Abstract
Animal social networks can be extremely complex and are characterized by highly non-random interactions between group members. However, very little is known about the underlying factors affecting interaction preferences, and hence network structure. One possibility is that behavioural differences between individuals, such as how bold or shy they are, can affect the frequency and distribution of their interactions within a network. We tested this using individually marked three-spined sticklebacks (Gasterosteus aculeatus), and found that bold individuals had fewer overall interactions than shy fish, but tended to distribute their interactions more evenly across all group members. Shy fish, on the other hand, tended to associate preferentially with a small number of other group members, leading to a highly skewed distribution of interactions. This was mediated by the reduced tendency of shy fish to move to a new location within the tank when they were interacting with another individual; bold fish showed no such tendency and were equally likely to move irrespective of whether they were interacting or not. The results show that animal social network structure can be affected by the behavioural composition of group members and have important implications for understanding the spread of information and disease in social groups.
Keywords: social network, behavioural syndrome, personality, temperament, animal group
1. Introduction
Animal social networks can be highly complex and connections between individuals are often non-random in distribution (Lusseau 2003; Croft et al. 2004, 2005; McDonald 2007). Even within large, interconnected groups, a highly structured network of interactions is evident, with consistent inter-individual variation in the number of social associations (Croft et al. 2005). These interactions are likely to play a key role in the transfer of information within the network, while significantly affecting an individual's access to resources and the probability of predation (Watts & Strogatz 1998). However, most investigations of social organization have been primarily concerned with interactions between pairs of individuals and so have provided an incomplete picture of the whole network of interactions that occur (Whitehead & Dufault 1999; Croft et al. 2005). As a consequence, very little is known about the factors influencing social network structure or the causes and consequences of the underlying social interactions. In particular, few studies have considered how individual variation between group members can affect network structure (Croft et al. 2005).
Studies on group-joining preferences have shown that individuals frequently assort by phenotypic characters, including body length, species, colour and parasite load (reviewed by Krause et al. 2000). Such assortative interactions may confer important adaptive benefits, including reduced predation risk through predator confusion and increased foraging efficiency, and are therefore likely to be important determinants of group structure (Krause & Ruxton 2002). However, the structure of groups may also be mediated by behavioural rather than morphological differences between individuals. One of the most commonly studied behaviours is boldness (Bell 2007). Where an individual lies along the bold–shy continuum defines their reaction to risky situations (Réale et al. 2007), and is considered to be a major and relatively stable component of behavioural variation (Wilson et al. 1994). Studies in both fishes and birds have demonstrated that bold or more assertive individuals tend to more readily approach novel objects (e.g. Verbeek et al. 1996; Budaev et al. 1999) and return more quickly to a food resource after having been startled (van Oers et al. 2004; Ward et al. 2004). Furthermore, boldness has been shown to covary with other behavioural traits such as exploratory behaviour, activity level and aggression (Verbeek et al. 1994, 1999; Marchetti & Drent 2000; Fraser et al. 2001; Dingemanse et al. 2003; Krackow 2003); although the extent to which behaviours are independent or form part of a behavioural syndrome (a suite of correlated behaviours that defines an individuals personality or temperament; Réale et al. 2007) is a controversial and important issue, since these relationships are not fixed and may vary between populations (Dingemanse et al. 2007).
An individual's behavioural phenotype appears to affect the various ways it interacts with its environment, whether in its reactions with predators, food sources and habitat, or in its social or sexual interactions with conspecifics (Réale et al. 2007). As a result, one can hypothesize that these behavioural differences could affect an individual's interactions with other members of a social group: for example, more active individuals may have a higher frequency of chance encounters with other individuals than less-active members of the group, while particularly aggressive individuals may be avoided. Indeed, empirical work on three-spined stickleback (Gasterosteus aculeatus) has shown that bold fish tend to adopt positions near the front of shoals (Ward et al. 2004) and associate with conspecifics that interact with a similar number of group members to themselves (Croft et al. 2005). Consequently, an individual's position along the bold–shy axis may play a key role in determining the extent to which an individual associates with conspecifics, and the structure of the network they occupy.
In the current study, we examined how the relative composition of bold and shy behavioural phenotypes within a social network affected the frequency and distribution of individual interactions, using a laboratory-based population of three-spined sticklebacks. Sticklebacks are a social species that are known to exhibit complex social interactions (Croft et al. 2005). Previous work has tended to focus on either shoal selection (e.g. Krause 1993; Frommen et al. 2007) or inferred intra-group associations from persistent occurrences of known individuals within a shoal (e.g. Croft et al. 2004, 2005). Here we adopted a different approach in which we tracked marked individuals over time in order to monitor interactions among group members. This technique allowed us to collect uniquely fine-scale information on individual interactions, and so provided a more complete understanding of network structure.
2. Material and methods
Three-spined sticklebacks were captured with dip nets from the River Endrick, Scotland (56°04′ N, 4°23′ W) during January 2007. On return to the laboratory, fish were randomly allocated to one of six holding tanks and held at 10±1°C under a 10 L : 14 D photoperiod (characteristic of winter conditions) to prevent the onset of sexual maturation. Since it is impossible to non-invasively sex sticklebacks outside the breeding season, all groups were assumed to be mixed sex. Group sex ratio was unlikely to impact on our results, however, because during the winter sticklebacks of both sexes are non-territorial and actively shoal together (Wootton 1984). Fish were held for at least two weeks under these conditions before the experiments began.
(a) Experimental design
We generated three types of network that differed in their relative composition of bold and shy phenotypes: (i) all bold, (ii) mixed (composed of randomly selected behavioural phenotypes), and (iii) all shy, with multiple replicates of each. Each network was composed of six fish, one taken from each of the six holding tanks in order to minimize the chance that our results would be influenced by prior association preferences. To avoid potential selection bias when assigning fish to networks (e.g. bold individuals being more likely to be captured first), we adopted the following randomization procedure. To assign individuals to mixed networks, all the fish in a given holding tank were caught, held individually and sequentially assigned a number; a random number generator was then used to select a single fish. When a fish had been selected from each of the holding tanks, they were individually assayed for boldness using the latency to feed following a mild startle (Réale et al. 2007) prior to the onset of the experiment. In order to select fish for bold or shy networks, we randomly selected several fish from each holding tank (using random numbers as above) and assayed them sequentially for boldness. The first fish from each tank to exceed the threshold criterion, which for bold fish was defined as having a latency to feed of less than 50 s and for shy fish a latency to feed greater than 150 s (based on the distribution of phenotypes in our population), was assigned to the relevant network.
Following boldness measurements, fish were measured (standard length, ±0.1 mm) under anaesthetic (benzocaine) and assigned a tag bearing a unique identifier. Tags (7 mm in diameter and weighing approx. 12 mg) were made from a thin disc of transparent acetate onto which was glued a white plastic symbol or letter. These were then placed over the posterior most dorsal spine of the anaesthetized fish so that the identifier faced upwards, and held in place with a small quantity of cyanoacetate adhesive that adhered the tag to the spine. Fish were then allowed to recover in individual tanks for at least 24 hours, during which time they showed no signs of distress and behaved normally.
When all fish had been tagged, they were fed to satiation (to standardize initial hunger levels) and released simultaneously into an experimental arena. The arena consisted of a 50×30 cm2 tank, with a water height of 15 cm. The floor and sides of the tank were painted black, and the whole arena was shaded with thin black plastic sheets in order to minimize stress and disturbance, and encourage natural behaviour. Food (bloodworm) was available throughout the experiment, scattered at random across the floor of the experimental arena. Using an infrared-sensitive CCD camera (Monacor TVCCD-624ECOL, Bremen, Germany) located directly above the arena, we captured still images of the fish at regular intervals over the subsequent 24 hours. Mixed networks were photographed at 1 min intervals. However, because initial analyses on the mixed networks found a strong relationship between the frequency of observed dyadic interactions recorded at 1 min intervals and those recorded at 5 min intervals (r=0.94, n=60, p<0.001), we opted to photograph bold and shy networks at 5 min intervals instead. Additional illumination was provided by infrared light-emitting diodes (peak emission at 875 nm; Loligo Systems, Tjele Denmark), which most fishes (including sticklebacks) are unable to perceive (Batty 1983; Higgs & Fuiman 1996). This allowed us to locate fish in low light levels during the day and also at night. The white identifiers on each fish's tag strongly reflected infrared light, allowing us to manually extract information on the two-dimensional spatial position (i.e. x- and y-coordinates) of each individual in each image; and hence individual interactions and movement over time. After the 24 hours observation period, we assayed each fish for boldness a second time in order to confirm repeatability of this behaviour over the time course of the experiment. Repeatability of boldness scores, measured as the coefficient of intraclass correlation (ρ; Sokal & Rohlf 1995, p. 213) from a hierarchical mixed model with individual nested within network id (both random effects), was very high (ρ=0.64, F1,99=103.75, p<0.001). We are thus confident that during the experiment fishes behaved in a consistently bold or shy manner.
Mixed networks were replicated a further nine times, using six different randomly selected fish each time. Bold and shy networks were each replicated five times. There was no difference in body size between fish allocated to bold, mixed or shy networks (F2,117=1.13, p=0.325) and no evidence that body size predicted individual boldness scores (F1,118=0.07, p=0.798).
(b) Boldness assay
Boldness was determined as the latency to feed following a mild startle, using the method described by Ward et al. (2004). Briefly, an aquarium tank was divided into two equal compartments (each measuring 33×18×19 cm, and filled with 10 cm water) by a one-way mirror. The rear compartment held a group of five non-experimental sticklebacks (mean±s.e. length: 46.4±0.4 mm), that acted as a companion group to reduce stress experienced by the focal fish. As a result of the one-way mirror, this group was unable to see into the front (experimental) compartment, although the rear compartment was visible from the front. The same companion group was used for all focal fish. A single focal fish, that had been starved for 24 hours in order to increase motivation to forage, was added to the front compartment and given 5 min to acclimatize, after which approximately six finely chopped bloodworms (Chironomus larvae) were pipetted into a Petri dish located at one end of the compartment. When the focal fish was observed to feed, a 50 g stainless steel bolt suspended by fishing line 20 cm above the centre of the front compartment was dropped, eliciting a fright response from the focal fish but not the stimulus group (which showed no behavioural reaction and continued to behave normally). Boldness was defined as the latency to resume feeding following the weight's release, with bolder individuals resuming feeding faster than shyer fish. If fishes had not fed after 3 min, the test was terminated and a recovery time of 180 s recorded for that trial (Ward et al. 2004). All fish were fed to satiation following the trials.
(c) Analysis
In each captured image, we determined the presence or absence of dyadic interactions between group members. An individual was assumed to be interacting with another individual if both fish were within one body length (i.e. less than 45 mm) of each other. This allowed us to build up a square interaction matrix for each network, where the value linking two individuals represented the number of frames per minute in which they were observed to be interacting. For each network, we then calculated the following metrics:
Strength. An individual's strength is the sum of all the interactions they had with all other individuals (Barthélemy et al. 2005). A high strength indicates that an individual interacted frequently with other fish in their network. The mean strength of each network is found by averaging over all individuals.
Clustering coefficient. We used the clustering coefficient developed for weighted networks by Zhang & Horvath (2005), which provides a measure of individual cliquishness. High values (the clustering coefficient is bounded by 0 and 1) indicate that an individual interacted with all other individuals with an approximately uniform frequency. Low values are indicative of highly non-uniform interactions, such as might occur if an individual interacted frequently with some group members but rarely with others. The mean clustering coefficient of a network is found by averaging over all individuals.
Activity. This was determined as the probability that an individual moved more than one body length (i.e. greater than 45 mm) between consecutive frames (i) when interacting with another individual (i.e. less than 45 mm from another fish) and (ii) when alone (i.e. no other fish within 45 mm).
Differences in the mean strength and the mean clustering coefficient between bold, mixed and shy networks were compared using ANOVAs, with post hoc pairwise comparisons made using Bonferroni-corrected two-sample t-tests. To look at individual variation in strength and clustering coefficient in relation to boldness, we focused on fishes in mixed networks. The relationships between boldness and each network metric were analysed using linear mixed models with network id as a random factor; however, because network metrics for individuals from the same network do not constitute statistically independent samples, p-values were based on the proportion of 106 Monte Carlo simulations (in which individuals were randomized within networks) with test statistics greater than the observed test statistic. Activity was analysed using linear mixed models with the probability of moving either when interacting or not interacting as a repeated measure and network id as a random factor. Means are presented ±1 s.e. and n denotes the sample size (the number of fish).
3. Results
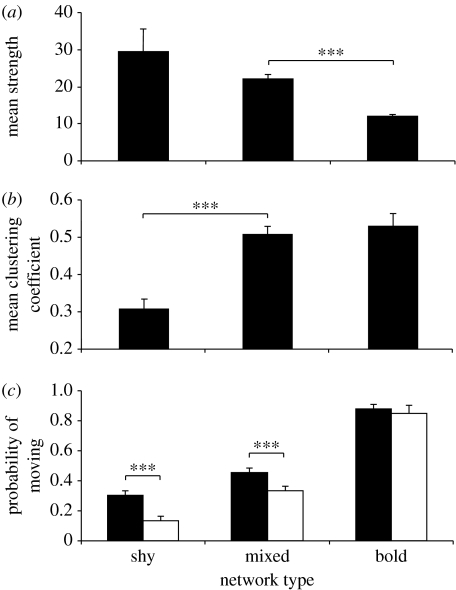
The composition of behavioural phenotypes within a network significantly affected both its mean strength (F2,17=6.91, p=0.006) and mean clustering coefficient (F2,17=15.48, p<0.001), such that as the proportion of shy fish in the network increased there was a concordant increase in the overall frequency of interactions within the network (figure 1a) but a decline in the uniformity of these interactions (figure 1b). Similarly, in mixed networks, variation in boldness significantly predicted an individual's strength (Monte Carlo test, p<0.001) and clustering coefficient (p<0.001), such that shy fish had a greater strength and a lower clustering coefficient than bolder individuals.
Figure 1.
Differences between networks composed of shy, mixed (i.e. randomly selected) and bold fish in terms of (a) mean strength, (b) mean clustering coefficient and (c) activity (the probability of moving when interacting with another individual (white bars) and when alone (black bars)). Bars denote mean±s.e. and asterisks indicate a significant difference between two groups; ***p<0.001. See text for full details.
There were also differences in activity between fish in bold, mixed and shy networks. Fish in networks composed entirely of bold fish were significantly more active on average than those in mixed or shy networks (F2,17=98.68, p<0.001; figure 1c) and, overall, fish were less active when interacting than when alone (F1,117=37.17, p<0.001). However, the significant interaction between network type (bold, mixed or shy) and interaction status (F2,117=3.53, p=0.033) highlights the fact that while fish in bold networks moved with an approximately equal probability whether they were interacting or not (F1,29=0.27, p=0.605), in networks of mixed or shy fish interacting individuals were significantly less likely to move than isolated fish (mixed: F1,29=34.27, p<0.001; shy: F1,29=76.32, p<0.001). Similarly, within mixed networks, there was a highly significant relationship between boldness and activity (F1,49=317.02, p<0.001), with bolder fish more likely to have moved between successive images than shy fish. There was also a significant interaction between boldness and interaction status (F1,49=4.18, p=0.046), suggesting that while bold fish were just as likely to move when interacting with another fish as when alone, shy fish were less likely to move when interacting. There was no evidence, however, that activity levels differed between bold fish in mixed networks and fish in all-bold networks (not-interacting: F1,25=0.79, p=0.383; interacting: F1,25=0.33, p=0.571), and similar results were found for shy fish (not-interacting: F1,32=0.96, p=0.334; interacting: F1,32=1.06, p=0.311).
4. Discussion
The results of this study show how the frequency and distribution of interactions within an animal social network can be affected by individual-level differences in behaviour between group members and, hence, how the flow of information and/or disease in animal social groups may be affected by the behavioural composition of individuals within that group. In particular, we show that networks composed of bold individuals are characterized by a low overall interaction frequency (i.e. a low mean strength) and uniform distribution of interactions (i.e. a high mean clustering coefficient). By contrast, fish in ‘shy’ networks tend to form long-lasting associations with one or two other individuals, leading to a highly non-uniform interaction distribution (i.e. a low mean clustering coefficient). These network-level results are mirrored in the individual-levels data from mixed networks, where shy fish had a greater strength but lower clustering coefficient than bold fish. Analysis of the activity data suggests that apparently complex interaction patterns can arise simply from differences in the tendency of bold and shy individuals to change location when they are interacting with another individual. Bold fish had approximately the same probability of moving irrespective of whether they were interacting or not, while shy fish showed a much lower probability (approaching zero) of moving when in association with one or more fishes. This is likely to lead to pairs of shy fish forming long-lasting associations (accounting for the highly non-uniform interaction distribution of shy fish) and thus having a greater overall interaction frequency (explaining the higher strength observed in shy fish). There was no evidence, however, that the composition of behavioural phenotypes within the network affected activity levels, since bold and shy fish in mixed networks showed comparable levels of activity to their counterparts in bold and shy networks. This suggests that in this, as in other stickleback populations (e.g. Ward et al. 2004), boldness and activity are correlated traits. Overall, these finding are broadly consistent with evidence from human social networks, where both similarity of personality (particularly the degree of extroversion) and proximity to others are key factors in the formation of network ties (e.g. Ying 2002).
Previous work in sticklebacks that considered the co-occurrence of individuals within a shoal as an interaction (Croft et al. 2005) has shown that repeated interactions between pairs of individuals occur more often than would be expected by chance. Our data suggest that a passive mechanism could generate assortative interactions without the need to invoke complex cognitive recognition mechanisms or any explicit preference mechanisms, although other mechanisms may also be important. For example, such association patterns could arise through preferences for similar spatial locations (Ward et al. 2007) or by active choice (Croft et al. 2004); perhaps individuals avoid associating with bold fish because they show increased levels of aggression, or paired shy fish perform better at certain tasks, such as foraging or predator avoidance. It is also conceivable that interactions were mediated by sex rather than boldness per se, if there were sex differences in boldness. However, although the sex ratio of fish in our networks was unknown, previous work on sticklebacks has failed to find any differences in boldness between the sexes (Ward et al. 2004) and so we consider this unlikely. In addition, because fish were wild caught we cannot completely rule out the possibility that individuals were preferentially associating with related conspecifics or individuals with which they had prior social experience. However, because kin-based shoaling preferences in sticklebacks are known to be based on familiarity, rather than relatedness per se (Frommen et al. 2007), and are probably mediated by ephemeral habitat-specific chemical cues (Ward et al. 2007), this also seems unlikely. Finally, because behavioural phenotypes in sticklebacks are largely environmentally determined (Bakker 1994; Bell 2005), and social experience may shape behaviours (Hemelrijk & Wantia 2005), it is unclear whether individual phenotype affects network structure (as we have assumed), or the position an individual preferentially adopts in a network has determined their prior experiences; and hence boldness. For instance, individuals that adopt peripheral positions in a group may have different experiences (e.g. with predators) and therefore also differ in boldness when given a standardized test. Our data cannot differentiate between these alternatives, and so this remains an exciting avenue for future research.
While networks consisting of entirely bold or entirely shy individuals must be considered extremes, and such biased compositions of behavioural phenotypes are perhaps unlikely to occur in nature (although this has not been tested explicitly, and the general tendency to assort phenotypically (e.g. Krause et al. 2000; Krause & Ruxton 2002) in social groups might lead to such group compositions), our data provide compelling evidence that the behavioural composition of individuals within a network could have major implications for the spread of information or disease. For instance, in networks with a high proportion of shy individuals, the spread of information is likely to be slow and highly localized with many individuals failing to receive it; indeed, the spread of epidemics within such networks is predicted to be largely contained among a few individuals that are highly interconnected with one another (Newman 2003). As the proportion of bold individuals increases, this information is likely to spread far more rapidly and reach many more members of the group (e.g. Szendroi & Csányi 2004). The composition of behavioural phenotypes within a social group may also affect individual access to resources. Bold sticklebacks, for example, have been shown to have greater access to (high quality) food and a greater competitive advantage over shy fish (Bell & Stamps 2004; Webster et al. 2007), although competition may be high in groups composed of predominantly bold members. By contrast, less-active shy fish may encounter high-quality prey less frequently, but enjoy lower competition. There may therefore be differential growth and survival between individuals within a group as a function of both individual behavioural type and mean phenotype within the group. However, the long-term implications of behavioural composition and group structure on individual fitness and a group's success in foraging, for instance, are unknown, and elucidating the interactions between them provide a major challenge for the future.
Acknowledgments
This research adhered to the Association for the Study of Animal Behaviour Guidelines for the use of Animals in Research and was performed under licence from the UK Home Office.
We would like to thank G. Law for assistance with animal husbandry, N. Herbert and S. Reebs for advice on using the infrared-sensitive cameras and M. Webster and four anonymous referees for valuable comments on the manuscript.
References
- Bakker T.C.M. Genetic correlations and the control of behaviour, exemplified by aggressiveness in sticklebacks. Adv. Study Behav. 1994;23:135–171. doi:10.1016/S0065-3454(08)60353-8 [Google Scholar]
- Barthélemy M, Barrat A, Pastor-Satorras R, Vespignani A. Characterization and modeling of weighted networks. Physica A. 2005;346:34–43. doi:10.1016/j.physa.2004.08.047 [Google Scholar]
- Batty R.S. Observation of fish larvae in the dark with television and infrared illumination. Mar. Biol. 1983;75:105–107. doi:10.1007/BF00393061 [Google Scholar]
- Bell A.M. Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus) J. Evol. Biol. 2005;18:464–473. doi: 10.1111/j.1420-9101.2004.00817.x. doi:10.1111/j.1420-9101.2004.00817.x [DOI] [PubMed] [Google Scholar]
- Bell A.M. Future directions in behavioural syndromes research. Proc. R. Soc. B. 2007;274:755–761. doi: 10.1098/rspb.2006.0199. doi:10.1098/rspb.2006.0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A.M, Stamps J.A. Development of behavioural differences between individuals and populations of sticklebacks, Gasterosteus aculeatus. Anim. Behav. 2004;68:1339–1348. doi:10.1016/j.anbehav.2004.05.007 [Google Scholar]
- Budaev S.V, Zworykin D.D, Mochek A.D. Individual differences in parental care and behavioural profile in the convict cichlid: a correlation study. Anim. Behav. 1999;58:195–202. doi: 10.1006/anbe.1999.1124. doi:10.1006/anbe.1999.1124 [DOI] [PubMed] [Google Scholar]
- Croft D.P, Krause J, James R. Social networks in the guppy (Poecilia reticulata) Proc. R. Soc. B. 2004;271(Suppl.):S516–S519. doi: 10.1098/rsbl.2004.0206. doi:10.1098/rsbl.2004.0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft D.P, James R, Ward A.J.W, Botham M.S, Mawdsley D, Krause J. The structure of social networks and association patterns in fish. Oecologia. 2005;143:211–219. doi: 10.1007/s00442-004-1796-8. doi:10.1007/s00442-004-1796-8 [DOI] [PubMed] [Google Scholar]
- Dingemanse N.J, Both C, van Noordwijk A.J, Rutten A.L, Drent P.J. Natal dispersal and personalities in great tits (Parus major) Proc. R. Soc. B. 2003;270:741–747. doi: 10.1098/rspb.2002.2300. doi:10.1098/rspb.2002.2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N.J, Wright J, Kazem A.J.N, Thomas D.K, Hickling R, Dawnay N. Behavioural syndromes differ predictably between 12 populations of stickleback. J. Anim. Ecol. 2007;76:1128–1138. doi: 10.1111/j.1365-2656.2007.01284.x. doi:10.1111/j.1365-2656.2007.01284.x [DOI] [PubMed] [Google Scholar]
- Fraser D.F, Gilliam J.F, Daley M.J, Le A.N, Skalski G.T. Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration. Am. Nat. 2001;158:124–135. doi: 10.1086/321307. doi:10.1086/321307 [DOI] [PubMed] [Google Scholar]
- Frommen J.G, Luz C, Bakker T.C.M. Kin discrimination in sticklebacks is mediated by social learning rather than innate recognition. Ethology. 2007;113:276–282. doi:10.1111/j.1439-0310.2006.01316.x [Google Scholar]
- Hemelrijk C.K, Wantia J. Individual variation by self-organisation. Neurosci. Biobehav. Rev. 2005;29:125–136. doi: 10.1016/j.neubiorev.2004.07.003. doi:10.1016/j.neubiorev.2004.07.003 [DOI] [PubMed] [Google Scholar]
- Higgs D.M, Fuiman L.A. Ontogeny of visual and mechanosensory structure and function in Atlantic menhaden Brevoortia tyrannus. Can. J. Fish Aquat. Sci. 1996;199:2619–2629. doi: 10.1242/jeb.199.12.2619. [DOI] [PubMed] [Google Scholar]
- Krackow S. Motivational and heritable determinants of dispersal latency in wild male house mice (Mus musculus musculus) Ethology. 2003;109:671–689. doi:10.1046/j.1439-0310.2003.00913.x [Google Scholar]
- Krause J. The influence of hunger on shoal size choice by three-spined sticklebacks, Gasterosteus aculeatus. J. Fish Biol. 1993;43:775–780. doi:10.1111/j.1095-8649.1993.tb01154.x [Google Scholar]
- Krause J, Ruxton G.D. Oxford University Press; Oxford, UK: 2002. Living in groups. [Google Scholar]
- Krause J, Butlin R, Peuhkuri N, Pritchard V.L. The social organisation of fish shoals: a test of the predictive power of laboratory experiments for the field. Biol. Rev. 2000;75:477–501. doi: 10.1111/j.1469-185x.2000.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Lusseau D. The emergent properties of a dolphin social network. Proc. R. Soc. B. 2003;270(Suppl.):S186–S188. doi: 10.1098/rsbl.2003.0057. doi:10.1098/rsbl.2003.0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C, Drent P.J. Individual differences in the use of social information in foraging by captive great tits. Anim. Behav. 2000;60:131–140. doi: 10.1006/anbe.2000.1443. doi:10.1006/anbe.2000.1443 [DOI] [PubMed] [Google Scholar]
- McDonald D.B. Predicting fate from early connectivity in a social network. Proc. Natl Acad. Sci. USA. 2007;104:10 910–10 914. doi: 10.1073/pnas.0701159104. doi:10.1073/pnas.0701159104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M.E.J. Properties of highly clustered networks. Phys. Rev. E. 2003;68:026 121. doi: 10.1103/PhysRevE.68.026121. doi:10.1103/PhysRevE.68.026121 [DOI] [PubMed] [Google Scholar]
- Réale D, Reader S.M, Sol D, McDougall P, Dingemanse N.J. Integrating animal temperament within ecology and evolutionary biology. Biol. Rev. 2007;82:1–28. doi: 10.1111/j.1469-185X.2007.00010.x. doi:10.1111/j.1469-185X.2007.00010.x [DOI] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. 3rd edn. Freeman; San Francisco, CA: 1995. Biometry. [Google Scholar]
- Szendroi B, Csányi G. Polynomial epidemics and clustering in contact networks. Proc. R. Soc. B. 2004;271(Suppl.):S364–S366. doi: 10.1098/rsbl.2004.0188. doi:10.1098/rsbl.2004.0188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers K, Drent P.J, de Goede P, van Noordwijk A.J. Realized heritability and repeatability of risk-taking behaviour in relation to avian personalities. Proc. R. Soc. B. 2004;271:65–73. doi: 10.1098/rspb.2003.2518. doi:10.1098/rspb.2003.2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek M.E.M, Drent P.J, Wiepkema P.R. Consistent individual-differences in early exploratory-behavior of male great tits. Anim. Behav. 1994;48:1113–1121. doi:10.1006/anbe.1994.1344 [Google Scholar]
- Verbeek M.E.M, Boon A, Drent P.J. Exploration, aggressive behavior and dominance in pair-wise confrontations of juvenile male great tits. Behaviour. 1996;133:945–963. doi:10.1163/156853996X00314 [Google Scholar]
- Verbeek M.E.M, de Goede P, Drent P.J, Wiepkema P.R. Individual behavioural characteristics and dominance in aviary groups of great tits. Behaviour. 1999;136:23–48. [Google Scholar]
- Ward A.J.W, Thomas P, Hart P.J.B, Krause J. Correlates of boldness in three-spined sticklebacks (Gasterosteus aculeatus) Behav. Ecol. Sociobiol. 2004;55:561–568. doi:10.1007/s00265-003-0751-8 [Google Scholar]
- Ward A.J.W, Webster M.M, Hart P.J.B. Social recognition in wild fish populations. Proc. R. Soc. B. 2007;274:1071–1077. doi: 10.1098/rspb.2006.0231. doi:10.1098/rspb.2006.0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D.J, Strogatz S.H. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. doi:10.1038/30918 [DOI] [PubMed] [Google Scholar]
- Webster M.M, Ward A.J.W, Hart P.J.B. Boldness is influenced by social context in threespine sticklebacks (Gasterosteus aculeatus) Behaviour. 2007;144:351–371. doi:10.1163/156853907780425721 [Google Scholar]
- Whitehead H, Dufault S. Techniques for analyzing vertebrate social structure using identified individuals: review and recommendations. Adv. Study Behav. 1999;28:33–74. doi:10.1016/S0065-3454(08)60215-6 [Google Scholar]
- Wilson D.S, Clark A.B, Coleman K, Dearstyne T. Shyness and boldness in humans and other animals. Trends Ecol. Evol. 1994;9:442–446. doi: 10.1016/0169-5347(94)90134-1. doi:10.1016/0169-5347(94)90134-1 [DOI] [PubMed] [Google Scholar]
- Wootton R.J. Croom Helm; London, UK: 1984. The functional biology of sticklebacks. [Google Scholar]
- Ying Y.W. Formation of cross-cultural relationships of Taiwanese international students in the United States. J. Commun. Psychol. 2002;30:45–55. doi:10.1002/jcop.1049 [Google Scholar]
- Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 2005;4:17. doi: 10.2202/1544-6115.1128. [DOI] [PubMed] [Google Scholar]