Abstract
Butterflies have competing demands for flight ability depending on, for example, mating system, predation pressure, the localization of host plants and dispersal needs. The flight apparatus, however, is costly to manufacture and therefore trade-offs are expected since resources are limited and must be allocated between flight ability and other functions, such as reproduction. Trade-offs between flight and reproduction may be difficult to reveal since they interact with other factors and can be confounded by differences in resource consumption. Previous studies have shown that adults of the summer generation of Pieris napi have relatively larger thoraxes compared with the spring generation. To study whether difference in thorax size results in a trade-off between flight ability and reproduction among the two generations, we conducted a split-brood experiment under common garden conditions. Our results show that summer generation adults have a higher dispersal capacity measured as flight duration in five different temperatures. Reproductive output differed between the two developmental pathways; spring generation females had a significantly higher output of eggs compared with summer generation females. We suggest that this is a consequence of a resource-allocation trade-off made during pupal development implemented by different demands for flight between the spring and summer generations. The significance of this finding is discussed in relation to reproduction and mobility in butterflies.
Keywords: phenotypic plasticity, Pieris napi, Lepidoptera, resource allocation
1. Introduction
Flying in insects provides many advantages, e.g. easier mate location, better predator avoidance, increased feeding opportunities and host-plant tracking. Nevertheless, flying also incurs costs since flight ability is resource demanding, and trade-offs with other costly traits are thus expected (Johnson 1969; Zera & Harshman 2001; Roff et al. 2003). The costs and benefits of flight are expected to influence life-history strategies. For example, in flight dimorphic insect species, with morphs either capable of flight or wingless, the flightless morph generally has a higher fecundity and/or earlier sexual maturation than morphs with flight ability (e.g. Roff 1977; Zera & Denno 1997). This trade-off between fecundity and flight has been explored less in wing-monomorphic insects such as butterflies. Butterflies are strongly dependent on flight throughout their adult lives for both reproduction and survival. Flight ability in butterflies may nevertheless differ among and within species depending on, for example, mating system (Wickman 1993), predation pressure (Chai & Srygley 1990), metapopulation structure (Hanski et al. 2004, 2006), geographical expansion (Hill et al. 1999; Hughes et al. 2003) and seasonal timing of adult appearance (Fric et al. 2006). Similar to many insects, it is predicted that there should be a trade-off between flight and reproductive output, since both muscles and eggs/spermatophores contain resources that are in short supply for adult butterflies feeding on nectar (Boggs 1981; Karlsson 1995).
In a comparative study on neotropical butterflies, Srygley & Chai (1990) showed that species with a low investment in flight muscles had a higher investment in reproductive abdominal reserves, perhaps suggesting a trade-off. Moreover, an intraspecific study on the speckled wood butterfly, Pararge aegeria, demonstrated that females in expanding populations invested more in thorax flight muscles (as a measure of flight ability and dispersal capacity) but showed reduced egg production compared with populations in core areas where females had higher reproductive performance but less mass allocated to the thorax (Hughes et al. 2003, 2007). These studies suggest that there may be a trade-off between fecundity and dispersal ability where selection on an increased dispersal affects reproduction negatively. Females in newly established populations of the Glanville fritillary butterfly Melitaea cinxia are known to be more dispersive compared with females in older populations (Hanski et al. 2002). Furthermore, Hanski et al. (2004) found that females in newly established populations showed reduced potential fecundity compared with females in old populations. However, the view of a general dispersal–fecundity trade-off in M. cinxia has been challenged by Hanski et al. (2006) and Saastamoinen (2007), because they found no evidence in outdoor cage experiments of a trade-off between mobility and fecundity.
At present, the contradictory results concerning the evolutionary trade-offs between flight and reproduction in butterflies suggest that further studies are needed to elucidate the underlying mechanisms. Studies of trade-offs are often correlative by nature (Roff & Fairbairn 2007); however, by conducting between-generation investigations, in which there are expected differences in adult dispersal patterns, this would provide an alternative way to study the trade-off between flight ability and reproduction in butterflies. Similar to many insects in seasonal environments, many butterflies have different developmental pathways depending on the season and adults often show phenotypic plasticity between generations. Different phenotypes are selected by the circumstances that exist during the period of the year when they appear (Brakefield et al. 2003) and this can provide each generation with the ability to perform at its best under the present environmental conditions. Hence, phenotypic plasticity gives an opportunity to explore internal developmental differences and causal trade-offs.
As one example, Karlsson et al. (2008) have found in a recent study that the short-lived summer morph butterflies of Polygonia c-album invest less in their soma, whereas the longer lived winter morph has a body constitution that is stronger and more durable. As a consequence, females of the summer morph have more resources available for egg laying and have a significantly higher reproductive output compared with the winter morph females.
In this study, we used spring and summer generations of the green-veined white butterfly, Pieris napi. These two phenotypes differ in several traits (Fric et al. 2006) and are expected to differ also in mobility pattern. The summer generation of P. napi is much more numerous compared with the spring generation (Emmet & Heath 1989; Thomas & Lewington 1991; Eliasson et al. 2005) and we thus predict this generation to be more dispersive. Furthermore, Fric et al. (2006) found that adults of the summer generation of P. napi have relatively larger thoraces than adults of the spring generation, something that potentially will increase flight ability (Marden 1989; Berwaerts & Van Dyck 2004). Srygley & Kingsolver (1998) also showed that manoeuvrability increases when the relative thorax mass increases in a set of pierid butterflies. This gives further support for our prediction that adult females of the summer generation should be more inclined to disperse and have a higher dispersal capacity to reduce the risk of crowding and to avoid male harassment during egg laying (Shapiro 1970; Baker 1984; Baguette et al. 1996; Gibbs et al. 2005; Fric et al. 2006). If differences in flight ability between generations occur, we will also predict a trade-off between flight ability and fecundity. Therefore, we conducted a split-brood experiment and measured flight ability and reproduction in spring and summer generation adults of P. napi.
2. Material and methods
(a) Study species
The green-veined white butterfly, P. napi, hibernates as pupae and in Sweden the first adults appear in April and May. A second (summer) generation flies in southern and middle Sweden during the end of June and July (Henriksen & Kreutzer 1982). Fifteen P. napi females were captured from the southern part of Sweden and allowed to lay eggs in indoor cages.
Females were captured at several sites to avoid the possibility of sampling relatives. The eggs were laid on leaves of garlic mustard, Alliaria petiolata. Newly hatched larvae originating from these females were put in 1.5 l plastic containers with fresh garlic mustard leaves to feed upon. Two larvae were reared in each container. The containers were kept at 20°C and either in a 22 L : 2 D or in a 12 L : 12 D regime, generating direct development (summer generation) or hibernating individuals (spring generation), respectively. Larvae were constantly given new leaves until pupation. The pupae coming from the 12 L : 12 D regime were transferred to +1°C for overwintering while those in the 22 L : 2 D regime stayed in the same temperature until the adults eclosed. On the day of eclosion, all adults were weighed on a Cahn 28 automatic electrobalance, labelled with a permanent marker on the right hind wing, and thereafter kept at +8°C until the start of the experiments.
(b) Direct developing pathway (summer generation)
When the adults were at least 1 day old, a cohort of the direct developing butterflies was transferred to mating cages (0.5×0.7×0.7 m) for mating. The sex ratio in the cages was approximately 1 : 1. In the cages, the butterflies had access to 20% sugar water. To avoid inbreeding, males and females from the same maternal line were always kept in separate cages. The sugar was soaked into cotton on sticks and dripped onto Chrysanthemum flowers in pots. The mating couples were removed from the cage as soon as they had started mating, thereby ensuring that each individual butterfly mated only once. The mating time was recorded in each case to ensure that it was a normal mating.
(c) Egg laying
Following mating, the females were immediately transferred to new 1.5 l plastic containers, where they were allowed to lay eggs on garlic mustard (A. petiolata) leaves. Since the two developmental pathways investigated in this experiment may differ in their propensities to lay eggs in relation to temperature, we divided the containers into environmental cabinets with five different temperatures: 15, 20, 25, 30 and 35°C. In the containers, the females had a piece of cotton soaked with water and through the covering net they could reach another piece of cotton soaked with 20% sugar water. The eggs laid by each individual female were counted every day and the females were given new leaves of garlic mustard on which to lay eggs on a daily basis. On the first day that a female laid eggs, 10 of those eggs were weighed on a Cahn 28 automatic electrobalance as a measure of mean egg weight. These eggs were then stored in 5 ml plastic cups with lids and later inspected to see that they were properly fertilized. The females were kept in the containers until they died or after a maximum of 7 days of egg laying. A maximum of 7 days was chosen since P. napi females mated to a virgin male remate after on average 5.5–7 days (Kaitala & Wiklund 1995).
(d) Flight ability
Another cohort of animals from 22 L : 2 D took part in a flight experiment. After emergence, adults were directly kept at 8°C (in order to keep the metabolism at a low level and more specifically to suppress oviposition) and they were not fed or mated prior to observations. For this flight experiment, we used five identical climate rooms (height×length×width: 2.4×4×2 m3) differing only in temperature: at 13, 15, 17, 19 and 21°C. Again, testing the animals in different temperatures was carried out in order to remove the confounding effects of unequal temperature flight dependence among developmental pathways. Prior to the experiment, there was an acclimatization period of 30 min. After this period, thorax temperature was considered to be at the prevailing ambient temperature in the climate room (cf. Merckx et al. 2006). Tested adults were held with a pair of tweezers by the wings and released from a standard height of 2 m and the time from release until alighting was noted by a stopwatch. Every butterfly got to fly in at least two different temperatures and maximum once in each temperature, but the order was randomized. Before and after flight, they were stored at 8°C.
(e) Overwintering pathway (spring generation)
Pupae of the spring generation butterflies were transferred after three months at 1°C to a climate room at 20°C. After eclosion, the adults were weighed, labelled and transferred to 8°C until the start of the experiments. For practical reasons, it was not possible to wait for more than three months, although a longer diapause would have increased the number of eclosed adults available for experiments (cf. Forsberg & Wiklund 1988). Both the egg laying and the flying experiments were repeated with spring generation animals. Identical procedures and protocols were used and to avoid unknown confounding effects, the same environmental cabinets and flight rooms were used for each temperature as for the summer generation animals.
(f) Statistical analysis
Egg number, egg weight and longevity were analysed in relation to developmental pathway (spring generation and summer generation), using ANCOVA (GLM, program Statistica v. 7.1, StatSoft 2005). Although eclosion size did not differ significantly among treatments, eclosion mass was entered into the models but, if insignificant, this factor was removed from the models. Flight duration (fd) was transformed as 1−(1/sqrt (fd)) to obtain homogeneous variances. Differences in flight ability were tested with repeated measures (each female could fly in several temperatures) ANOVA (GLM, program Statistica v. 7.1, StatSoft 2005).
3. Results
(a) Flight duration
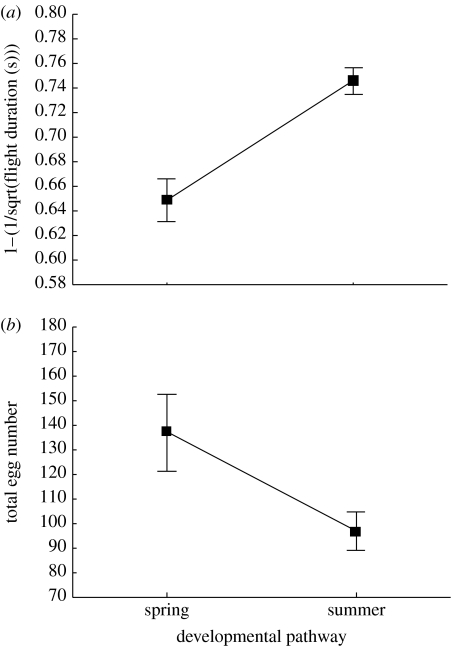
The overall effect of temperature on flight duration of the two investigated developmental pathways was highly significant and flight duration increased with temperature (table 1). When females of the spring and summer generations were compared, there was a significant difference in flight duration; summer females had a significantly higher duration of flight compared with spring generation females (table 1; figure 1). The effect of temperature on flight duration of males was similar to that of females, again with a significant difference in flight duration when summer and spring generations were compared (table 1), although males generally had longer flight durations compared with females (F1,102=8.58, p=0.0042).
Table 1.
Summary of the analyses of total flight duration (a), total egg number (b), egg weight (c) and longevity (d) in relation to developmental pathway (spring or summer) and temperature of the green-veined white butterflies using GLM procedure.
| variable | effect | F-value | p | |
|---|---|---|---|---|
| (a) flight duration (s) | ||||
| females | developmental pathway | F1,48 | 18.67 | <0.001 |
| temperature | F4,192 | 34.37 | <0.001 | |
| female size | F1,47 | 0.14 | 0.709 | |
| developmental pathway×temperature | F4,192 | 1.77 | 0.136 | |
| males | developmental pathway | F1,54 | 13.30 | <0.001 |
| temperature | F4,216 | 37.95 | <0.001 | |
| male size | F1,53 | 2.24 | 0.141 | |
| developmental pathway×temperature | F4,216 | 1 | 0.406 | |
| (b) total egg number | developmental pathway | F1,118 | 5.76 | 0.018 |
| temperature | F4,118 | 8.51 | <0.001 | |
| female size | F1,117 | 0.98 | 0.326 | |
| developmental pathway×temperature | F4,118 | 1.84 | 0.126 | |
| (c) egg weight (mg) | developmental pathway | F1,110 | 1.13 | 0.29 |
| temperature | F4,110 | 8.30 | <0.001 | |
| female size | F1,109 | 6.31 | 0.013 | |
| developmental pathway×temperature | F1,109 | 0.85 | 0.495 | |
| (d) longevity | developmental pathway | F1,118 | 2.11 | 0.15 |
| temperature | F4,118 | 21.30 | <0.001 | |
| female size | F1,117 | 3.03 | 0.085 | |
| developmental pathway×temperature | F4,118 | 1.41 | 0.235 | |
Figure 1.
(a) Flight duration (s) (transformed as 1−1/sqrt) and (b) total egg number of P. napi females belonging to spring or summer developing pathways. Mean values±s.e.
(b) Egg number and egg size
There was a significant effect of temperature on egg laying among both generations and the number of eggs increased with temperature (table 1). A comparison between the generations showed a significant difference in the number of eggs laid; (figure 1; table 1) the summer generation females laid significantly fewer eggs compared with the spring generation females (table 1; figure 1).
The egg size decreased significantly with increasing temperature, but there was no difference between the developmental pathways (table 1).
(c) Longevity
Longevity decreased significantly with increasing temperature and there was no significant difference in the lifespan between the pathways (table 1).
4. Discussion
Our experiment was conducted by comparing two different generations (or developmental pathways) of the green-veined white butterfly that experience different demographic and ecological circumstances in the wild. The results clearly demonstrate that there is phenotypic plasticity in flight ability and fecundity, and that increased flight ability is traded off against egg-laying capacity (figure 1; table 1). Hence, our result supports the idea that females equipped with a more powerful flight apparatus (enabling higher flight ability) pay a cost in terms of reduced egg production.
We argue that our method to explore environmentally cued alternative phenotypes can be seen as an alternative way to experimental manipulation of, for example, resources or selection experiments since developmental pathways affect the developmental investment of reproductive resources. Hence, we can use variation in reproductive effort among pathways to test for correlated changes in flight. A further interesting question would be to examine the genetic variation underlying our observed trade-off (Roff & Fairbairn 2007; Suzuki & Nijhout 2008; Marden 2008; Leimar 2007), i.e. to regard the plasticity/trade-off itself as a trait that varies and is subject to natural selection.
To avoid confounding effects due to different temperature-dependent propensities in egg laying and flight ability, we investigated these traits along a temperature gradient from relatively low to relatively high temperatures. As expected, there was a positive relationship between temperature and performance (the thermal reaction norm) regarding both flight duration and fecundity, but there were no significant interactions (table 1). There was no difference in egg size when comparing summer and spring generations and thus there were no indications showing that the summer generation, laying fewer eggs, also laid larger eggs. This excludes the possibility of a trade-off in egg size versus egg number between the generations, which could affect the fecundity–flight capacity trade-off. The pattern of flight duration of males was similar to that of females, again with a significant difference in flight duration when summer and spring generations were compared, indicating that both sexes of the summer generation invest more in flight muscles and therefore put a greater investment into potential dispersal capacity.
One requisite for an internal resource allocation trade-off between flight and fecundity is that the resources connected to the trade-off parameters are in some sense limited. Both eggs and flight muscles contain high concentrations of nitrogen, and adult nectar-feeding butterflies cannot significantly enhance larval-derived nitrogen stores as adults since adult food sources consist mainly of carbohydrates (Boggs 1981; Karlsson 1995; but see Mevi-Schütz & Erhardt 2005). Further support for the idea that nitrogen is a resource that is in limited supply for P. napi comes from Karlsson (1998), who found that body stores of nitrogen set an upper limit for egg production in P. napi. We suggest that there is an internal competition during development between resources allocated either to eggs or to flight muscles, and that our example of a developmental trade-off suggests a causal relationship.
One potential confounding factor in our study is the possibility that flight muscles from the thorax can be reallocated and to some extent be used for egg production in butterflies (Stjernholm et al. 2005), something that also applies to P. napi (Karlsson 1998; Stjernholm & Karlsson 2000). This could reduce the importance of a causal relationship between flight ability and egg production. However, we have no indications that summer versus spring generation females would differ in their use of thorax resources. On the contrary, both spring generation females (Stjernholm & Karlsson 2000) and summer generation females (Karlsson 1998) seem to use thorax resources in a similar way, with approximately a 10% increase of lifetime reproductive output due to breakdown of flight muscles.
Our finding that a wing-monomorphic insect shows a trade-off between reproduction and flight is also found in some other studies but, as outlined in §1, there are also studies that do not find such trade-off. However, there is a discrepancy in the methods used among the studies that, to some extent, might explain the different results.
Our study and the studies by Hughes et al. (2003, 2007) concern differences in body design and allocation patterns taken during development whereas the studies by Hanski et al. (2006) and Saastamoinen (2007) on M. cinxia involve trade-offs taken during adult life. In the studies by Hanski et al. (2006) and Saastamoinen (2007), individuals of newly established populations of this butterfly are more dispersive compared with individuals in older populations and a prediction from their studies is a dispersal–fecundity trade-off, partly since Hanski et al. (2004) found a negative association between dispersal ability and potential fecundity. Furthermore, Baguette & Schtickzelle (2006) found, in a comparative study of five butterflies (four of these species were fritillaries, closely related to M. cinxia), that there was a strong negative relationship between investment into reproduction measured as maximum growth rate and dispersal distance, supporting a trade-off between these two traits. However, Hanski et al. (2006) and Saastamoinen (2007) could not find a general trade-off between egg production and mobility (measured as the distance moved in an enclosed cage). Hanski et al. (2006) suggested that since butterflies in newly established populations have a higher metabolic performance but a shorter maximal lifespan compared with butterflies in old populations, this could explain why butterflies in newly established populations have both a higher mobility as well as a higher fecundity. It is thus possible that the underlying flight ability–fecundity trade-off disappears when these two populations are compared but it is still possible that there is a functional trade-off between mobility and fecundity within each population but that the trade-off is concealed by environmental conditions that constrain realized fecundity (cf. Saastamoinen 2007).
In conclusion, our study finds support for a functional trade-off between flight ability and reproduction in P. napi. Since our study system includes a comparison between a summer morph that is known to allocate more resources to flight muscles (Fric et al. 2006) and is more dispersive, and a spring morph that is allocating less to flight muscles and is more sedentary, we suggest that the trade-off we find supports the idea that butterflies designed for a more dispersive lifestyle pay a cost in reduced reproductive output (cf. Hughes et al. 2003, 2007).
Acknowledgments
We thank Magne Friberg, Mike Singer, Christer Wiklund and three anonymous referees for their comments on the manuscript.
References
- Baguette M, Schtickzelle N. Negative relationship between dispersal distance and demography in butterfly metapopulations. Ecology. 2006;87:648–654. doi: 10.1890/04-1631. doi:10.1890/04-1631 [DOI] [PubMed] [Google Scholar]
- Baguette M, Convie I, Neve G. Male density affects female spatial behaviour in the butterfly Proclossiana eunomia. Acta Oecol. 1996;17:225–232. [Google Scholar]
- Baker R.R. The dilemma: when and how to go or stay. In: Vane-Wright R.I, Ackery P.R, editors. Biology of butterflies; Symp. the Royal Entomological Society of London, No. 11. Academic Press; London, UK: 1984. pp. 279–296. [Google Scholar]
- Boggs C.L. Nutritional and life history determinants of resource allocation in holometabolous insects. Am. Nat. 1981;117:692–709. doi:10.1086/283753 [Google Scholar]
- Berwaerts K, Van Dyck H. Take-off performance under optimal and suboptimal thermal conditions in the butterfly Pararge aegeria. Oecologia. 2004;141:536–545. doi: 10.1007/s00442-004-1661-9. doi:10.1007/s00442-004-1661-9 [DOI] [PubMed] [Google Scholar]
- Brakefield P.M, French V, Zwaan B.J. Development and the genetics of evolutionary change within insect species. Annu. Rev. Ecol. Evol. Syst. 2003;34:633–660. doi:10.1146/annurev.ecolsys.34.011802.132425 [Google Scholar]
- Chai P, Srygley R.B. Predation and the flight. Morphology, and temperature of neotropical rain-forest butterflies. Am. Nat. 1990;135:748–765. doi:10.1086/285072 [Google Scholar]
- Emmet A.M, Heath J. Part 1, Hesperiidae-Nymphalidae, The butterflies. vol. 7. Harley Books; Essex, UK: 1989. The moths and butterflies of Great Britain and Ireland. [Google Scholar]
- Eliasson C.U, Ryrholm N, Holmer M, Jilg K, Gärdenfors U. Artdatabanken, SLU; Uppsala, Sweden: 2005. Encyclopedia of the Swedish flora and fauna. Butterflies. Hesperiidae-Nymphalidae. [Google Scholar]
- Forsberg J, Wiklund C. Protandry in the green-veined white butterfly, Pieris napi L. (Lepidoptera;Pieridae) Funct. Ecol. 1988;2:81–88. doi:10.2307/2389464 [Google Scholar]
- Fric Z, Klimova M, Konvicka M. Mechanical design indicates differences in mobility among butterfly generations. Evol. Ecol. Res. 2006;8:1511–1522. [Google Scholar]
- Gibbs M, Lace L.A, Jones M.J, Moore A.J. Egg size-number trade-off and a decline in oviposition site choice quality: female Pararge aegeria butterflies pay a cost of having males present at oviposition. J. Insect. Sci. 2005;5:1–9. doi: 10.1093/jis/5.1.39. doi:10.1673/1536-2442(2005)5[1:ESTAAD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski I, Breuker C.J, Schops K, Setchfield R, Nieminen M. Population history and life history influence the migration rate of female Glanville fritillary butterflies. Oikos. 2002;98:87–97. doi:10.1034/j.1600-0706.2002.980109.x [Google Scholar]
- Hanski I, Erälathi C, Kankare M, Ovaskainen O, Sirén H. Variation in migration propensity among individuals maintained by landscape structure. Ecol. Lett. 2004;7:958–966. doi:10.1111/j.1461-0248.2004.00654.x [Google Scholar]
- Hanski I, Saastamoinen M, Ovaskainen O. Dispersal-related life-history trade-offs in a butterfly metapopulation. J. Anim. Ecol. 2006;75:91–100. doi: 10.1111/j.1365-2656.2005.01024.x. doi:10.1111/j.1365-2656.2005.01024.x [DOI] [PubMed] [Google Scholar]
- Henriksen H.J, Kreutzer I.B. Skandinavisk bogforlag; Odense, Denmark: 1982. The butterflies in Scandinavia in nature. [Google Scholar]
- Hill J.K, Thomas C.D, Blakelev D.S. Evolution of flight morphology in a butterfly that has recently expanded its geographic range. Oecologia. 1999;121:165–170. doi: 10.1007/s004420050918. doi:10.1007/s004420050918 [DOI] [PubMed] [Google Scholar]
- Hughes C.L, Hill J.K, Dytham C. Evolution trade-offs between reproduction and dispersal in populations at expanding range boundaries. Proc. R. Soc. B. 2003;270:147–150. doi: 10.1098/rsbl.2003.0049. doi:10.1098/rsbl.2003.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C.L, Dytham C, Hill J.K. Modelling and analysing evolution of dispersal in populations at expanding range boundaries. Ecol. Entomol. 2007;32:437–445. doi:10.1111/j.1365-2311.2007.00890.x [Google Scholar]
- Johnson C.G.Migration and dispersal of insects by flight1969Methuen; London, UK [Google Scholar]
- Kaitala A, Wiklund C. Female mate choice and mating costs in the polyandrous butterfly Pieris napi (Lepidioptera:Pieridae) J. Insect Behav. 1995;8:355–363. doi:10.1007/BF01989364 [Google Scholar]
- Karlsson B. Resource allocation and mating systems in butterflies. Evolution. 1995;49:955–961. doi: 10.1111/j.1558-5646.1995.tb02330.x. doi:10.2307/2410417 [DOI] [PubMed] [Google Scholar]
- Karlsson B. Nuptial gifts, resource budgets, and reproductive output in a polyandrous butterfly. Ecology. 1998;79:2931–2940. [Google Scholar]
- Karlsson B, Stjernholm F, Wiklund C. Test of a developmental trade-off in a polyphenic butterfly: direct development favours reproductive output. Funct. Ecol. 2008;22:121–126. doi:10.1111/j.1365-2435.2007.01334.x [Google Scholar]
- Leimar, O. 2007 Environmental and genetic cues in the evolution of phenotypic polymorphism. Evol. Ecol. (doi:10.1007/s10682-007-9194-4)
- Marden J.H. Bodybuilding dragonflies: costs and benefits of maximizing flight muscle. Physiol. Zool. 1989;62:505–521. [Google Scholar]
- Marden J.H. Quantitative and evolutionary biology of alternative splicing: how changing the mix of alternative transcripts affects phenotypic plasticity and reaction norms. Heredity. 2008;100:111–120. doi: 10.1038/sj.hdy.6800904. doi:10.1038/sj.hdy.6800904 [DOI] [PubMed] [Google Scholar]
- Merckx T, Karlsson B, Van Dyck H. Sex- and landscape-related differences in flight ability under suboptimal temperatures in a woodland butterfly. Funct. Ecol. 2006;20:436–441. doi:10.1111/j.1365-2435.2006.01124.x [Google Scholar]
- Mevi-Schütz J, Erhardt A. Amino acids in nectar enhance butterfly fecundity: a long-awaited link. Am. Nat. 2005;165:411–419. doi: 10.1086/429150. doi:10.1086/429150 [DOI] [PubMed] [Google Scholar]
- Roff D.A. Dispersal in dipterans: its costs and consequences. J. Anim. Ecol. 1977;46:443–456. doi:10.2307/3822 [Google Scholar]
- Roff D.A, Crnokrak P, Fairbairn D.J. The evolution of trade-offs: geographic variation in call duration and flight ability in the sand cricket, Gryllus firmus. J. Evol. Biol. 2003;164:744–753. doi: 10.1046/j.1420-9101.2003.00570.x. doi:10.1046/j.1420-9101.2003.00570.x [DOI] [PubMed] [Google Scholar]
- Roff D.A, Fairbairn D.J. The evolution of trade-offs: where are we? J. Evol. Biol. 2007;20:433–447. doi: 10.1111/j.1420-9101.2006.01255.x. doi:10.1111/j.1420-9101.2006.01255.x [DOI] [PubMed] [Google Scholar]
- Saastamoinen M. Mobility and lifetime fecundity in new versus old populations of the Glanville fritillary butterfly. Oecologia. 2007;153:569–578. doi: 10.1007/s00442-007-0772-5. doi:10.1007/s00442-007-0772-5 [DOI] [PubMed] [Google Scholar]
- Shapiro A.M. The role of sexual behavior in density-related dispersal of pierid butterflies. Am. Nat. 1970;104:367–372. [Google Scholar]
- Srygley R.B, Chai P. Flight morphology of neotropical butterflies: palatability and distribution of mass to the thorax and abdomen. Oecologia. 1990;84:491–499. doi: 10.1007/BF00328165. doi:10.1007/BF00328165 [DOI] [PubMed] [Google Scholar]
- Srygley R.B, Kingsolver J.G. Red-winged blackbird reproductive behaviour and the palability, flight performance, and morphology of temperate butterflies (Colias, Pieris, and Pontia) Biol. J. Linn. Soc. 1998;64:41–55. [Google Scholar]
- StatSoft 1999 Statistica for Windows. See http://www.statsoft.com
- Stjernholm F, Karlsson B. Nuptial gifts and the use of body resources for reproduction in the green-veined white butterfly Pieris napi. Proc. R. Soc. B. 2000;267:807–811. doi: 10.1098/rspb.2000.1075. doi:10.1098/rspb.2000.1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernholm F, Karlsson B, Boggs C.L. Age-related changes in thoracic mass: possible reallocation of resources to reproduction in butterflies. Bio. J. Linn. Soc. 2005;86:363–380. doi:10.1111/j.1095-8312.2005.00542.x [Google Scholar]
- Suzuki Y, Nijhout F. Genetic basis of adaptive evolution of a polyphenism by genetic accommodation. J. Evol. Biol. 2008;21:57–66. doi: 10.1111/j.1420-9101.2007.01464.x. doi:10.1111/j.1420-9101.2007.01464.x [DOI] [PubMed] [Google Scholar]
- Thomas J, Lewington R. Dorling Kindersley; London, UK: 1991. The butterflies of Britain and Ireland. [Google Scholar]
- Wickman P.O. Sexual selection and butterfly design: a comparative study. Evolution. 1993;46:1525–1536. doi: 10.1111/j.1558-5646.1992.tb01142.x. doi:10.2307/2409955 [DOI] [PubMed] [Google Scholar]
- Zera A.J, Denno R.F. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 1997;42:207–230. doi: 10.1146/annurev.ento.42.1.207. doi:10.1146/annurev.ento.42.1.207 [DOI] [PubMed] [Google Scholar]
- Zera A.J, Harshman L.G. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 2001;32:95–126. doi:10.1146/annurev.ecolsys.32.081501.114006 [Google Scholar]