Abstract
In a climate of increased funding for vaccines, chemotherapy, and prevention of vector-borne diseases, fewer resources have been directed toward improving disease and vector surveillance. Recently developed light-emitting diode (LED) technology was applied to standard insect-vector traps to produce a more effective lighting system. This approach improved phlebotomine sand fly capture rates by 50%, and simultaneously reduced the energy consumption by 50–60%. The LEDs were incorporated into 2 lighting designs, 1) a LED combination bulb for current light traps and 2) a chip-based LED design for a modified Centers for Disease Control and Prevention light trap. Detailed descriptions of the 2 designs are presented.
Keywords: Light trap, light-emitting diode (LED), insect trapping, disease vector, ultraviolet (UV)
Vector-borne disease and insect surveillance throughout the world are most often conducted with Centers for Disease Control and Prevention (CDC) light traps. These traps were developed originally by Sudia and Chamberlain (1962) and have provided a reliable method for monitoring disease vectors with minimal human exposure. Modifications have improved the CDC trap’s effectiveness (Stewart et al. 1970, Johnston et al. 1973, Elston and Apperson 1977, Addison et al. 1979), but power consumption and bulkiness continue to be disadvantages, especially during field collections in rural areas and in developing countries. Burkett et al. (1998) first used light-emitting diodes (LEDs) to improve energy efficiency; however, the traps had several deficiencies—the bulbs were not easily interchangeable, the bandwidths were broad (±50 nm wide), the lighting array was limited to 1 color, and the reflected light diminished luminosity. To address these concerns in the new design, a newly designed LED light bulb was substituted for the standard incandescent bulb in current CDC traps. In a 2nd iteration, a new LED platform-based lighting design was incorporated into a modified CDC light trap body. Both designs increased capture rates of medically important nocturnally flying dipterans and provided for a much longer battery life.
The principle attractant of the CDC trap to insects is the light source, usually a 4–6-W incandescent light bulb. When a nocturnal insect flies near the bulb, it is drawn into a collection bag or killing jar by a downward current of air propelled by a fan mounted below the light source. The impact of the visual cues provided by the light is key to trapping effectiveness. Vision in insects has evolved only once; therefore insects generally see in 3 specific colors—ultraviolet (UV), blue, and green (Birscoe and Chitka 2001). However, incandescent light bulbs emit most strongly in the infrared spectra and weakly in the visible light spectra of blue, green, and red; furthermore, their efficiency of converting electrical current to light is very low, approximately 6%. The remainder (94%) is emitted as infrared radiation or heat (General Electric 2008). Not only are red and infrared light (heat) invisible to most insect eyes (Fig. 1), tungsten filament incandescent light bulbs do not produce UV light, which is visible to insects.
Fig. 1.
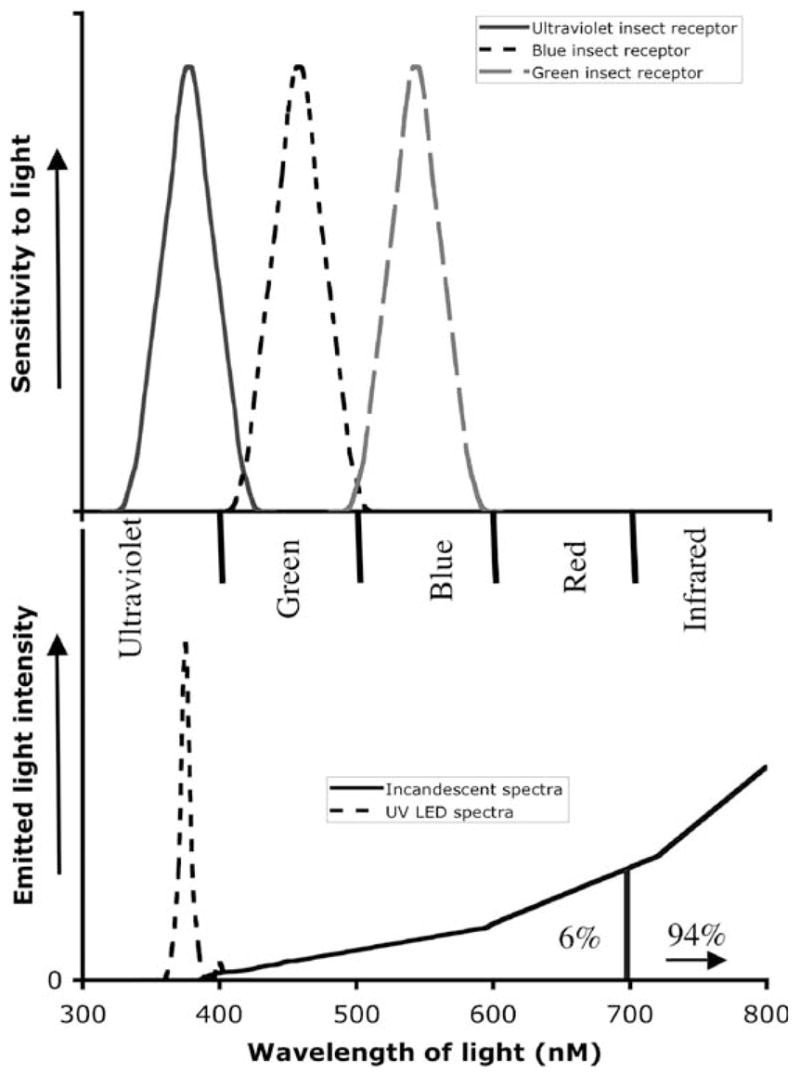
Insect vision and lighting spectra. The upper graph represents a schematic of the conserved insect photoreceptors and their maximum sensitivities in the ultraviolet, blue, and green spectra. The lower graph indicates the spectra emitted from ultraviolet light-emitting diode and incandescent lights. The overlap of the insect vision and the lighting system yields the light visible to insect eyes. Note that 94% of the incandescent bulb energy is expended in the infrared.
In recent years, manufacturing and technological advances have reduced the retail cost of high-efficiency LEDs. The LED is a solid-state unit that converts electricity to light with minimal generation of heat and therefore is a very efficient source of light. Typical modern LEDs produce emissions within a narrow 5-nm bandwidth. Light-emitting diode color can range from UV (350 nm) to infrared (700 nm) depending on the chemical composition of the LED. The angle of dispersion or cone of illumination from the bulb depends on the bulb structure and ranges from very narrow (as with laser pointers) to more broadly diffuse (up to 120° for omnidirectional lights). Brightness level is determined by the electrical current (measured in milliamperes) passing through the LED. Higher current produces brighter light, but the longevity of the bulbs is reduced. A LED will function for several thousand hours if not subjected to electrical overload. The solid-state design of the LEDs makes them durable under field conditions; they are difficult to shatter and rarely need to be replaced.
In the current application, LED technology has been integrated into CDC light traps in 2 innovative modes. The 1st is the creation of a combo LED light bulb replacement for current incandescent CDC light traps. The 2nd is a platform-based design for use of LEDs in a modified CDC light trap body. Both lighting designs allow for flexibility in the selection of the number of LED bulbs (4–16) with varying colors, viewing angles, or light intensity. Specific lighting arrangements can be used to maximize either capture rates or battery life, depending on the field application. Whereas incandescent bulbs produce a broad spectrum of light to attract insects, LEDs can be selected to emit a narrow bandwidth or specific color. Previous studies have found that mosquitoes (Wilton and Fay 1972, Burkett and Butler 2005), phlebotomine sand flies (Mellor and Hamilton 2003), and Culicoide flies (Bishop et al. 2004) are attracted preferentially to specific wavelengths of light. Finally, LED bulbs are advantageous in that they can be changed quickly in the field to configure the trap to the particular needs of a trapping environment.
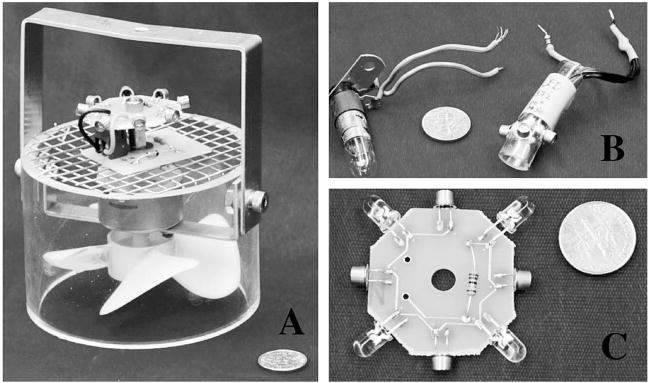
The combo LED replacement fitted for the incandescent bulb socket consists of a metal or plastic housing that holds 4–8 LED bulbs in a circular alignment (Fig. 2, 2B). A resistor on the LED leads regulates the 6-V current to the LEDs. The combo LED arrangement is fully self-contained and fits into the incandescent bulb socket. The LED leads plug directly into the power unit.
Fig. 2.
Light-emitting diode (LED) lighting platform configurations. (A) A standard Centers for Disease Control and Prevention light trap reconfigured with power circuit board and ultraviolet LED platform with 8 bulbs. (B) Standard incandescent bulb and socket (left) and its LED replacement with same socket (right). (C) Lighting platform with 8 LED bulbs of 2 types—clear bulbs (λ = 360 nm) with a 45° angle of dispersion, and metallic-collared bulbs (λ = 370 nm) with a 90° angle of dispersion.
The 2nd modification consists of a LED lighting platform, which is incorporated into a modified CDC trap body. The platform is an octagonal printed circuit board with LED ports on each side (Fig. 2, 2C). Each lighting platform is self-contained and carries 8 ports, each capable of holding a standard 5-mm LED bulb and connected to one another by embedded circuits. Several lighting platforms can be used in parallel by stacking them—either to create brighter light emission or to produce a trap that simultaneously emits light of multiple wavelengths. The power circuit board is mounted to the metal insect screen directly above the fan motor; it serves as the interface for the lighting chips, fan, and power pack. The footprint of the power circuit board and lighting chips are equal to the circumference of the motor and located within the down-draft eddy created by the motor. The size and location of these components keep them from interfering with the trap suction.
The electrical power required to drive the light traps can limit the scope of rural field collections. In this setting, the primary advantage of LED lighting is decreased power consumption (Table 1). Conventional CDC traps run on 6-V power supplies, usually 4 D-cell batteries or a rechargeable dry cell. A standard battery pack of 4 D-cells (17.5 Ah) will provide power to an incandescent bulb and motor for 4 nights and to a UV fluorescent bulb and motor for 1.5 nights in typical CDC light traps. In contrast, the LED lighting system is more efficient and produces an equivalent luminescence for 8 nights. A further advantage of the LED lighting system is that the bulbs and lighting chips will automatically shut off if the voltage drops below 3.2 V. In this event, the motor and fan continue to operate, thereby preventing escape of the collected specimens. The 4-bulb LED combo configuration trap under some circumstances can attract substantially more insects than incandescent traps. For example, in a paired trap comparison (tropical forests of French Guiana), the LED traps collected 30% more sand flies than the incandescent traps (Table 2). In a second comparison in dry forest habitats in Colombia, 2 stacked LED lighting chips (16 LED bulbs) were used. Although this configuration consumed nearly as much energy as the incandescent bulb trap, the stacked-chip trap attracted 50% more sand flies (Table 2). In other geographic settings, the traps have successfully captured mosquitoes (United States, Equatorial Guinea) and Culicoides flies (Colombia) as well.
Table 1.
Light-trap power consumption and weight considerations.
| Light source | Energy efficiency1 | Batteries (power unit) | Trap-nights per power unit2 | Energy needed per week | Weight per week per trap |
|---|---|---|---|---|---|
| Standard incandescent white light bulb | 6% | 4 D-cell battery pack | 4 | 8 batteries | 3.2 lb |
| Fluorescent light bulb | 10%3 | Special battery (single unit) | 1.5 | 4 recharges | 8 lb |
| LED4 replacement bulb | 35% | 4 D-cell battery pack | 8 | 4 batteries | 1.6 lb |
Conversion of electrical current to visible light.
Trap-nights are the average time a trap can function on 1 battery pack (calculated from field tests). Each battery pack weighs 1.6 lb.
This does not include the additional power required to initiate the fluorescent tube illumination.
LED, light-emitting diode.
Table 2.
Sand fly capture rates (average number of sand flies per trap-night).1
| Light source | French Guiana (4 LED bulbs) | Colombia (16 LEDs) |
|---|---|---|
| Incandescent white light bulb | 21.5 | 64.8 |
| Fluorescent UV light bulb | 42 | 46 |
| LED UV replacement bulb | 28.25 | 91.8 |
LED, light-emitting diode; UV, ultraviolet.
In the current environment of new and emergent vector-borne diseases, significant resources are being designated for research on insect-transmitted diseases. Unfortunately, little progress has been made in the ability to collect field specimens—an essential activity for monitoring disease vector prevalence. The new lighting systems described herein use LED technology to improve disease vector capture rates and to increases battery life. By a reconfiguration of the bulb arrangements, specific wavelengths of light can be customized to maximize target species capture. The ability to swap components in the field provides flexibility for fluctuating collection conditions and, thereby, makes the trap ideal for field work in rural or remote areas. In sum, the new system will improve insect surveillance and help target control measures in areas of difficult access, in rural areas, and in developing countries.
For their assistance in collecting phlebotomine sand flies, we acknowledge Fred Le Corre, Lorenza Beati, and Ly Kasper in French Guiana and Dairo Marin, Horacio Ramirez, and Clara Ocampo in Colombia. William M. Cohnstaedt provided valuable input into the lighting trap design. The LED lighting platforms are patent pending (11/936,540). Funding was provided by National Institutes of Health grants R01 AI-56254 and U19 AI065866 (Project 2) to LEM.
REFERENCES CITED
- Addison LD, Watson BJ, Webber LA. Apparatus for the use of CO2 gas with a CDC light trap. Mosq News. 1979;39:803–804. [Google Scholar]
- Birscoe AD, Chitka L. The evolution of color vision in insects. Ann Rev Entomol. 2001;46:471–510. doi: 10.1146/annurev.ento.46.1.471. [DOI] [PubMed] [Google Scholar]
- Bishop AL, Worrall R, Spohr LJ, McKenzie HJ, Barchia IM. Response of Culicoides spp. (Diptera: Ceratopogonidae) to light-emitting diodes. Aust J Entomol. 2004;43:184–188. [Google Scholar]
- Burkett DA, Butler JF. Laboratory evaluation of colored light as an attractant for female Aedes aegypti, Aedes albopictus, Anopheles quadrimaculatus, and Culex nigripalpus. Fla Entomol. 2005;88(4):383–389. [Google Scholar]
- Burkett DA, Butler JF, Kline DL. Field evaluation of colored light-emitting diodes as attractants for woodland mosquitoes and other diptera in north central Florida. J Am Mosq Control Assoc. 1998;14(2):186–195. [PubMed] [Google Scholar]
- Elston R, Apperson C. Light-activated on–off switch for CDC light trap. J Med Entomol. 1977;14:254–255. doi: 10.1093/jmedent/14.2.254. [DOI] [PubMed] [Google Scholar]
- General Electric. Introduction to lighting. [accessed 5 February 2008];General Electric. 2008 Available from: http://www.gelighting.com/na/business_lighting/education_resources/learn_about_light/light_intro.htm.
- Johnston J, Weaver J, Sudia W. Flashlight batteries as a power source for CDC miniature light traps. Mosq News. 1973;33:190–194. [Google Scholar]
- Mellor HE, Hamilton JGC. Navigation of Lutzomyia longipalpis (Diptera: Psychodidae) under dusk or starlight conditions. Bull Entomol Res. 2003;93:315–322. doi: 10.1079/ber2003248. [DOI] [PubMed] [Google Scholar]
- Stewart WWA. A modified CDC light trap. Mosq News. 1970;30:188–189. [Google Scholar]
- Wilton DP, Fay RW. Responses of adult Anopheles stephensi to light of various wavelengths. J Med Entomol. 1972;9:301–304. doi: 10.1093/jmedent/9.4.301. [DOI] [PubMed] [Google Scholar]