Abstract
Competition between young of the same brood or litter is of particular interest in the fields of behavioural and evolutionary ecology, because the competing individuals are likely to be closely related, where evolutionary theory predicts a greater degree of cooperation. Studies of cooperative breeding species typically concentrate on who contributes care to rearing young, and assume a passive role of the young. Relatively, little attention has been devoted to considering how intralitter competition between young affects the distribution of care in cooperative breeders. In banded mongoose (Mungos mungo) groups, the majority of pups each form a stable exclusive one-to-one association with an adult group member (its ‘escort’) that is its principal care provider. This paper presents experimental evidence that each pup aggressively defends access to its escort, preventing other pups approaching, and therefore monopolizes the care provided by its escort. Each pup travels with the group and follows its escort, around which its exclusion zone is fixed: a form of mobile territoriality. This represents a novel system of care of young in a mammal species, but has general implications for the study of the distribution of care of young, particularly in cooperative breeding species. Parents and helpers may provide biased care to young, not due to preference but due to the competitive actions of the young within the brood or litter.
Keywords: aggression, competition, sibling rivalry, Mungos mungo, parent–offspring conflict, provisioning
1. Introduction
Intraspecific competition is evident across the animal kingdom, and forms the basis of Darwin's theory of evolution by natural selection, where individuals strive to maximize their personal genetic contribution to subsequent gene pools. Competition within species can occur across the developmental spectrum, from gestation (Bruce & Wellstead 1992), to early post-hatch/birth (Mock & Parker 1997, 1998; Drummond 2006), to adulthood (Clutton-Brock et al. 1982; van Schaik & van Noordwijk 1988). Competition between young of the same brood or litter is of particular interest, because the competing individuals are likely to be closely related. While Hamilton's kin selection theory predicts cooperation between relatives where the benefits outweigh the costs (Hamilton 1964), competition can be most intense between relatives (West et al. 2001, 2002).
In cooperative breeding species, individuals exhibit apparent altruism by caring for young that are not their own (Stacey & Koenig 1990; Solomon & French 1997; Koenig & Dickinson 2004). In studies of such systems, the interest generally lies in who cares for young and which young they care for (Jennions & Macdonald 1994; Solomon & French 1997; Cockburn 1998; Clutton-Brock 2002). By contrast, in non-cooperative breeding species, there has been much attention on competitive interactions between young and how this can affect the distribution of care delivered by parents and received by young (Mock & Parker 1997, 1998; Drummond 2006). Such competition can lead directly or indirectly to biased care provided to superior competitors. However, little attention has been given to the possibility that the distribution of care of young within cooperative breeding groups may be determined by the actions of the young rather than those of the carers (but see Ostreiher 1997; Hodge et al. 2007). Parents and helpers may provide biased care to young, not due to the preference but due to the competitive actions of the young within the brood or litter.
Until recently, research on intralitter competition in mammals was under-researched relative to avian species, and data on interference competition (incorporating evidence of dominance relationships) between littermates are rare (Drummond 2006; Hudson & Trillmich 2007). In this study, temporary removal of young of a cooperative breeding mammal, the banded mongoose (Mungos mungo) was used to test whether interference competition between littermates prevents pups interacting freely with potential carers.
The banded mongoose is a communal breeding species that forms mixed sex groups with up to 10 females breeding synchronously, producing communal litters of mixed parentage (Cant 2000; Gilchrist et al. 2004; Gilchrist 2006). After a month spent in the den, the pups emerge and travel with the foraging group. For approximately the next two months, the pups are dependent upon group members for care, in particular provisioning and protection (Gilchrist 2004; Gilchrist & Russell 2007). The majority of pups form exclusive one-to-one associations with specific group members, escorts (Cant 1998), that are likely to be breeders, but unlikely to be a parent of the specific associated pup (Gilchrist 2004; Gilchrist & Russell 2007).
This study examines the dynamics of the pup–escort system in the banded mongoose, in particular the role of pup–pup competition, by conducting temporary removal experiments. Temporary removal of associating pups was used to test whether other pups experienced increased proximity and direct interaction (e.g. contact, grooming and provisions) with potential carers. Analysis of aggressive interaction between pups was used to evaluate whether associating pups use force to monopolize potential carers.
2. Material and methods
(a) Data collection
The study was conducted in Queen Elizabeth National Park, Uganda (0°12′ S, 27°54′ E) between 29 April 2002 and 19 April 2003 on individually marked mongooses. All individual mongooses were located, trapped and marked using methods outlined elsewhere (Cant 2000; Cant et al. 2001). There is no evidence of negative effects of temporary pup removal upon removed pups (see electronic supplementary material). All procedures were licensed by the Uganda Wildlife Authority and the Uganda National Council for Science and Technology. The study followed the code of ethics and guidelines outlined by the Association for the Study of Animal Behaviour and the Animal Behaviour Society (ASAB 2006).
Twenty-five pup removal trials were conducted over 10 communal litters in four groups. Each trial incorporated two focal pups and two focal escorts, with at least one other dependent pup present within the group at the time of focal pup removal. An escort was defined as a group member (over 90 days old) that regularly associates with a specific pup (aged 90 days or less), where association was defined as close proximity (30 cm or less) between group member and pup. All trials were conducted on post-weaned pups, after emergence from the den, within the period of pup dependence upon adults (30–90 days), with pups aged 48±1.17 days (mean±s.e.), range 34–66. For each experimental trial, two escorts were identified with a strong stable association with a pup, i.e. they consistently maintained close proximity to a specific pup over a period of days (within and between days). The two escorts were classified as focal escorts and the two pups in association as focal pups (other pups are referred to as control pups). Observations were conducted on the focal escorts and all pups for 6 days. Data were collected with all individuals present for the first 2 days, the two focal pups were then removed for 2 days and then returned to their group, continuing observations for a further 2 days. These three periods are referred to (according to the status of the focal pups) as (i) pre-removal, (ii) post-removal, and (iii) post-release. Data collection during each trial usually comprised a minimum of 1 hour in both the morning and afternoon activity periods.
(b) Scan data collection
Nearest neighbour scans were conducted at 5 min intervals to score the presence and identity of the nearest adult within 10 cm for each pup and the nearest pup within 10 cm for the two focal escorts. In addition, for 7 of the 25 experiments, scan data were also collected on four to eight control escorts (other adults in association with a pup). From these data, an association index was calculated for each individual. The association index is the proportion of scans that an individual was scored as in association (within 10 cm) with another individual.
(c) Focal data collection
Focal data collection applied to focal escorts enabled evaluation of the frequency of behaviour donated to and received from pups and also recorded pup–pup interactions within 1 m of the focal escort. Focal duration was 15 min, with the observer switching between the two focal escorts in a group. Interactive behaviour types selected for data analysis are described below. Follows were recorded when the focal escort followed a pup (within 100 cm) in or out of cover (escort-initiated follows), or the focal escort was followed by a pup (within 100 cm) in or out of cover (pup-initiated follows). Contact involved any physical touching of adult and pup, and included adult contact sniffing pups, and pups climbing on the back of or sheltering under the belly of an adult. Contacts were recorded as focal escort to pup (escort-initiated) or pup to focal escort (pup-initiated). Grooming (licking of the fur) occurred between the focal escort and pups. Successful provision events were recorded where the focal escort provided a pup with a food item (mainly invertebrate) or piece thereof. Provision events where the escort approached the pup were classed as escort-initiated and provision events where the pup approached the escort were classed as pup-initiated.
(d) Aggression
Aggression between pups occurring within 1 m of the focal escort was recorded. Where possible the cause of the aggression was classified. The majority of pup–pup aggression was motivated by access to an escort (there was no other resource nearby). Food items were the other major cause. Aggressive events were classified as attack where the initiator was further away from the escort than the attacked pup, and classified as defence where the initiator was closer to the escort than the attacked pup. The initiator was classified as the winner if it repelled the other pup, and the loser if it was repelled by the other pup. The event was classed as a draw where there was no clear winner.
(e) Statistical analysis
Data analyses were performed using GenStat v. 6.0. For scan data analyses, generalized linear mixed models were fitted to the data using the IRREML procedure with logit link function. The dependent variate was fitted using a binomial function, with the number of scans that an individual was within 10 cm of an adult or pup as the numerator, and the total number of scans for the individual as the denominator. For focal data analyses, linear mixed models were fitted to the data using the REML procedure. The dependent variate was the mean rate (per min) of behavioural interaction. Analyses and rates reflect interaction between each focal escort and all available control pups (the number of which vary from 1 to 11 dependent upon the experiment), or (in the case of aggression analysis) between focal pup and all control pups. All rates are given as per minute. For comparison between periods, REML analyses were used due to the differing duration of observations between periods. Wilcoxon signed-rank tests were used to compare the rates of interaction initiated by the focal escort and control pups. For these tests, comparison was restricted to the post-removal period and the unit of pairing was the experimental replicate (with two replicates per experiment, n=46 replicates minus the number of zero scores). Chi-squared tests were used to compare overall counts of behaviour between control pups and focal escorts or between focal and control pups where the count for one category was less than 10. Comparing the overall count for two categories uses d.f. 1.
In the majority of statistical models, treatment was fitted as the main fixed effect. There were three treatments per experiment, denoting the status of the two focal pups: pre-removal; post-removal; and post-release. In all models, group identity (n=4), experiment (n=25, including two pup removals per experiment), communal litter (n=10) and escort identity (n=37 individuals across the 50 pup removals) were included as terms in the random model. This accounts for repeated sampling across error terms (Schall 1991). Random terms identified as negative or null components of variance were dropped from the random model. None of the random terms fitted were significant (all p>0.05).
Where the fixed effect in a model was significant and contained three or more levels, post hoc pairwise comparisons were made between the levels using the t-test with the d.f. set to 25 (the number of experiments, the random term with highest component of variance). Using this value for the degrees of freedom is a conservative approximation (Brown & Prescott 1999). Exact degrees of freedom are not available because the main effect means are estimated across the random terms. The critical value applied to post hoc pairwise t-tests was 2.06 (with d.f.=25 for all pairwise tests). Mean values are provided ±s.e. in text and figures (error bars). All tests are two tailed with significance defined as p<=0.05.
3. Results
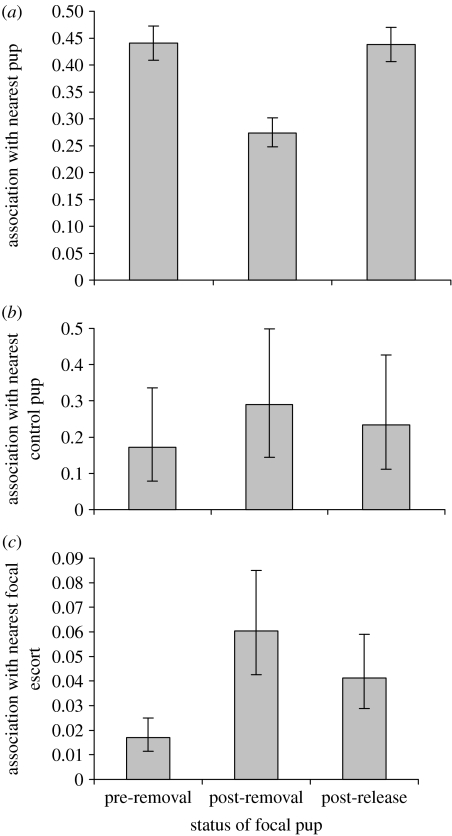
As expected, the overall incidence of close association between focal escorts and pups decreased upon removal of focal pups (figure 1a), arguably due in part to the lower number of available pups. Association index for focal escorts with pups differed significantly between treatments (IRREML: Χ22=80.72, p<0.001, n=146), and was lowest during the post-removal period.
Figure 1.
Factors affecting association (proportion of scans within 10 cm) between focal escorts and pups during the pre-removal, post-removal and post-release treatment of focal pups. (a) Association index for focal escorts with pups (pre-removal versus post-removal t=8.42, p<0.001; pre-removal versus post-release t=0.15, p=0.88 and post-removal versus post-release t=7.76, p<0.001). (b) Association index with control pups for focal escorts (pre-removal versus post-removal t=3.76, p<0.001; pre-removal versus post-release t=1.99, p=0.058 and post-removal versus post-release t=1.57, p=0.13). (c) Association index with focal escort for control pups (pre-removal versus post-removal t=6.19, p<0.001; pre-removal versus post-release t=4.16, p<0.001 and post-removal versus post-release t=2.50, p=0.019).
(a) Does the presence of a pup in stable association with an escorting adult reduce the ability of other pups to attain close proximity with the escort?
The association between focal escorts and control pups increased upon removal of focal pups. Focal escorts had a greater probability of having a control pup in close proximity as their nearest neighbour when the focal pups were removed (figure 1b), and control pups had a greater probability of having a focal escort in close proximity as their nearest neighbour when the focal pups were removed (figure 1c). Association index with control pups for focal escorts differed significantly between treatments (IRREML: Χ22=14.16, p=0.001, n=146), and was highest during the post-removal period (figure 1b). Association index with focal escort for control pups differed significantly between treatments (IRREML: Χ22=38.60, p<0.001, n=269), and was highest during the post-removal period (figure 1c). By contrast, the association of control escorts (escorts whose paired pups were not removed) was unaffected by removal of the focal pups (overall pup association IRREML: Χ22=0.28, p=0.87, n=123; association with control pups IRREML: Χ22=0.27, p=0.87, n=123). Together, these results indicate an active behavioural response on the part of either the vacant escorts or the control pups.
(b) Does the presence of a pup in stable association with an escorting adult reduce the ability of other pups to interact with that escort?
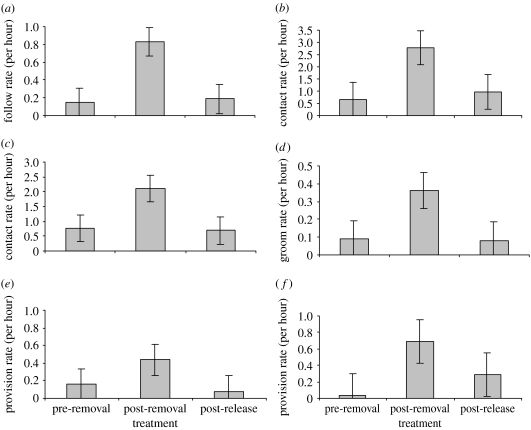
Upon removal of a focal pup, the rate of behavioural interaction between the focal escort and the control pups increased. For focal escorts, the rate of control-pup-initiated follows differed between treatments (REML: Χ22=16.50, p<0.0001, n=135), and was highest during the post-removal period (figure 2a). By contrast, focal escorts rarely followed control pups (Wilcoxon signed-rank test: W=18.00, n=20, p=0.001; control-pup-initiated 0.83±0.23 follows per hour, focal-escort-initiated 0.083±0.074). For focal escorts, the rate of control-pup-initiated contact differed between treatments (REML: Χ22=10.63, p=0.005, n=135), and was highest during the post-removal period (figure 2b). For control pups, the rate of focal-escort-initiated contact differed between treatments (REML: Χ22=19.50, p<0.0001, n=135), and was similarly highest during the post-removal period (figure 2c). During the post-removal period, control pups were responsible for initiating contact with focal escorts as frequently as focal escorts initiated contact with control pups (Wilcoxon signed-rank test: W=282, n=36, p=0.42; control-pup-initiated 2.90±0.94 contacts per hour, focal-escort-initiated 2.33±0.45). For control pups, the rate of focal escort-initiated grooming differed between treatments (REML: Χ22=8.00, p=0.018, n=135), and was highest during the post-removal period (figure 2d). Focal escorts groomed control pups at a significantly higher rate than control pups groomed focal escorts, with the latter never observed (Χ12=35.1, p<0.0001; control-pup-initiated 0±0 grooms per hour, focal-escort-initiated 0.38±0.13). For control pups, the rate of focal-escort-initiated provisioning differed between treatments (REML: Χ22=5.97, p=0.050, n=135) and was highest during the post-removal period (figure 2e). For control pups, the rate of self-initiated provisioning differed between treatments (REML: Χ22=10.40, p=0.006, n=135), and was similarly highest during the post-removal period (figure 2f). Control pups initiated successful provisions at a significantly higher rate than focal escorts (Wilcoxon signed-rank test: W=93.00, n=28, p=0.012; control-pup-initiated 0.97±0.18 provisions per hour, focal-escort-initiated 0.52±0.20).
Figure 2.
Rates of interactive behaviour between focal escorts and control pups during the pre-removal, post-removal and post-release treatments of focal pups. (a) For focal escorts, the rate of control-pup-initiated follows (pre-removal versus post-removal t=3.65, p=0.0012; pre-removal versus post-release t=0.23, p=0.82 and post-removal versus post-release t=3.34, p=0.0026). (b) For focal escorts, the rate of control-pup-initiated contact (pre-removal versus post-removal t=3.03, p=0.0056; pre-removal versus post-release t=0.44, p=0.66 and post-removal versus post-release t=2.53, p=0.018). (c) For control pups, the rate of focal-escort-initiated contact (pre-removal versus post-removal t=3.76, p<0.001; pre-removal versus post-release t=0.17, p=0.87 and post-removal versus post-release t=3.86, p<0.001). (d) For control pups, the rate of focal-escort-initiated grooming (pre-removal versus post-removal t=2.43, p=0.023; pre-removal versus post-release t=0.08, p=0.094 and post-removal versus post-release t=2.46, p=0.021). (e) For control pups, the rate of focal-escort-initiated provisioning (pre-removal versus post-removal t=1.86, p=0.075; pre-removal versus post-release t=0.48, p=0.64 and post-removal versus post-release t=2.29, p=0.031). (f) For control pups, the rate of control-pup-initiated provisioning (pre-removal versus post-removal t=3.20, p=0.0037; pre-removal versus post-release t=1.23, p=0.23 and post-removal versus post-release t=1.92, p=0.067).
(c) Is the increased association between focal escorts and control pups in the absence of the focal pups evenly distributed between control pups?
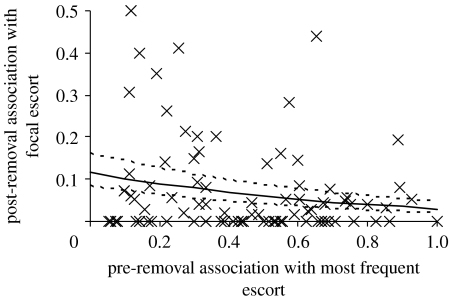
Pups with low or unstable association in the pre-removal period would be expected to be more likely (than pups with high or reliable association) to increase association with the focal escorts upon removal of focal pups. While there was no significant correlation between pre- and post-removal associations with focal escorts for control pups (IRREML: Χ12=0.28, p=0.60, n=91), control pups with relatively unstable associations (low escort fidelity) during the pre-removal period were more likely (than those with a relatively stable association) to associate with a vacant focal escort during the post-removal period (figure 3). Association index with a focal escort during the post-removal period was significantly negatively correlated with association index with a pup's most frequent escort during the pre-removal period (IRREML: Χ12=4.30, p=0.038, n=91).
Figure 3.
Relationship between each control pup's pre-removal association (proportion of scans within 10 cm) with its most frequent escort and its post-removal association with focal escorts (where pre- and post-removal periods refer to removal of the focal pups).
It is likely that these low association control pups increase interaction with vacant focal escorts either because they are sampling potential escorts and can access the vacant focal escort more, or because they increase interaction with the focal escort because it is a vacant ‘good’ carer, i.e. the pup shows a preference. At least some pups employ the latter strategy, with a control pup forming a notable association (control pup post-removal association index with focal escort exceeding 0.17, the minimum focal pup pre-removal association index) in 31% (21/47) of trials.
(d) Do removed pups re-establish association with their original escort upon reintroduction?
On reintroduction of the removed focal pup, it usually resumed strong association with its original escort, the focal pup post-release association index exceeding 0.17 in 83% (38/46) of trials. However, a control pup maintained principal access to the focal escort (monopolizing the escort, with an association index exceeding 0.17) into the post-release period in 15% (7/46) of trials. The control pup was significantly more likely to form a post-release association with an escort when it had formed a post-removal association with the same escort (Χ12=4.03, p=0.045). In these instances, the control pup often defended its new escort (the focal escort) from approach by the released focal pup. Aggressive interaction was noted between released focal pup and a control pup during the post-release period in 19% (8/43) of trials. There was a tendency for those trials with occurrence of post-release aggression to occur where a control pup had formed a notable post-removal association with the vacant focal escort (Χ12=3.78, p=0.052).
(e) The role of pup aggression in escort monopolization
The reason for the increased association and interaction between focal escorts and control pups during the post-removal period was the release of the latter from aggressive competitive exclusion by focal pups. When present, focal pups sustain relatively high rates of escort defence-motivated aggression against control pups and win substantially more aggressive interactions. There were 152 records of focal pup to control pup aggression (chasing, snapping, lunging or wrestling) with the focal escort the cause. By far, the majority of such aggressive events were in defence of their escort (91%), and the focal pup won 95% of all aggressive encounters (table 1). By contrast, there were only 40 records of control pup to focal pup aggression with the focal escort the cause (table 1). Notably, 32 of these events occurred during the post-release period, with the majority of these (19) occurring in cases where a control pup had formed an association with a focal escort in the absence of the focal pup, and a conflict of ownership resulted during the post-release period. The majority of cases where the control pup won the aggressive encounter occurred where a control pup had formed an association with the focal escort: 14 of the 21 wins by control pups during the post-release period.
Table 1.
Count of escort-motivated aggression between focal and control pups. Total duration of observations: 312 hours, 42 min.
| aggression type | result | count | percentage |
|---|---|---|---|
| focal-pup-initiated | |||
| attack | win | 11 | 7.3 |
| attack | draw | 2 | 1.3 |
| defence | win | 133 | 87.5 |
| defence | draw | 3 | 2 |
| defence | loss | 2 | 1.3 |
| unknown | win | 1 | 0.6 |
| total | 152 | 100 | |
| control-pup-initiated | |||
| attack | win | 8 | 20 |
| attack | draw | 1 | 2.5 |
| attack | loss | 8 | 20 |
| defence | win | 19 | 47.5 |
| defence | draw | 3 | 7.5 |
| defence | loss | 1 | 2.5 |
| total | 40 | 100 | |
For focal escort-motivated aggression, focal pups were responsible for initiating a greater rate of aggressive events towards control pups than vice versa (Wilcoxon signed-rank test: W=35.00, n=30, p<0.001; focal pup-initiated aggression rate 2.00±0.51 aggressive behaviours per hour, control-pup-initiated aggression rate 1.13±0.43). Overall, focal pups won a greater proportion of focal escort-motivated aggressive events against control pups than vice versa (80% versus 15%, Χ12=85.4, p<0.0001). Overall, pup–pup focal escort-motivated aggression rates did not differ significantly between pre-removal and post-release periods (REML: Χ12=0.36, p=0.55, n=89; pre-removal aggression rate 0.89±0.90 aggressive behaviours per hour, post-release aggression rate 1.37±0.91).
4. Discussion
The presence of a pup in stable association with an escorting adult reduces the ability of other pups to attain close proximity to and interact with the escort. It is aggression by the associating pup that prevents other pups getting too close to their escort. If an escort becomes vacant (e.g. due to temporary removal of its associated pup), there is an increased likelihood of interaction and association with other pups, in particular with pups that lack a stable escort themselves. These ‘drifters’ may have most to gain by initiating association with vacant escorts. It is these pups that are the principal driver of the increased association and interaction between focal escorts and control pups upon removal of focal pups.
Upon removal of focal pups, the control pups substantially increased rates of active following of the focal escorts; by contrast, escorts almost never follow pups. In addition, control pups increased the rate at which they contacted focal escorts. By reducing proximity to focal escorts, control pups also receive a greater frequency of focal-escort-initiated behaviours, e.g. contacts, grooms and provisions. This demonstrates that focal escorts are willing to provide active care to other pups in the communal litter and suggests that the normal restriction of care to an individual pup is driven by that pup (maintaining presence) and not by the escort. Nevertheless, research also indicates that escorts can recognize and exhibit preferential response towards the pup with which they normally associate (Gilchrist et al. 2008; Muller & Manser 2008).
In a natural situation, each escorted pup is dominant in interactions with other pups with regard to access to its escort (Gilchrist 2004). Similarly, in this study, focal pups were dominant to control pups prior to removal. In addition, on some of the occasions when an escorted focal pup was temporarily removed, a control pup formed an association with the vacated escort, and the new pup actively and successfully defended the escort on reintroduction of the original escorted pup. These observations support the competitive exclusion principle—that the presence of a dominant pup with an escort actively prevents other pups approaching or accessing the defended escort. It also supports an ownership principle, when an individual perceives itself to be the owner of a resource, it behaves as dominant in interactions with others (Tobias 1997).
By actively maintaining association with an adult escort, a pup increases the rate at which it receives provisions, and probably increases its probability of evading predation, with long-term fitness consequences. Escorts contact, shelter, groom, play with, protect and provision pups (Gilchrist 2004). Pups maximize the care they receive by maintaining close proximity to an adult (their escort). In the banded mongoose, like many altricial bird species (Malacarne et al. 1994; Kacelnik et al. 1995; Cotton et al. 1996), carers tend to feed the closest young (Gilchrist 2004). By actively defending access to their escort, each pup gains primary access to the resources provided by the carer, in particular food items. Escorted pups are fed more food items, the majority of which come from their escort (Gilchrist 2004; Hodge 2005; Bell 2007). A pup receives twice as many provisions when it initiates provision by approaching its escort, as by relying upon escort-initiated provisioning.
An escort is therefore a resource worth defending. Pups with stable association are generally heavier, have higher survival (probably due to decreased predation), and probably experience earlier reproductive maturation (Gilchrist 2004; Hodge 2005). It is likely that, as for other species, early nutritional provision influences survival and lifetime reproductive success (Lindström 1999; Lummaa & Clutton-Brock 2002; Russell et al. 2007), with dominance hierarchies developed during the period of dependence possibly correlated with status or competitive ability later in life (Boag & Alway 1980; Taylor 1980; Rajecki et al. 1981). In addition, the system of exclusive territoriality among littermates also probably minimizes costly competition between pups for access to food items (Bell 2007).
By using temporary removal of pups, this study demonstrates that the presence of specific pups prevents other pups from associating and interacting with their preferred escort. Each pup forms an exclusive mobile territory around its carer, which it defends versus approach by other pups. This system is akin to mate guarding in adults of many species (Birkhead & Møller 1998). However, it differs fundamentally from the systems of care of young in other species. For example, altricial bird chicks defend a static position within the nest (Kacelnik et al. 1995), domestic piglets (Sus scrofa) compete for access to and ownership of a fixed resource (teats; De Passile et al. 1988) and spotted hyena (Crocuta crocuta) pups display a consistent individual-based dominance hierarchy (Smale et al. 1999). Competition between litter- or broodmates has been shown to influence the distribution of provisioning in a variety of altricial bird species (e.g. McRae et al. 1993; Tanner et al. 2008). However, in these cases, the chicks defend an optimal position in the nest rather than following and defending an unpredictable, mobile provisioner. To accomplish the latter, the pups must be capable of individual recognition between adults in their group (Muller & Manser 2008).
In another cooperative breeding mongoose species, the meerkat (Suricata suricatta), pups are provisioned by numerous adults; principally non-breeding helpers. However, meerkat pups do not form exclusive stable associations with adults (Brotherton et al. 2001; Hodge et al. 2007). Nevertheless, meerkat pups often defend access to the nearest helper and, similar to banded mongoose pups, ownership usually equates to dominance in aggressive interactions (Hodge et al. 2007). Why then do banded mongoose pups form stable associations, while meerkat pups employ a switching strategy? It may be that an individual banded mongoose can provision at a sufficiently high rate to supply a pup, whereas a meerkat helper cannot (Gilchrist 2001, 2004), or that switching enables each meerkat pup to get more food than staying with a single helper if there are many helpers present (Hodge et al. 2007). However, banded mongoose pups do not appear to switch more when the ratio of adults to pups is high (J. S. Gilchrist 1997–2000, personal observation).
In a recent paper, Bell (2007) highlights how begging by multiple pups can benefit the individual pup within a communal litter. My findings suggest that a pup benefits from other callers (nearby) only as long as the littermates are not so close as to receive food from the pup's escort that it would otherwise receive.
While communal littermates will usually not all be the offspring of a single mother or father, the high intrasexual relatedness within breeders in banded mongoose groups means it is likely that pups within a communal litter are closely related. As such, at least some pup–pup competition represents a form of sibling rivalry (Mock & Parker 1997; Slagsvold 1997). Competitive interactions between pups within a communal litter mediate the ability of adults to make decisions on the distribution of pup care (as within bird broods; Kacelnik et al. 1995). An adult may therefore be unable to provide care to those pups that would afford it the greatest fitness gain. Within cooperative breeding species, parents and helpers can provide biased care to young, not only due to preference but due to the competitive actions of the young in the brood or litter. This has important implications for studies evaluating the distribution of care from carers to young in cooperative breeding species. In addition, this study presents further evidence of the intense competition that can occur between relatives and within cooperative breeding societies—conflict can be as rife as cooperation.
Acknowledgments
All procedures were licensed by the Uganda Wildlife Authority and the Uganda National Council for Science and Technology. The study followed the code of ethics and guidelines outlined by the Association for the Study of Animal Behaviour and the Animal Behaviour Society (ASAB 2006).
I thank Francis Mwanguhya, Emily Otali and Solomon Kyabulima for their assistance with data collection; Professor Tim Clutton-Brock the Department of Zoology, University of Cambridge for logistical support; the Uganda Wildlife Authority and Uganda Institute for Science and Technology for research permission and Mike Cant, Alison Craig and Giacomo Tavecchia and the reviewers for commenting on the manuscript. This study was funded by the British Ecological Society.
Supplementary Material
Supplementary methods
References
- ASAB. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 2006;71:245–253. doi: 10.1006/anbe.1999.1349. doi:10.1016/j.anbehav.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Bell M.B.V. Cooperative begging in banded mongoose pups. Curr. Biol. 2007;17:717–721. doi: 10.1016/j.cub.2007.03.015. doi:10.1016/j.cub.2007.03.015 [DOI] [PubMed] [Google Scholar]
- Birkhead T.R, Møller A.P. Academic Press; London, UK: 1998. Sperm competition and sexual selection. [Google Scholar]
- Boag D.A, Alway J.H. Effect of social-environment within the brood on dominance rank in gallinaceous birds (Tetraonidae and Phasianidae) Can. J. Zool. Rev. Can. Zool. 1980;58:44–49. [Google Scholar]
- Brotherton P.N.M, Clutton-Brock T.H, O'Riain M.J, Gaynor D, Sharpe L, Kansky R, McIlrath G.M. Offspring food allocation by parents and helpers in a cooperative mammal. Behav. Ecol. 2001;12:590–599. doi:10.1093/beheco/12.5.590 [Google Scholar]
- Brown H, Prescott R. Wiley; Chichester, UK: 1999. Applied mixed models in medicine. [Google Scholar]
- Bruce N.W, Wellstead J.R. Spacing of fetuses and local competition in strains of mice with large, medium and small litters. J. Reprod. Fertil. 1992;95:783–789. doi: 10.1530/jrf.0.0950783. [DOI] [PubMed] [Google Scholar]
- Cant, M. A. 1998 Communal breeding in banded mongooses and the theory of reproductive skew. PhD thesis, University of Cambridge, Cambridge, United Kingdom.
- Cant M.A. Social control of reproduction in banded mongooses. Anim. Behav. 2000;59:147–158. doi: 10.1006/anbe.1999.1279. doi:10.1006/anbe.1999.1279 [DOI] [PubMed] [Google Scholar]
- Cant M.A, Otali E, Mwanguhya F. Eviction and dispersal in co-operatively breeding banded mongooses (Mungos mungo) J. Zool. 2001;254:155–162. doi:10.1017/S0952836901000668 [Google Scholar]
- Clutton-Brock T. Behavioral ecology—breeding together: kin selection and mutualism in cooperative vertebrates. Science. 2002;296:69–72. doi: 10.1126/science.296.5565.69. doi:10.1126/science.296.5565.69 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H, Albon S.D, Guinness F.E. Competition between female relatives in a matrilocal mammal. Nature. 1982;300:178–180. doi:10.1038/300178a0 [Google Scholar]
- Cockburn A. Evolution of helping behavior in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 1998;29:141–177. doi:10.1146/annurev.ecolsys.29.1.141 [Google Scholar]
- Cotton P.A, Kacelnik A, Wright J. Chick begging as a signal: are nestlings honest? Behav. Ecol. 1996;7:178–182. doi:10.1093/beheco/7.2.178 [Google Scholar]
- De Passile A.M.B, Rushen J, Hartsock T.G. Ontogeny of teat fidelity in pigs and its relation to competition at suckling. Can. J. Anim. Sci. 1988;68:325–338. [Google Scholar]
- Drummond H. Dominance in vertebrate broods and litters. Q. Rev. Biol. 2006;81:3–32. doi: 10.1086/503922. doi:10.1086/503922 [DOI] [PubMed] [Google Scholar]
- Gilchrist, J. S. 2001 Reproduction and pup care in the communal breeding banded mongoose. PhD thesis, University of Cambridge, Cambridge, United Kingdom.
- Gilchrist J.S. Pup escorting in the communal breeding banded mongoose: behavior, benefits, and maintenance. Behav. Ecol. 2004;15:952–960. doi:10.1093/beheco/arh071 [Google Scholar]
- Gilchrist J.S. Reproductive success in a low skew, communal breeding mammal: the banded mongoose, Mungos mungo. Behav. Ecol. Sociobiol. 2006;60:854–863. doi:10.1007/s00265-006-0229-6 [Google Scholar]
- Gilchrist J.S, Russell A.F. Who cares? Individual contributions to pup care by breeders vs non-breeders in the cooperatively breeding banded mongoose (Mungos mungo) Behav. Ecol. Sociobiol. 2007;61:1053–1060. doi:10.1007/s00265-006-0338-2 [Google Scholar]
- Gilchrist J.S, Otali E, Mwanguhya F. Why breed communally? Factors affecting fecundity in a communal breeding mammal: the banded mongoose (Mungos mungo) Behav. Ecol. Sociobiol. 2004;57:119–131. doi:10.1007/s00265-004-0837-y [Google Scholar]
- Gilchrist J.S, Otali E.O, Mwanguhya F. Caregivers recognize and bias response towards individual young in a cooperative breeding mammal, the banded mongoose. J. Zool. 2008;275:41–46. doi:10.1111/j.1469-7998.2007.00405.x [Google Scholar]
- Hamilton W.D. The genetical evolution of social behaviour. J. Theor. Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. doi:10.1016/0022-5193(64)90038-4 [DOI] [PubMed] [Google Scholar]
- Hodge S.J. Helpers benefit offspring in both the short and long-term in the cooperatively breeding banded mongoose. Proc. R. Soc. B. 2005;272:2479–2484. doi: 10.1098/rspb.2005.3255. doi:10.1098/rspb.2005.3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge S.J, Flower T.P, Clutton-Brock T.H. Offspring competition and helper associations in cooperative meerkats. Anim. Behav. 2007;74:957–964. doi:10.1016/j.anbehav.2006.10.029 [Google Scholar]
- Hudson R, Trillmich F. Sibling competition and cooperation in mammals: challenges, developments and prospects. Behav. Ecol. Sociobiol. 2007;62:299–307. doi:10.1007/s00265-007-0417-z [Google Scholar]
- Jennions M.D, Macdonald D.W. Cooperative breeding in mammals. Trends Ecol. Evol. 1994;9:89–93. doi: 10.1016/0169-5347(94)90202-X. doi:10.1016/0169-5347(94)90202-X [DOI] [PubMed] [Google Scholar]
- Kacelnik A, Cotton P.A, Stirling L, Wright J. Food allocation among nestling starlings—sibling competition and the scope of parental choice. Proc. R. Soc. B. 1995;259:259–263. doi:10.1098/rspb.1995.0038 [Google Scholar]
- Koenig W.D, Dickinson J.L, editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2004. [Google Scholar]
- Lindström J. Early development and fitness in birds and mammals. Trends Ecol. Evol. 1999;14:34–36. doi: 10.1016/s0169-5347(99)01639-0. doi:10.1016/S0169-5347(99)01639-0 [DOI] [PubMed] [Google Scholar]
- Lummaa V, Clutton-Brock T. Early development, survival and reproduction in humans. Trends Ecol. Evol. 2002;17:141–147. doi:10.1016/S0169-5347(01)02414-4 [Google Scholar]
- Malacarne G, Cucco M, Bertolo E. Sibling competition in asynchronously hatched broods of the pallid-swift (Apus pallidus) Ethol. Ecol. Evol. 1994;6:293–300. [Google Scholar]
- McRae S.B, Weatherhead P.J, Montgomerie R. American robin nestlings compete by jockeying for position. Behav. Ecol. Sociobiol. 1993;33:101–106. doi:10.1007/BF00171661 [Google Scholar]
- Mock D.W, Parker G.A. Oxford University Press; Oxford, UK: 1997. The evolution of sibling rivalry. [Google Scholar]
- Mock D.W, Parker G.A. Siblicide, family conflict and the evolutionary limits of selfishness. Anim. Behav. 1998;56:1–10. doi: 10.1006/anbe.1998.0842. doi:10.1006/anbe.1998.0842 [DOI] [PubMed] [Google Scholar]
- Muller C.A, Manser M.B. Mutual recognition of pups and providers in the cooperatively breeding banded mongoose. Anim. Behav. 2008;75:1683–1692. doi:10.1016/j.anbehav.2007.10.021 [Google Scholar]
- Ostreiher R. Food division in the Arabian babbler nest: adult choice or nestling competition? Behav. Ecol. 1997;8:233–238. doi:10.1093/beheco/8.2.233 [Google Scholar]
- Rajecki D.W, Nerenz D.R, Hoff S.J, Newman E.R, Volbrecht V.J. Early development of aggression in chickens—the relative importance of pecking and leaping. Behav. Process. 1981;6:239–248. doi: 10.1016/0376-6357(81)90003-6. doi:10.1016/0376-6357(81)90003-6 [DOI] [PubMed] [Google Scholar]
- Russell A.F, Young A.J, Spong G, Jordan N.R, Clutton-Brock T.H. Helpers increase the reproductive potential of offspring in cooperative meerkats. Proc. R. Soc. B. 2007;274:513–520. doi: 10.1098/rspb.2006.3698. doi:10.1098/rspb.2006.3698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall R. Estimation of generalised linear models with random effects. Biometrika. 1991;78:719–727. doi:10.1093/biomet/78.4.719 [Google Scholar]
- Slagsvold T. Brood division in birds in relation to offspring size: sibling rivalry and parental control. Anim. Behav. 1997;54:1357–1368. doi: 10.1006/anbe.1997.0530. doi:10.1006/anbe.1997.0530 [DOI] [PubMed] [Google Scholar]
- Smale L, Holekamp K.E, White P.A. Siblicide revisited in the spotted hyaena: does it conform to obligate or facultative models? Anim. Behav. 1999;58:545–551. doi: 10.1006/anbe.1999.1207. doi:10.1006/anbe.1999.1207 [DOI] [PubMed] [Google Scholar]
- Solomon N.G, French J.A, editors. Cooperative breeding in mammals. Cambridge University Press; Cambridge, UK: 1997. [Google Scholar]
- Stacey P.B, Koenig W.D, editors. Cooperative breeding in birds: long-term studies of ecology and behavior. Cambridge University Press; Cambridge, UK: 1990. [Google Scholar]
- Tanner M, Koelliker M, Richner H. Differential food allocation by male and female great tit, Parus major, parents: are parents or offspring in control? Anim. Behav. 2008;75:1563–1569. doi:10.1016/j.anbehav.2007.10.010 [Google Scholar]
- Taylor G.T. Fighting in juvenile rats and the ontogeny of agonistic behavior. J. Comp. Physiol. Psychol. 1980;94:953–961. doi:10.1037/h0077816 [Google Scholar]
- Tobias J. Asymmetric territorial contests in the European robin: the role of settlement costs. Anim. Behav. 1997;54:9–21. doi: 10.1006/anbe.1996.0383. doi:10.1006/anbe.1996.0383 [DOI] [PubMed] [Google Scholar]
- van Schaik C.P, van Noordwijk M.A. Scramble and contest in feeding competition among female long-tailed macaques (Macaca fascicularis) Behaviour. 1988;105:77–98. doi:10.1163/156853988X00458 [Google Scholar]
- West S.A, Murray M.G, Machado C.A, Griffin A.S, Herre E.A. Testing Hamilton's rule with competition between relatives. Nature. 2001;409:510–513. doi: 10.1038/35054057. doi:10.1038/35054057 [DOI] [PubMed] [Google Scholar]
- West S.A, Pen I, Griffin A.S. Conflict and cooperation—cooperation and competition between relatives. Science. 2002;296:72–75. doi: 10.1126/science.1065507. doi:10.1126/science.1065507 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods