanations as to how the bases might influence sugar damage. Here we speculate on one of these by referring to Scheme 1. All reactions leading to unaltered base release proceed through the sugar radical III, which is formed primarily by deprotonation of sugar-phosphate radical cation II and secondarily by deprotonation of base radical cation I. A small fraction of the radicals initially trapped as III will persist or undergo unimolecular rearrangement to form related sugar radicals; before and after dissolution these radicals will undergo disproportionation reactions resulting in free base plus strand break products. A much larger fraction of III undergoes a second one-electron oxidation, reaction 3, yielding the carbocation IV. Reaction 3 occurs under two distinctly different circumstances (both within the solid phase), as a spur reaction prior to radical trapping or as a thermally activated reaction dependent on hole mobility through the base stack, bringing Base•+ within a reaction distance that favors electron transfer from III. The degree to which 3 occurs as a spur reaction has a direct bearing on whether or not III will be trapped and thus the value of Gsugar(fr). While this hypothesis, entailing double oxidation of deoxyribose, was advanced to explain the shortfall between strand breaks and trapped sugar radicals, it might also explain the influence of the bases on sugar damage.
There are three reactions in Scheme 1 where the structure of the base and/or the stereochemistry between the base and sugar may influence the reaction rate. Reaction 1 competes with hole transfer to another base. Current thinking is that transfer to, and subsequent trapping by, Gua is the predominant reaction. But deprotonation at C1′ of a base radical cation cannot be ruled out entirely; it is known to occur in cytosine mononucleotides (27). Reaction 3, as it occurs within a spur, may compete with hole transfer to another base. Reaction 3 may also occur as the films are annealed to room temperature; in this case, Gua•+ will be the favored site of Base•+ because Gua is the deepest hole trap and holes hop from Gua to Gua. Last, the rate of reaction 4, which may occur as a spur reaction, or as the film is annealed, or when the film is dissolved in water, will depend on the base as a leaving group. If cleavage of the glycosidic bond by IV occurs in the solid state, base release may occur prior to dissolution, and thereby depend on the nature of the base. An example of base elimination upon dissolution of IV is shown in Scheme 2. The C1′ carbocation reacts with water to give 2′-deoxyribonolactone (VI) and V. 2′-Deoxyribonolactone has been detected by Roginskaya et al. in irradiated d(CGCG)2 films (28).
Base Release is Independent of Sample Temperature during Irradiation
It is remarkable that base release in d(CGCGCG)2 films is unaffected by the sample temperature during irradiation, whether it be at 4 K or room temperature. In contrast, the trapped-free-radical yield in crystalline oligodeoxynucleotides varies by an order of magnitude (29). Either irradiation at room temperature or warming to room temperature after irradiation at 4 K results in trapped-radical yields that are 1% to 18% of the yields measured at 4 K. This underscores the relative importance of the chemistry, with regard to base release, that must take place in the solid phase prior to dissolution. Furthermore, the lack of a difference in free base yields, whether the postirradiated dissolved films were heated to 90°C or not, suggests that base release is predominantly from prompt sugar damage or, alternatively, that C1′ damage proceeds primarily through a different mechanism than that observed for the indirect effect (30-32).
There are far-reaching implications to the finding that irradiation at 4 K followed by annealing to room temperature gives the same yields as obtained by irradiation at room temperature. In terms of understanding direct damage mechanisms, it indicates that alternative reaction pathways that might be favored by slow annealing from 4 K to room temperature do not exist or, if they do exist, they play a minor role. In terms of applications to radiation biology, it means the information determined in model systems irradiated at low temperatures is directly applicable to understanding the direct effect in vivo.
This observation is, of course, for just one oligomer sequence and one set of stable end products. It remains to be seen if it holds true for high molecular weight B-form DNA and other end products.
CONCLUSIONS
The present results demonstrate that in d(CGCGCG)2 (a Z-form duplex) the yield of unaltered guanine, G(Gua), is more than twofold greater than the yield of unaltered cytosine, G(Cyt). This inequality persists for two sample types, films and polycrystalline, and for irradiation at two different temperatures, 4 K and room temperature. It is concluded that damage of a specific sugar is influenced by the base attached to that sugar and/or the adjacent bases. To the best of our knowledge, this is the first report showing a strong dependence between the probability of a DNA sugar being damaged and the base attached to that sugar.
There exists a shortfall of 0.02-0.09 μmol/J between the yield of trappable sugar radicals, Gsugar(fr), and the yield of free base release, G(fbr). This is consistent with our working hypothesis that formation of strand breaks entails two one-electron oxidations of the same sugar.
ACKNOWLEDGMENTS
We thank Dr. Steven G. Swarts and Dr. Yuriy Razskazovskiy for useful discussions and Kermit R. Mercer and Katerina A. Naumenko for their invaluable technical assistance. The investigation was supported by PHS grant 2-R01-CA32546, awarded by National Cancer Institute, DHHS. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
REFERENCES
- 1.von Sonntag C. Free-Radical-Induced DNA Damage. Springer-Verlag; Berlin: 2006. [Google Scholar]
- 2.Krisch RE, Flick MB, Trumbore CN. Radiation chemical mechanisms of single- and double-strand break formation in irradiated SV40 DNA. Radiat. Res. 1991;126:251–259. [PubMed] [Google Scholar]
- 3.Debije MG, Milano MT, Bernhard WA. DNA responds to ionizing radiation as an insulator, not as a “molecular wire”. Angew. Chem. Int. Ed. 1999;38:2752–2756. [PMC free article] [PubMed] [Google Scholar]
- 4.Henle ES, Roots R, Holley WR, Chatterjee A. DNA strand breakage is correlated with unaltered base release after gamma irradiation. Radiat. Res. 1995;143:144–150. [PubMed] [Google Scholar]
- 5.Swarts SG, Sevilla MD, Becker D, Tokar CJ, Wheeler KT. Radiation-induced DNA damage as a function of hydration. I. Release of unaltered bases. Radiat. Res. 1992;129:333–344. [PubMed] [Google Scholar]
- 6.Razskazovskiy Y, Debije MG, Bernhard WA. Direct radiation damage to crystalline DNA: What is the source of unaltered base release? Radiat. Res. 2000;153:436–441. doi: 10.1667/0033-7587(2000)153[0436:drdtcd]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pogozelski WK, Tullius TD. Oxidative strand scission of nucleic acids: Routes initiated by hydrogen abstraction from the sugar moiety. Chem. Rev. 1998;98:1089–1107. doi: 10.1021/cr960437i. [DOI] [PubMed] [Google Scholar]
- 8.Breen A, Murphy J. Reactions of oxyl radicals with DNA. Free Radic. Biol. Med. 1995;18:1033–1077. doi: 10.1016/0891-5849(94)00209-3. [DOI] [PubMed] [Google Scholar]
- 9.Rashid R, Langfinger D, Wagner R, Schuchmann HP, von Sonntag C. Bleomycin versus OH-radical-induced malonaldehydic-product formation in DNA. Int. J. Radiat. Biol. 1999;75:101–109. doi: 10.1080/095530099140852. [DOI] [PubMed] [Google Scholar]
- 10.Roginskaya M, Bernhard WA, Marion RT, Razskazovskiy Y. The release of 5-methylene-2-furanone from irradiated DNA catalyzed by cationic polyamines and divalent metal cations. Radiat. Res. 2005;163:85–89. doi: 10.1667/rr3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukla LI, Pazdro R, Becker D, Sevilla MD. Sugar radicals in DNA: Isolation of neutral radicals in gamma-irradiated DNA by hole and electron scavenging. Radiat. Res. 2005;163:591–602. doi: 10.1667/rr3347. [DOI] [PubMed] [Google Scholar]
- 12.Purkayastha S, Bernhard WA. What is the initial chemical precursor of DNA strand breaks generated by direct-type effects? J. Phys. Chem. B. 2004;108:18377–18382. doi: 10.1021/jp048539x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purkayastha S, Milligan JR, Bernhard WA. Correlation of free radical yields with strand break yields produced in plasmid DNA by the direct effect of ionizing radiation. J. Phys. Chem. B. 2005;109:16967–16973. doi: 10.1021/jp0518409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenspan L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. 1977;81A:89–96. [Google Scholar]
- 15.Wang A, Quigley G, Kolpak F, Crawford J, van Boom J, van der Marel G, Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979;282:680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- 16.Mercer KR, Bernhard WA. Design and operation of a variable temperature accessory for Q-band ESR. J. Magn. Reson. 1987;74:66–71. [Google Scholar]
- 17.Voet D, Gratzer WB, Cox RA, Doty P. Absorption spectra of nucleotides, polynucleotides, and nucleic acids in the far ultraviolet. Biopolymers. 1963;1:193–208. [Google Scholar]
- 18.Purkayastha S, Milligan JR, Bernhard WA. An investigation into the mechanisms of DNA strand breakage by direct ionization of variably hydrated plasmid DNA. J. Phys. Chem. B. doi: 10.1021/jp065489i. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debije MG, Strickler MD, Bernhard WA. On the efficiency of hole and electron transfer from the hydration layer to DNA: An EPR study of crystalline DNA X-irradiated at 4 K. Radiat. Res. 2000;154:163–170. doi: 10.1667/0033-7587(2000)154[0163:oteoha]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purkayastha S, Milligan JR, Bernhard WA. The role of hydration in the distribution of free radical trapping in directly ionized DNA. Radiat. Res. 2006;166:1–8. doi: 10.1667/RR3585.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Debije MG, Razskazovskiy Y, Bernhard WA. The yield of strand breaks resulting from direct-type effects in crystalline DNA X-irradiated at 4 K and room temperature. J. Am. Chem. Soc. 2001;123:2917–2918. doi: 10.1021/ja005790r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Debije MG, Bernhard WA. Free radical yields in crystalline DNA X-irradiated at 4 K. Radiat. Res. 1999;152:583–589. [PMC free article] [PubMed] [Google Scholar]
- 23.Milano MT, Bernhard WA. The influence of packing on free radical yields in solid-state DNA: Film compared to lyophilized frozen solution. Radiat. Res. 1999;152:196–201. [PMC free article] [PubMed] [Google Scholar]
- 24.Milano MT, Bernhard WA. The effect of packing and conformation on free radical yields in films of variably hydrated DNA. Radiat. Res. 1999;151:39–49. [PMC free article] [PubMed] [Google Scholar]
- 25.Roginskaya M, Bernhard WA, Razskazovskiy Y. Protection of DNA against direct radiation damage by complex formation with positively charged polypeptides. Radiat. Res. 2006;166:9–18. doi: 10.1667/RR3571.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razskazovskiy Y, Debije MG, Bernhard WA. Strand breaks produced in X-irradiated crystalline DNA: Influence of base sequence. Radiat. Res. 2003;159:663–669. doi: 10.1667/0033-7587(2003)159[0663:sbpixc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiland B, Hüttermann J, Malone ME, Cullis PM. Formation of C1′ located sugar radicals from X-irradiated cytosine nucleosides and -tides in BeF2 glasses and frozen aqueous solutions. Int. J. Radiat. Biol. 1996;70:327–336. doi: 10.1080/095530096145067. [DOI] [PubMed] [Google Scholar]
- 28.Roginskaya M, Razskazovskiy Y, Bernhard WA. 2-Deoxyribonolactone lesions in X-ray-irradiated DNA: Quantitative determination by catalytic 5-methylene-2-furanone release. Angew. Chem. Int. Ed. 2005;44:6210–6213. doi: 10.1002/anie.200501956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debije MG, Bernhard WA. Electron and hole transfer induced by thermal annealing of crystalline DNA X-irradiated at 4 K. J. Phys. Chem. B. 2000;104:7845–7851. [Google Scholar]
- 30.Deeble DJ, Schulz D, von Sonntag C. Reactions of OH radicals with poly(U) in deoxygenated solutions: Sites of OH radical attack and the kinetics of base release. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1986;49:915–926. doi: 10.1080/09553008514553151. [DOI] [PubMed] [Google Scholar]
- 31.Deeble DJ, von Sonntag C. Radiolysis of poly(U) in oxygenated solution. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1986;49:927–936. doi: 10.1080/09553008514553161. [DOI] [PubMed] [Google Scholar]
- 32.Ward JF, Kuo I. Strand breaks, base release, and post irradiation changes in DNA γ-irradiated in dilute oxygen-saturated aqueous solution. Radiat. Res. 1976;66:485–498. [PubMed] [Google Scholar]