Abstract
Alleles of the human dopamine D4 receptor (D4R) gene (DRD4.7) have repeatedly been found to correlate with novelty seeking, substance abuse, pathological gambling, and attention deficit hyperactivity disorder (ADHD). If these various psychopathologies are a result of attenuated D4R-mediated signaling, mice lacking D4Rs (D4KO) should be more impulsive than wild-type (WT) mice and exhibit more novelty seeking. However, in our study, D4KO and WT mice showed similar levels of impulsivity as measured by delay discounting performance and response inhibition on a Go/No-go test, suggesting that D4R-mediated signaling may not affect impulsivity. D4KO mice were more active than WT mice in the first 5 min of a novel open field test, suggesting greater novelty seeking but for both genotypes, with the more impulsive D4KO mice habituated less readily in the novel open field. These data suggest that the absence of D4Rs is not sufficient to cause psychopathologies associated with heightened impulsivity and novelty seeking.
Keywords: impulsivity, D4 receptors, mice, delay discounting, Go/No-go, inhibition, novelty seeking, locomotion
Psychopathologies including pathological gambling (Comings et al., 2001), substance abuse (Vandenbergh et al., 2000), opiate dependence (Kotler et al., 1997), and attention-deficit hyperactivity disorder (ADHD; Faraone et al., 2001; Grady et al., 2003) are associated with the presence of dopamine D4 receptor (D4R) gene (DRD4) polymorphisms. Novelty-seeking is often associated with these psychopathologies and is also observed in individuals possessing DRD4 alleles containing 7 repeats of a 48-nucleotide sequence (DRD4.7) (Benjamin et al., 1996; Ebstein et al., 1996, 1997). Heightened levels of impulsivity is another common trait among the above-mentioned psychopathologies (Evenden, 1999), but its association with the DRD4 gene is unknown.
Impulsivity is thought to encompass multiple subcomponents functioning through distinct neural pathways. “Choice” impulsivity and “motor” impulsivity are two such subcomponents (Winstanley et al., 2004). “Choice” impulsivity refers to an intolerance for reward delays and is assessed by measuring relative preference for small, immediate over large, delayed rewards (Rachlin & Green, 1972; Ainslie, 1975; Logue, 1988) in rats (Bradshaw & Szabadi, 1992), mice (Isles et al., 2004; Helms et al., 2006), pigeons (Mazur, 1987), and humans (Rachlin et al., 1991; Green et al., 1994). Exaggerated preference for immediate rewards (heightened “delay discounting”) is found in many of the clinical populations in which DRD4.7 is observed (opioid abuse: Madden et al., 1997; pathological gambling: Petry, 2001b; alcohol abuse: Petry, 2001a; 5-to 11-year old children with ADHD: Tripp & Alsop, 2001). One procedure used to characterize impulsive choice in a variety of species is the adjusting amount procedure (humans: Richards et al., 1999; rats: Richards et al., 1997; mice: Mitchell et al., 2006). In prior studies using the adjusting amount procedure, subjects chose between a smaller immediate reward and a larger delayed reward. Choice of the immediate reward caused its size to decrease. Choice of the delayed reward caused the size of the immediate reward to increase. In this way, the size of the immediate reward when animals became indifferent between the rewards could be used to index the value of the delayed reward. While no differences were found between lines of mice selected for high or low alcohol drinking (Wilhelm et al., 2007), we have shown that DBA/2J mice are more impulsive than C57BL/6J mice (Helms et al., 2006), suggesting that this procedure is sensitive to strain differences.
“Motor” impulsivity refers to the inability to withhold responses and can be measured using a Go/No-go task, in which subjects respond to a specific cue (e.g., a light) and withhold responding when presented with an alternate cue (e.g., a tone). Subjects that show either a stronger relative preference for immediate rewards or less behavioral inhibition are characterized as being more impulsive. The unique prefrontal localization of D4Rs (Ariano et al., 1997; Oak et al., 2000), and the importance of mesocortical dopamine pathways for delay discounting (see Cardinal 2006 for review) and Go/No-go (Robbins, 2002), suggest that D4Rs may be involved in these subcomponents of impulsivity.
The present study measured impulsivity, response to a novel object and locomotor activity in mice genetically engineered to express a truncated D4R protein lacking putative transmembrane domains III-VII (Rubinstein et al., 1997). Domain III is affected by polymorphisms in human DRD4 and participates in coupling the receptor to G proteins and in receptor trafficking (Oldenhof et al., 1998). How polymorphisms affect receptor function in humans is unclear (Paterson, 1999). The absence of functional D4Rs in D4KO mice models is one possible functional consequence of the DRD4.7 polymorphism in humans.
Methods
Subjects
All mice were produced as described by Rubinstein et al. (1997). The D4KO genotype was originally created in C57Bl/6J × 129/Ola F1 animals. The subjects used in Experiment 1 were male D4KO (N = 12) and male WT (N = 11) mice from litters produced after 10 generations of backcrossing to C57Bl/6J wild-type mice (N10). The mice were backcrossed on the C57Bl/6J WT mice to eliminate possible confounding effects of the 129 genotype. The subjects used in Experiment 2 were male D4KO (N = 5), female D4KO (N = 5), female WT (N = 7) and male WT (N = 5) from litters produced after 20 generations of backcrossing to C57Bl/6J wild-type mice (N20). Due to availability, both male and female mice were used in Experiment 2. Practical considerations precluded the exclusive use of littermates, but for each experiment the mice were of the same generational cohort and housed in the same room during approximately the same period. On receipt, the mice used in Experiment 1 were 60–155 days of age (D4KO median = 113.5; WT median = 73), and the mice used in Experiment 2 were 76–139 days of age (D4KO median = 87; WT median = 86). An independent samples t-test indicated, for mice used in Experiment 1, D4KO mice were significantly older than WT mice, t(21) = 2.13, p < 0.05, so age was used a between groups covariate in all Experiment 1 analyses.
All mice were weighed for 5–10 days to obtain stable free-feeding weights. The first day of operant training occurred after a minimum of 48 hours on a food-restricted diet. Mice were maintained at approximately 90% of subject age adjusted free-feeding weight with standard laboratory mouse chow. The median free feeding weights at the start of Experiment 1 were 29.2 and 27.6 g for D4KO and WT mice, respectively. For Experiment 2, weights were 22.0 and 27.5 g for D4KO females and males, respectively, and 23.0 and 29.1 g for WT females and males, respectively.
The mice were housed 2–9 per cage under a 12:12-h light: dark cycle (lights on at 6 a.m.) in a temperature-controlled vivarium (21.7 ± 1°C), and maintained according to guidelines provided by the Oregon Health & Science University Department of Comparative Medicine. The Institutional Animal Care and Use Committee approved all procedures.
Apparatus
Delay discounting and Go/No-go tasks
Behavior was assessed in eight identical Med-Associates (St. Albans, VT) operant chambers (ENV-307A) housed in sound-attenuating ventilated boxes. Chamber floors consisted of 25 0.32-cm diameter stainless steel rods set 0.53 cm apart above a litter pan. In the panel to the left of the door was mounted a 100 mA house light protected by a metal cylinder. The panel to the right of the door contained a nose poke hole (ENV-313M) mounted 1.27 cm above the grid floor; when scheduled, the hole was illuminated by a rear 0.50-cm diameter yellow LED. This panel also contained two 0.50-cm diameter yellow LED lights; each light was centered 1.91 cm above a head-entry detector (ENV-303HDLP). Each head-entry recess contained a liquid reward cup (ENV-303LP). Eighteen-gauge stainless steel pipes continuous with the cups fed into plastic tubing attached to a syringe, which was filled with 10% (w/v) sucrose dissolved in de-ionized water and secured in a Med-Associates pump (PHM-100; 3.33 RPM).
Locomotor Activity
A single San Diego Instruments (San Diego, CA) activity chamber was used (40 × 40 × 37.5 cm), with eight equidistant photo beams spanning the length and width of the chamber, 5 cm apart. The locomotor activity apparatus was illuminated with an overhead fluorescent bulb and placed underneath a sound-attenuating curtain.
Procedures
Experiment 1a: Delay discounting
Choice behavior was measured using the adjusting amount procedure. Sessions occurred during the light cycle, 5–7 days per week, one session per day, and lasted for 60 min or 80 choice trials, whichever occurred first.
The mice experienced several training stages before beginning the adjusting amount procedure (Table 1). The experimental task procedures were identical to the final stage of training (Stage 5) except that the large sucrose amount was delivered after a delay (0, 2, 4, 8 or 12 s). The delay was consistent within a session, but varied across sessions according to a randomized block design. During the delay, both the house light and the stimulus light above the trough to which sucrose was scheduled for delivery were illuminated. Figure 1 illustrates the sequence of events in a single trial of the adjusting amount procedure. Each trial began with illumination of the center nose poke LED. After a center poke, the LED shut off and the trough lights were turned on. A poke to either trough shut off the corresponding trough light and caused the corresponding pump to advance the syringe plunger, delivering sucrose solution into the trough cup. An external sound-generator produced a 10-Hz click when sucrose was delivered. Horizontal infrared beams were broken when the animal put its head 0.64 cm into the center hole or the troughs. An IBM-compatible computer, using Med-PC software, recorded beam breaks and pump activity. The procedure was continued for approximately 90 sessions (18 sessions per delay condition). Due to experimenter error or equipment failure, the number of sessions per delay condition ranged from 16–20. Data analyses were conducted on the last 5 sessions at each delay.
Table 1.
Training protocol for the adjusting amount procedure used in Experiment 1a.
| Stage1 and days per stage2 | Conditions | Mean (± SEM) sessions to completion |
|---|---|---|
| 1 (3 sessions) | Nose pokes in the left or right troughs reinforced with 9.76 µl sucrose; trough light briefly extinguished after each reinforced nose poke | D4KO: 5.0 ± 5.1 |
| WT: 8.0 ± 1.9 | ||
| 2 (1 session) | 15-s time out3 between trials | D4KO: 1.0 ± 0.0 |
| WT: 1.1 ± 0.1 | ||
| 3 (2 sessions) | Nose pokes not reinforced after two consecutive choices of the same alternative until the other option is sampled (forced choice) | D4KO: 3.1 ± 0.3 |
| WT: 2.6 ± 0.4 | ||
| 4 (2 sessions) | Nose poke required in center trough before left and right trough choice permitted | D4KO: 14.0 ± 3.3 |
| WT: 9.2 ± 2.0 | ||
| 5 (10 sessions) | Choice between adjusting quantity (4.88 µl on trial 1) and standard quantity of 9.76 µl; adjusting quantity decreases or increases by 10%4 with each choice of the adjusting and standard alternative, respectively |
Notes. Changes in each stage were preserved in subsequent stages.
For Stages 1–4, the mice advanced to the next stage after completing a criterion of 80 trials in 60 minutes or less for the number of consecutive sessions listed in the left-hand column. There was no performance criterion for Stage 5 training.
No stimuli were presented and nose pokes were not reinforced.
Minimum quantity: 0.12 µl; maximum quantity: 19.52 µl. The location of the cup delivering the adjusting quantity was counterbalanced across genotype. Forced choices did not affect the adjusting quantity.
Figure 1.
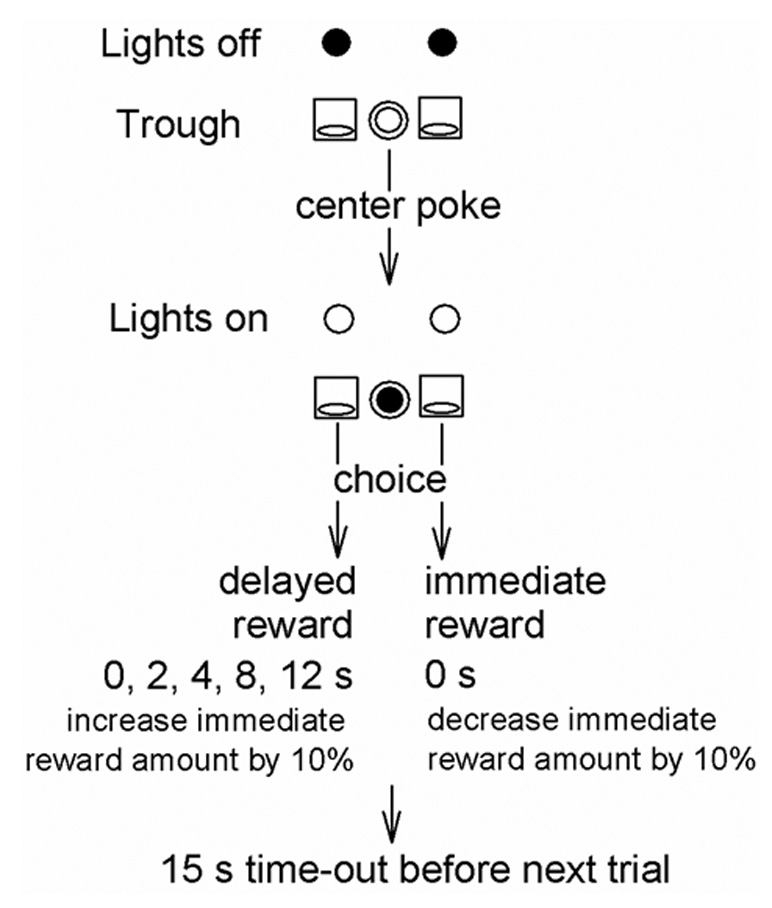
The nose poke contingencies of a single trial in the adjusting amount procedure (based on Richards et al., 1997); see methods for a complete description.
The main dependent variable was the “indifference point”. Indifference points were calculated as the median immediate sucrose amount for trials 40–80 (the point at which the immediate and delayed options were chosen with roughly equal frequency) for each mouse, and for each delay. To quantify the effect of delay on indifference points, the discounting rate (k) was obtained by fitting a hyperbolic equation: V = bA/(1 + kD) (Mazur, 1987), where V represents the value of the delayed reward measured by the median size of the immediate reward over trials 40–80 (indifference point), b represents side bias, D represents the reward delay, and A represents the amount of the delayed reward (9.76 µl).
Repeated measures ANOVA with Huynh-Feldt corrections for violations of the sphericity assumption were used to evaluate effects of delay and genotype on delay discounting (indifference points and the discounting rate, k), latency to initiate a trial (response latency), and latency to make a choice (choice latency). Examining the various measures indicated that discounting rates were non-normally distributed and so were loge transformed for all analyses. Main effects were evaluated with pair-wise comparisons using the Bonferroni correction or post-hoc Tukey tests.
Experiment 1b: Locomotor activity and response to novel object
Locomotor activity was measured 4–42 days after mice finished Experiment 1a because time and equipment limitations required that the mice be tested in cohorts. These cohorts were configured to include both D4KO and WT mice.
Testing occurred during the light cycle. As in Dulawa et al.’s (1999) novel object test, non-food-deprived mice were placed in the center of a novel open field and their activity (number of beam breaks) was recorded for 30 min in the novel open field. The mouse was then removed from the chamber, which was wiped clean with a solution of 10% isopropyl alcohol and de-ionized water. A white paper cup (height: 9.50 cm; diameter: 7.50 cm at the rim) was secured upside down in the center of the open field via tape inside of the cup. The mouse was then returned to the open field. Activity was recorded for an additional 30 min (novel object exploration). In each session, activity was recorded in 5-min bins.
The effect of genotype on total locomotor activity was evaluated with repeated measures ANOVA for which 5-min bin and session type (novel open field, exploration) were within-subjects factors. The total change in locomotor activity was calculated by subtracting activity in the final from the first 5-minute bin.
Experiment 2: Go/No-go task
The Go/No-go paradigm was modeled after that used by MacDonald et al (1998). Mice in this experiment experienced two training stages and an experimental phase (Table 2). Figure 2 shows a schematic representation of the Go/No-go task. During each of 60 trials, there was a variable duration pre-cue period (9–24 s) during which the house light was illuminated. Responses during the final 3 s of this period reset the trial to prevent premature responding. The pre-cue period was followed by a 5-s cue period, where distinct cues were used to differentiate Go trials from No-go trials. During a Go trial, the light above the left or right trough was illuminated (counterbalanced between subjects). During a No-go trial a continuous 65-dB 2.9-kHz tone was played. The first nose-poke response that occurred during the Go period terminated the Go cue and was reinforced by 19.95 µl of sucrose solution. A “click” signaled the delivery of the reward and the start of the 3-s reward period. This was followed by a 10-s inter-trial interval (ITI) during which the house light was off. If no nose poke responses occurred during the No-go period, a 19.95 µl reinforcer was delivered at the end of the period, signaled by a click. After a 3-s reward period, the 10-s ITI began. If a response occurred during the No-go period the tone was terminated and the ITI began without a reinforcer being delivered.
Table 2.
Training and experimental protocol for the Go/No-go procedure used in Experiment 2.
| Stage and days per stage 1 | Conditions | Mean (±SEM) sessions to completion |
|---|---|---|
| 1 (2 sessions) | Go trials only: Nose pokes during the light cue reinforced with 19.95 µl sucrose2. Each session consisted of 60 Go trials with cue periods of 30 s. | Female D4KO: 5.4 ± 0.7 |
| Female WT: 6.4 ± 1.0 | ||
| Male D4KO: 6.0 ± 0.6 | ||
| Male WT: 4.8 ± 0.4 | ||
| 2 (2 sessions) | Go trials only. Light cue period reduced to 10 s. | Female D4KO: 3.0 ± 0.44 |
| Female WT: 3.4 ± 0.9 | ||
| Male D4KO: 3.8 ± 1.4 | ||
| Male WT: 2.6 ± 0.4 | ||
| 3 (15 sessions) | No-go trials introduced: 19.95 µl sucrose delivered if no nose pokes occurred during 5-s tone cue. Each session consisted of 30 Go trials and 30 No-go trials with cue periods of 5 s. |
Notes. For Stages 1–2, mice advanced to the next stage after completing 2 consecutive sessions with 30 or more trials completed within 40 minutes.
The side location of the cup delivering the reward was counterbalanced across mice and across genotype.
Figure 2.
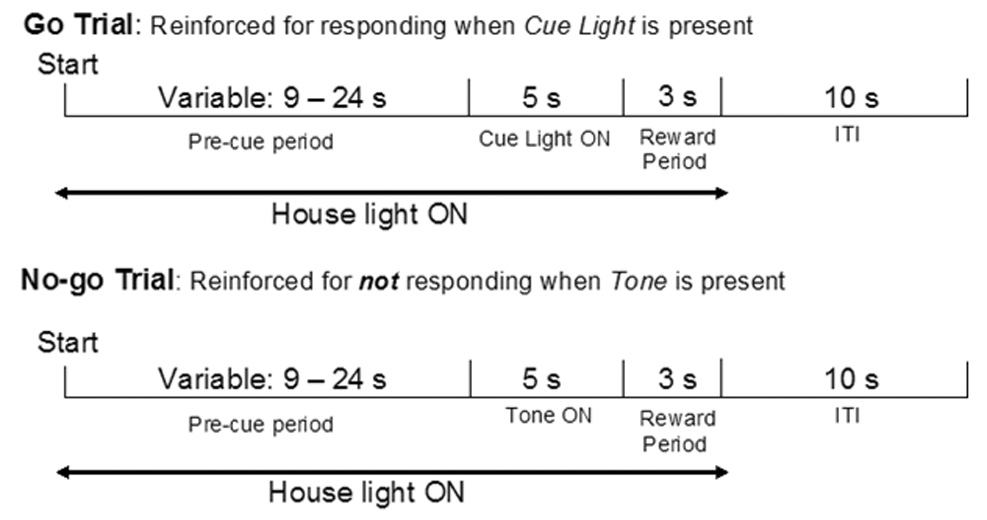
The nose poke contingencies of a single trial in the Go/ No-go procedure (based on McDonald et al., 1998); see methods for a complete description.
There were three main dependent measures for the Go/No-go task: the number of responses made during the variable length pre-cue period (pre-cue responses), the number of No-go trials on which mice responded during the No-go period (false alarms), and the number of rewards earned/total number of responses (efficiency).
We conducted mixed factor repeated measures ANOVAs with genotype as the between subjects factors and days as the within subjects factor for each of the three measures across days 6–10. Huynh-Feldt corrections were performed if there were violations of the sphericity assumption, and in those cases the adjusted degrees of freedom are reported.
Results
Experiment 1a: Delay discounting
Analyses of variance with age as a covariate revealed that the genotypes did not differ in the number of sessions to complete each training stage (Table 1). After training, D4KO and WT mice finished more than 40 of 80 trials on 15.77 ± 0.27 and 16.96 ± 0.20 sessions per delay condition, respectively. A 2 (genotype) × 5 (delay) repeated measures ANOVA revealed a main effect of delay on indifference points, F(2.29, 47.40) = 1.86, p< 0.001. That is, choice of the large, delayed reward systematically decreased as the delay increased. Pair-wise comparisons showed that indifference points on all delay conditions were significantly different except for 2 versus 4, and 8 versus 12 s. However, there was no main effect of genotype, F(1, 20) < 1.0, nor was there a delay × genotype interaction, F(2.29, 45.85) < 1.0 (Figure 3).
Figure 3.
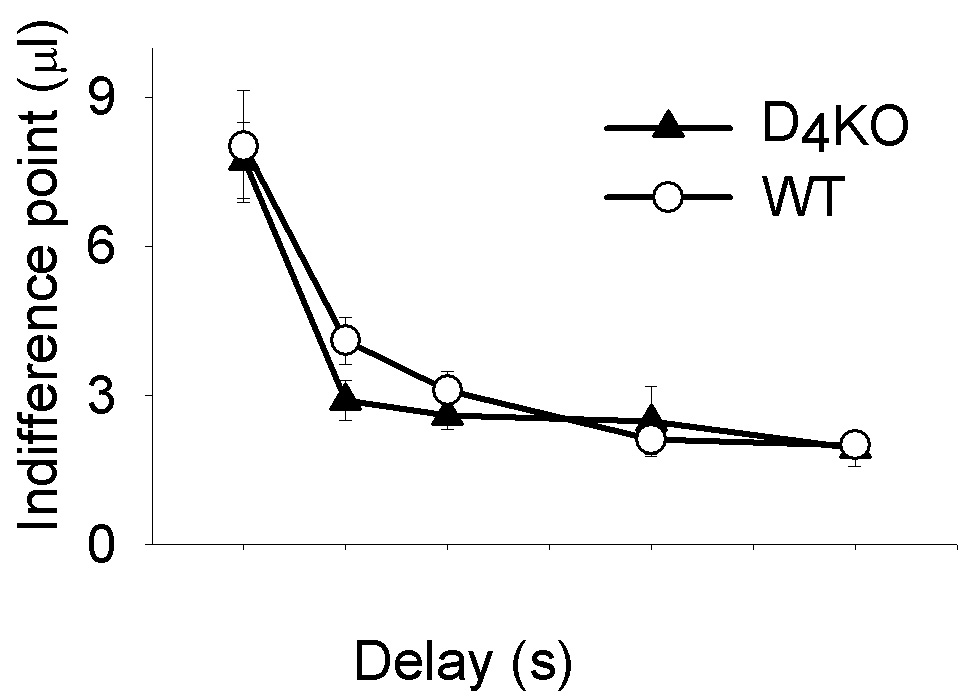
Mean (± SEM) of median adjusting sucrose amounts at indifference (µl) by delay (s) to 9.76 µl 10% sucrose for D4KO and WT mice from the last 40 of 80 choice trials for the final 5 sessions at each delay. Lower indifference points indicate greater impulsivity. There were no genotype differences in side bias (b; preference when Delay = 0 s) or in delay sensitivity (k; gradient of the discount function) as clearly shown in the figure (see text for statistical details).
A one-way ANOVA with age as a covariate indicated that impulsivity (logarithmically-transformed discounting rate, k) did not differ between the genotypes, F(1, 20) < 1.0 (mean ± SEM k values: D4KO,0.81 ± 0.29; WT, 0.46 ± 0.11). The genotypes were similarly biased away from the delayed reward side, F(1, 20) < 1.0 (mean ± SEM b values: D4KO, 0.78 ± 0.07; WT, 0.83 ± 0.11). Despite this preference for the “immediate” side, the mice were clearly affected by delaying sucrose delivery, as shown in Figure 3. The discounting rate, indexed by k values, is similar to that obtained from genetically heterogeneous WSC-1 and WSC-2 mice (Mitchell et al., 2006), DBA/2J and C57BL/6J mice (Helms et al., 2006) and rats (Richards et al., 1997). The side bias (b) values are also within range of those obtained from rats and WSC mice.
As shown in Figure 4, there were no main effects of genotype or interactions involving genotype for either response latency or choice latency, indicating that the temporal properties of behavior did not differ between the genotypes. Further, a 2 (genotype) × 5 (delay) repeated measures ANOVA revealed that choice latency did not vary with delay, as indicated by the absence of any main effects or interactions. Response latency, on the other hand, showed a main effect of delay, F(2.52, 53.00) = 35.46, p < 0.001, indicating that latency to initiate a trial increased as the delay to the large reward increased.
Figure 4.
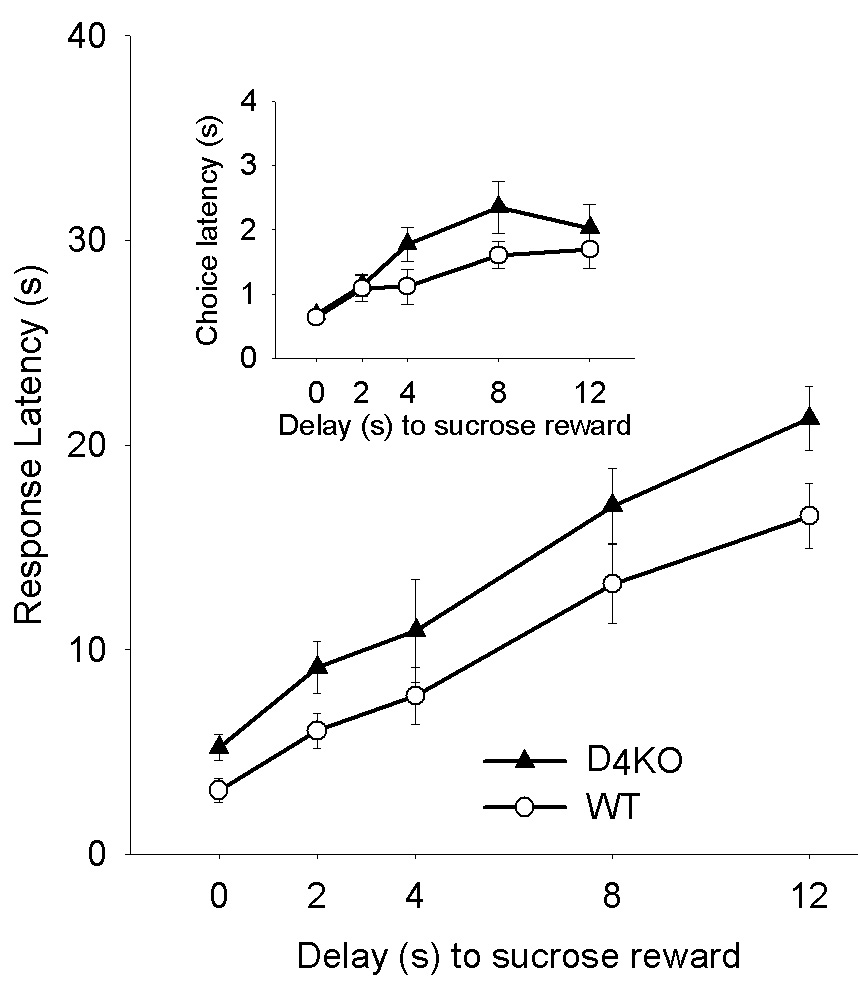
Mean (± SEM) latency (s) to initiate a trial after the time-out (reaction time) for D4KO and WT mice as a function of the Delay to the large reward in the adjusting amount procedure. The inset represents latency to choose the immediate or delayed reward (choice reaction time).
Experiment 1b: Locomotor activity and response to novel object
Total activity (beam breaks) did not differ between the genotypes as indicated by the absence of a main effect of genotype in a 2 (genotype) × 6 (5-min bin) × 2 (session type: novel open field, exploration) repeated measures ANOVA, (mean ± SEM: D4KO 373.34 ± 16.28; WT 353.55 ± 17.06). The D4KO mice were more active, however, during the first 5 minutes of the novel open field test (Figure 5), as indicated by a genotype × 5-min bin × session type (novel open field, exploration) interaction, F(5, 100) = 2.99, p < 0.05. Post hoc Tukey tests confirmed that D4KO mice were more active than WT mice only during the first 5 minutes of the novel open field test.
Figure 5.
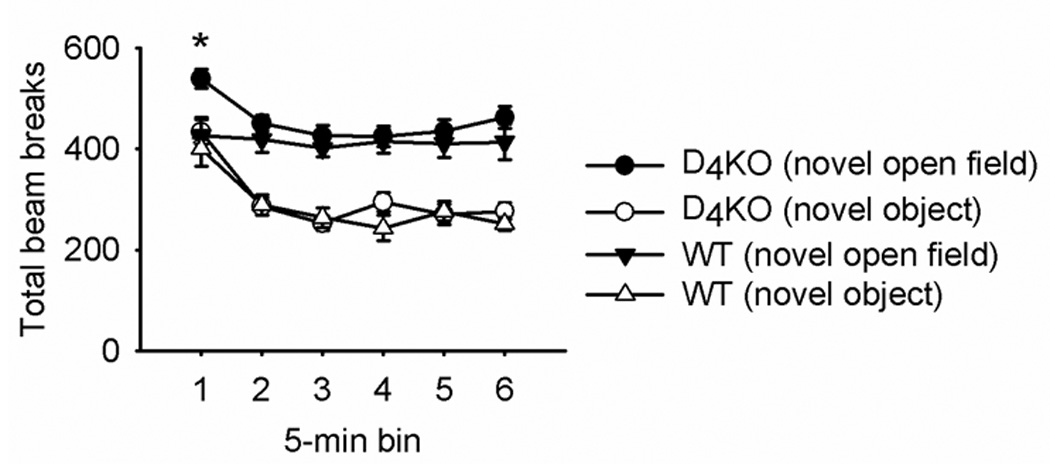
Mean (± SEM) total number of beam breaks in the entire arena across 5-min bins for the 30-min novel open field and novel object sessions for D4KO and WT mice. After 30 minutes in the open field, a novel paper cup was placed upside down in the center of the arena.
Activity decreased across the 6 5-min bins of the session as indicated by a main effect of bin, F(4.27, 85.34) = 6.05, p < 0.01. This decrease occurred for both the novel open field and novel object phase; there was no bin × session type interaction. Bonferroni-corrected pair-wise comparisons indicated that activity was significantly greater in the first, second, and third 5-min bins relative to the fourth, fifth, and sixth bins. Although the genotypes did not significantly differ, Figure 5 suggests that the activity of WT mice did not decrease substantially with time in the novel open field. Furthermore, the decrease in activity in D4KO mice was small and primarily occurred in the first 5 minutes due to a slightly higher baseline activity in D4KO mice.
Total activity decreased when the novel object (paper cup) was introduced. A 2 (session type: novel open field, exploration) × 6 (5-min bin) × 2 (genotype) repeated measures ANOVA revealed a main effect of session type, F(1, 20) = 8.29, p = 0.009. Session type did not interact with genotype, indicating that activity decreased similarly in both sessions independent of the two genotypes, F(1, 20) = 0.72 (novel open field: D4KO 453.67 ± 20.18; WT 417.81 ± 21.17; novel object: D4KO 301.46 ± 17.37; WT 288.78 ± 18.22). Casual observation suggested that the decrease in activity might have been due to the mice standing on the cup.
Discounting and Locomotor Activity Correlations
For the novel open field session, discounting rate (k) for D4KO mice was negatively correlated with their magnitude of the decrease in total activity from the first to the last 5-min bin (Spearman’s rho = −0.67, p < 0.05), indicating that more impulsive mice (greater discounting, large k) showed a smaller decrease in activity (less habituation) relative to less impulsive mice (lower discounting, smaller k). No correlation was observed for WT mice and change in total activity and delay discounting (k) did not correlate for either genotype during the novel object session.
Experiment 2: Go/No-go task
The genotypes did not differ in the number of sessions to complete each training stage according to ANOVAs (Table 2). Two female D4KO mice did not complete the first phase of training after 20 sessions and were omitted from the experiment.
There were no main effects of genotype on any measure of impulsivity on this task: pre-cue responses F(4, 76) = 0.12, p > 0.05, false alarms F(4, 76) = 1.71, p > 0.05, or efficiency F(4, 76) = 0.92, p > 0.05 (Figure 6). In addition, there were no significant sex × days effect for pre-cue responses F(4, 76)=2.45, p > 0.05, false alarms F(4, 76)=1.55, p > 0.05 or efficiency F(4, 76)=2.51, p > 0.05. The was no significant days effect for pre-cue response F(4, 76)=2.21, p > 0.05 or false alarms F(4, 76)=1.69, p > 0.05 but there was a significant overall increase in efficiency over days F(4, 76)=3.82, p < 0.01.
Figure 6.
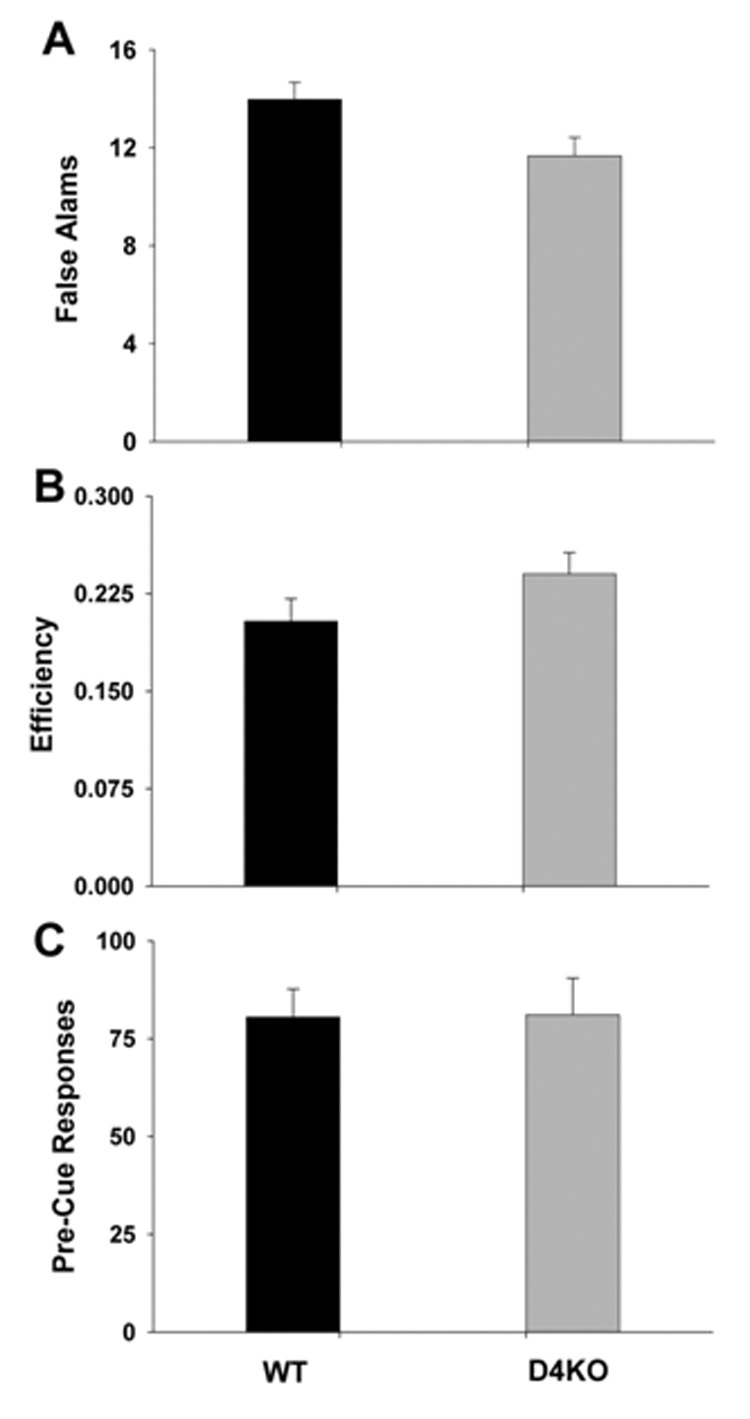
Mean (± SEM) pre-cue responses (A), false alarms (B) and efficiency (C) scores for D4KO and WT on the Go/ No-go procedure averaged over days 6–10. For all three variables there was no significant effect of genotype.
Discussion
It was hypothesized that mice lacking the D4R gene would score higher on measures of impulsivity. However, our study indicates that both “choice” impulsivity and “motor” impulsivity did not differ between D4KO and WT mice. In Experiment 1a (delay discounting), the genotypes exhibited similar bias towards the side associated with the immediate reward and similar systematic aversions to delayed rewards. In Experiment 2 (Go/No-go), the genotypes exhibited similar response inhibition. These data suggest that decreased D4R function does not result in greater impulsivity, however other explanations are also possible. For instance, alterations in other receptors or neurotransmitters may compensate for the lack of the D4R, which may also explain these results. The use of conditional knock-outs or selective D4R antagonists would be useful to confirm these findings.
The number of days required for the mice to learn the adjusting amount procedure or the Go/No-go task did not differ between the genotypes. This implies that efficacy of the sucrose reward was not affected by eliminating D4R signaling, consistent with other studies of reward efficacy (Falzone et al. 2002; Caine et al., 2002). Further, the lack of differences between D4KO and WT mice suggests that several processes involved in delay discounting and response inhibition are not affected by elimination of D4Rs, including auditory and visual perception, discrimination between reward magnitudes, delay discrimination, timing, and the effect of reward magnitude on response latency.
Delay discounting studies implicate serotonin and dopamine systems in impulsivity (Cardinal et al., 2004; Winstanley et al., 2004). For example, antagonism of dopamine D2Rs increases impulsivity in rats (Wade et al., 2000). Dopamine D2R binding appears not to be altered in D4KO mice (Rubinstein et al., 1997), supporting its role in choice impulsivity. Further, to the best of our knowledge, the serotonin system has not been investigated in D4KO mice, suggesting another preserved mechanism that may underlie choice impulsivity.
However, what is known of the neurobiological adaptations in D4KO mice has some parallels with the neurobiology of impulsivity, making the absence of an effect surprising. Thus, lesion studies have implicated the nucleus accumbens in impulsivity (Cardinal et al., 2004) and a recent study showed reduced dopamine turnover and KCl-evoked dopamine release in the nucleus accumbens of D4KO compared to WT mice (Thomas et al., 2007). Mammalian D4Rs are abundant in the prefrontal cortex (Tarazi & Baldessarini, 1999), residing on pyramidal and γ-aminobutyric acid neurons (Mrzljak et al., 1996; Wedzony et al., 2000). In D4KO mice, prefrontal cortex pyramidal neurons are hyperexcitable because dopamine activity at D4Rs normally inhibits cortical activity (Rubinstein et al., 2001). The prefrontal cortex has been characterized as supporting working memory (Goldman-Rakic, 1996), a cognitive process which might be expected to be critical in both delay discounting and go/no-go tasks. However, the lack of differences between WT and D4KO mice in either choice or motor impulsivity suggests that any possible effects of the D4R knockout on prefrontal-mediated working memory function did not affect behavior. In rodents, D4Rs are also expressed in the striatum (Van Tol et al., 1991), hippocampus, amygdala and hypothalamus (Mrzljak et al., 1996; Ariano et al., 1997). In D4KO mice, basal ganglia output neurons are not inhibited by dopamine (Shin et al., 2003) and striatal dopamine D1Rs and N-methyl-d-aspartate (NMDA) receptors show increased binding (Gan et al., 2004), suggesting that the consequences of knocking out D4Rs extend beyond the prefrontal cortex to brain regions that mediate learning and locomotor activity.
Pharmacological data (Powell et al., 2003) and D4KO mouse data (Dulawa et al., 1999) suggest that decreased D4R signaling results in decreased sensitivity to novel stimuli. In the current study, D4KO mice were more active than WT mice during the first five minutes of the novel open field test, suggesting greater sensitivity to novelty (Figure 5). Little change was observed for WT mice and the reasons for this are unclear. However, D4KO and WT mice exhibited similar novel object exploration. Dulawa et al. (1999) reported that, following the introduction of a novel cup, 8-week old F2 generation hybrid D4KO mice spent less time in the center of the chamber compared to WT mice. In Dulawa et al.’s protocol, activity habituated over a slightly longer time period and then increased when the cup was introduced. For our mice, the sensory effects of the novel object may have been blunted because the arena was relatively unfamiliar. Locomotor activity continued to decrease across the open field and novel object phases. Other procedural differences including breeding generation, light-dark cycle, age and training experience could account for the differential results. For example, Dulawa et al. tested the mice in an open-field for 3 days, each 30-min test separated by 1 day, then tested the mice in a separate open-field protocol, and then 2 weeks later conducted the novel object test. The cup used as a novel object in the current study had the same height and rim diameter as the cup used by Dulawa et al. Although not reported by Dulawa et al., the mice in the current study were observed standing on the cup suggesting that locomotor activity in this test may not have reflected the novelty of the cup. Future studies could assess novelty sensitivity after greater habituation to the arena and could use an object that does not interfere with locomotor activity measurements.
Total activity was unrelated to discounting rate, consistent with Isles et al.’s (2004) report that locomotor activity within inbred strains does not correlate with delay discounting in mice. The decrease in total activity from the first to the last 5 minutes of the novel open field test was, however, negatively correlated with discounting rate in D4KO mice, indicating that greater impulsivity is associated with slower habituation. Prolonged locomotor activity in the current study could also be interpreted as heightened sensation-seeking, as the animals presumably searched for stimulation (Antrop et al. 2000). This is consistent with O’Sullivan et al. (2005), which reported a slight but significant delay in the habituation of sifting behavior in D4KO compared to WT mice.
These data demonstrate that the absence of D4R activity in mice does not affect delay discounting or response inhibition measures of impulsivity. Additional studies could be conducted by verify these results. For example, future studies could assess the effects of pharmacological agonism and over-expression of D4Rs on impulsivity. In addition, studies using conditional D4R knockout mice could provide data not confounded by the possible developmental adaptations in mice born without D4Rs. Lastly, environmental conditions may interact with the neurobiological consequences of the D4KO in mice or D4R receptor polymorphisms in humans to affect behavior. For example, blood levels of lead positively correlated with the ADHD symptoms hyperactivity-impulsivity (Nigg et al., 2008). The functional consequences of DRD4 polymorphisms, and therefore possible mechanisms for interaction with environmental insults, are unclear (Paterson, 1999). In vitro, the receptor coded by DRD4.7 is slightly less sensitive to dopamine as indicated by reduced inhibition of cAMP relative to DRD4.2 or DRD4.4 (Asghari et al., 1995). Furthermore, children with the DRD4.7 allele show reduced sensitivity to the indirect dopamine agonist methylphenidate (Hamarman et al., 2007). Thus, additional studies may uncover the functional consequences of DRD4 polymorphisms and provide information about the conditions under which they influence behavioral phenotypes.
Acknowledgments
The authors thank William Guethlein, Tom Keck, Jamie Reeves, Katherine Suchland and Vanessa Wilson for outstanding technical assistance, and Jerry Richards for an initial version of the program, which was modified for collecting behavioral data in the Mitchell Lab. MH67497 (DKG), NIDA 5 T32-AA07468 (CMH) and MH070219 (CMH) supported this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainslie G. Specious reward: A behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Antrop I, Roeyers H, Von Oost P, Buysee A. Stimulation seeking and hyperactivity in children with ADHD. J Child Psychol Psychiatry. 2000;41:225–231. [PubMed] [Google Scholar]
- Ariano MA, Wang J, Noblett KL, Larson ER, Sibley DR. Cellular distribution of the rat D4 dopamine receptor protein in the CNS using anti-receptor antisera. Brain Res. 1997;752:26–34. doi: 10.1016/s0006-8993(96)01422-9. [DOI] [PubMed] [Google Scholar]
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Benjamin J, Li L, Patterson C, Greenberg BD, Murphy DL, Hamer DH. Population and familial association between the D4 dopamine receptor gene and measures of novelty seeking. Nat Genet. 1996;12:81–84. doi: 10.1038/ng0196-81. [DOI] [PubMed] [Google Scholar]
- Blough DS. Delayed matching in the pigeon. J Exp Anal Behav. 1959;2:151–160. doi: 10.1901/jeab.1959.2-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw CM, Szabadi E. Choice between delayed rewards in a discrete-trials schedule: The effect of deprivation level. Q J Exp Psychol B. 1992;44:1–16. doi: 10.1080/02724999208250599. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci. 2002;22:2977–2988. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural Networks. 2006;19:1277–1301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Comings DE, Gade-Andavolu R, Gonzalez N, Wu S, Muhleman D, Chen C, Koh P, Farwell K, Blake H, Dietz G, MacMurray JP, Lesieur HR, Rugle LJ, Rosenthal RJ. The additive effect of neurotransmitter genes in pathological gambling. Clin Genet. 2001;60:107–116. doi: 10.1034/j.1399-0004.2001.600204.x. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J Neurosci. 1999;19:9550–9556. doi: 10.1523/JNEUROSCI.19-21-09550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein RP, Nemanov L, Klotz I, Gritsenko I, Belmaker RH. Additional evidence for an association between the dopamine D4 receptor (D4DR) exon III repeat polymorphism and the human personality trait of Novelty Seeking. Mol Psychiatry. 1997;2:472–477. doi: 10.1038/sj.mp.4000333. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, Bennett ER, Nemanov L, Katz M, Belmaker RH. Dopamine D4 receptor (DRD4) exon III polymorphism associated with the human personality trait of novelty seeking. Nat Genet. 1996;12:78–80. doi: 10.1038/ng0196-78. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Falzone TL, Gelman DM, Young JI, Grandy DK, Low MJ, Rubinstein M. Absence of dopamine D4 receptors results in enhanced reactivity to unconditioned, but not conditioned, fear. Eur J Neurosci. 2002;15:158–164. doi: 10.1046/j.0953-816x.2001.01842.x. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Doyle AE, Mick E, Biederman J. Meta-analysis of the association between the 7-repeat allele of the dopamine D4 receptor gene and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1052–1057. doi: 10.1176/appi.ajp.158.7.1052. [DOI] [PubMed] [Google Scholar]
- Gan L, Falzone TL, Zhang K, Rubinstein M, Baldessarini RJ, Tarazi FI. Enhanced expression of dopamine D1 and glutamate NMDA receptors in dopamine D4 receptor knockout mice. J Molec Neurosci. 2004;22:167–177. doi: 10.1385/JMN:22:3:167. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Phil Trans: Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Grady DL, Chi HC, Ding YC, Smith M, Wang E, Schuck S, Flodman P, Spence MA, Swanson JM, Moyzis RK. High prevalence of rare dopamine receptor D4 alleles in children diagnosed with attention-deficit hyperactivity disorder. Mol Psychiatry. 2003;8:536–545. doi: 10.1038/sj.mp.4001350. [DOI] [PubMed] [Google Scholar]
- Green L, Fry A, Myerson J. Discounting of delayed rewards: A life-span comparison. Psychol Sci. 1994;5:33–36. [Google Scholar]
- Hamarman S, Fossella J, Ulger C, Brimacombe M, Dermody J. Dopamine receptor 4 (DRD4) 7-repeat allele predicts methylphenidate dose response in children witih attention deficit hyperactivity disorder: a pharmacogenetic study. J Child Adol Psychopharmacology. 2004;14:564–574. doi: 10.1089/cap.2004.14.564. [DOI] [PubMed] [Google Scholar]
- Helms CM, Reeves JM, Mitchell SH. Impact of strain and d-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology. 2006;188:144–151. doi: 10.1007/s00213-006-0478-0. [DOI] [PubMed] [Google Scholar]
- Isles AR, Humby T, Walters E, Wilkinson LS. Common genetic effects on variation in impulsivity and activity in mice. J Neurosci. 2004;24:6733–6640. doi: 10.1523/JNEUROSCI.1650-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler M, Cohen H, Segman R, Gritsenko I, Nemanov L, Lerer B, Kramer I, Zer-Zion M, Kletz I, Ebstein RP. Excess dopamine D4 receptor (D4DR) exon III seven repeat allele in opioid-dependent subjects. Mol Psychiatry. 1997;2:251–254. doi: 10.1038/sj.mp.4000248. [DOI] [PubMed] [Google Scholar]
- Logue AW. Research on self-control: An integrating framework. Behav Brain Sci. 1988;11:665–709. [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative analysis of behavior 1987; Vol 5. The effect of delay and intervening events on reinforcement value. Hillsdale, NJ: Erlbaum; pp. 55–73. [Google Scholar]
- McDonald MP, Wong R, Goldstein G, Weintraub B, Cheng SY, Crawley JN. Hyperactivity and learning deficits in transgenic mice bearing a human mutant thyroid hormone beta1 receptor gene. Learn Mem. 1998;5:289–301. [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Bergson M, Pappy R, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:246–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, Savelly JM, Li N, Phillips TJ. Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcoholism Alcohol Clin Exp Res. 2006;30:429–437. doi: 10.1111/j.1530-0277.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- Nigg J, Knottnerus GM, Martel MM, Nikolas M, Cavanagh K, Karmaus W, Rappley MD. Low blood lead levels associated with clinically diagnosed attention-deficit/hyperactivity disorder and mediated by weak cognitive control. Biol Psychiatry. 2008;63:325–331. doi: 10.1016/j.biopsych.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oak JN, Oldenhof J, Van Tol HHM. The dopamine D4 receptor: one decade of research. Eur J Pharmacol. 2000;405:303–327. doi: 10.1016/s0014-2999(00)00562-8. [DOI] [PubMed] [Google Scholar]
- Oldenhof J, Vickery R, Anafi M, Oak J, Ray A, Schoots O, Pawson T, von Zastrow M, Van Tol HHM. SH3 binding domains in the dopamine D4 receptor. Biochemistry. 1998;37:15726–15736. doi: 10.1021/bi981634+. [DOI] [PubMed] [Google Scholar]
- O’Sullivan GJ, Kinsella A, Grandy SK, Tighe O, Croke DT, Waddington JL. Ethological resolution of behavioral topography and D2-like vs D1-like agonist responses in congenic D4 dopamine receptor “knockouts”: identification of D4:D1-like interactions. Synapse. 2006;59:107–118. doi: 10.1002/syn.20225. [DOI] [PubMed] [Google Scholar]
- Paterson AD, Sunohara GA, Kennedy JL. Dopamine D4 receptor gene: novelty or nonsense? Neuropsychopharmacology. 1999;21:3–16. doi: 10.1016/S0893-133X(98)00115-8. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001a;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM. Pathological gamblers, with and without substance abuse disorders, discount delayed rewards at high rates. J Abnorm Psychol. 2001b;110:482–487. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- Powell SB, Paulus MP, Hartman DS, Godel T, Geyer M. RO-10-5824 is a selective dopamine D4 receptor agonist that increases novel object exploration in C57 mice. Neuropharmacology. 2003;44:473–481. doi: 10.1016/s0028-3908(02)00412-4. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice and self-control. J Exp Anal Anim Behav. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Rainieri A, Cross D. Subjective probability and delay. J Exp Anal Anim Behav. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, Seiden LS. Determination of discount functions in rats with an adjusting-amount procedure. J Exp Anal Anim Behav. 1997;67:353–366. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Anim Behav. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animal. Exp Brain Res. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Cepeda C, Hurst RS, Hernandez-Flores J, Ariano MA, Falzone TL, Kozell LB, Meshul CK, Bunzow JR, Low MJ, Levine MS, Grandy DK. Dopamine D4 receptor-deficient mice display cortical hyperexcitability. J Neurosci Res. 2001;21:3756–3763. doi: 10.1523/JNEUROSCI.21-11-03756.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, Saez C, Pugsley TA, Gershanik O, Low MJ, Grandy DK. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997;90:991–1001. doi: 10.1016/s0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- Shin R-Y, Masuda M, Miura M, Sano H, Shirasawa T, Song W-J, Kobayashi K, Aosaki T. Dopamine D4 receptor-induced postsynaptic inhibition of GABAergic currents in mouse globus pallidus neurons. J Neurosci. 2003;23:11662–11672. doi: 10.1523/JNEUROSCI.23-37-11662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Dopamine D4 receptors: significance for molecular psychiatry at the millennium. Mol Psychiatry. 1999;4:529–538. doi: 10.1038/sj.mp.4000674. [DOI] [PubMed] [Google Scholar]
- Thomas CT, Kruzich PJ, Joyce BM, Gash CR, Suchland K, Surgener SP, Rutherford EC, Grandy DK, Gerhardt GA, Glaser PEA. Dopamine D4 receptor knockout mice exhibit neurochemical changes consistent with decreased dopamine release. J Neurosci Methods. 2007;166:306–314. doi: 10.1016/j.jneumeth.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward delay in children with attention deficit hyperactivity disorder (ADHD) J Child Psychol Psychiatry. 2001;42:691–698. [PubMed] [Google Scholar]
- Vandenbergh DJ, Rodriguez LA, Hivert E, Schiller JH, Villareal G, Pugh EW, Lachman H, Uhl GR. Long forms of the dopamine receptor (DRD4) gene VNTR are more prevalent in substance abusers: No interaction with functional alleles of the catechol-o-methyltransferase (COMT) gene. Am J Med Genet. 2000;96:678–683. doi: 10.1002/1096-8628(20001009)96:5<678::aid-ajmg15>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Van Tol HHM, Wu CM, Guan HC, Sunahara RK, Seeman P, Niznik HB, Civelli O. Cloning of the gene for a human D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Chocyk A, Mackowiak M, Fijal K, Czyrak A. Cortical localization of dopamine D4 receptors in the rat brain-immunocytochemical study. J Physiol Pharmacol. 2000;51:205–221. [PubMed] [Google Scholar]
- Wilhelm CJ, Johnson RA, Lysko PG, Eshleman AJ, Janowsky A. Effects of methamphetamine and lobeline on vesicular monoamine and dopamine transporter-mediated dopamine release in a cotransfected model system. J Pharmacol Exp Ther. 2004;310:1142–1151. doi: 10.1124/jpet.104.067314. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DEH, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- Zhang K, Grady CJ, Tsapakis EM, Andersen SL, Tarazi FI, Baldessarini RJ. Regulation of working memory by dopamine D4 receptor in rats. Neuropsychopharmacology. 2004;29:1648–1655. doi: 10.1038/sj.npp.1300491. [DOI] [PubMed] [Google Scholar]


