Abstract
A perennial question in ornithology is whether flight has evolved mostly to facilitate access to food or as an anti-predator strategy. However, flight is an expensive mode of locomotion and species using flight regularly are associated with an expensive lifestyle. Using heart rate (HR) data loggers implanted in 13 female common eiders (Somateria mollissima), our objective was to test the hypothesis that a high level of flight activity increases their energy budget. We used the long-term recording (seven months) of HR as an index of energy expenditure and the HR flight signature to compile all flight events. Our results indicate that the eider is one of the thriftiest volant birds with only 10 minutes of flight time per day. Consequently, we were not able to detect any effect of flight activity on their energy budget despite very high flight costs (123–149 W), suggesting that flight was controlled by energy budget limitations. However, the low flight activity of that species may also be related to their prey landscape requiring few or no large-scale movements. Nevertheless, we suggest that the (fitness) benefits of keeping flight ability in this species exceed the costs by allowing a higher survival in relation to predation and environmental harshness.
Keywords: common eider, daily heart rate, data logger, energy budget, flight costs, flight behaviour
1. Introduction
Birds developed a sophisticated flight apparatus (flight muscles, wing bones, sternum and feathers) in the course of evolution that enable them to escape predation, avoid harsh weather or gain access to a large array of food sources. Flight is the most expensive mode of locomotion among vertebrates (Butler & Bishop 2000) and Ellis (1984) has shown that basal metabolic rate (BMR) is higher for species of sea birds engaged in costly activities, including flight, suggesting that more active lifestyles were associated with higher values of BMR. A similar analysis conducted by Birt-Friesen et al. (1989) revealed that field metabolic rate (MR) was higher for species using flapping flight and living in cold waters (but see Ellis & Gabrielsen 2002). The possible mechanism here is that breast muscle of birds is large and costly to maintain and use on a regular basis. Thus, preserving a functional flight apparatus incurs some penalty in terms of BMR, which is higher for flighted compared with permanently flightless species (McNab 1994, 2002). In addition, the energetic cost of flight cannot be increased indefinitely as it would drive the energy budget of an individual bird to some unacceptable level. In order to conserve flight ability, the fitness benefits of flight must exceed its costs (high-energy expenditure and the required food base), otherwise flightlessness, the permanent inability to fly, might evolve under certain ecological conditions.
Empirical and theoretical approaches exist to estimate flight energy expenditure of wild birds: those based on respirometry and doubly labelled water from which various allometric equations are derived (Masman & Klaassen 1987; Butler 1991; Rayner 1995), those using heart rate (HR), heart mass and the Fick principle (Bishop 1997) and, finally, those using aerodynamic theory (Pennycuick 1989). Which of these methods better quantifies the total cost of flight is an open debate (Pelletier 2006), but it seems so far that most studies estimate flight costs and then multiply them by any available approximation of the total time spent flying (TSF). However, any estimation of the total energy demand for flight is likely to be questionable without accurate quantification of flight time. For instance, short-term studies of intraspecific time–energy budgets have shown that when high-cost flyers increase their TSF per day they considerably increase their daily energy expenditure (Flint & Nagy 1984; Tatner & Bryant 1986; Masman & Klaasen 1987; Birt-Friesen et al. 1989; Carlson & Moreno 1992; Nudds & Bryant 2000). On the other hand, Masman & Klaassen's (1987) review of flight costs and flight time revealed an interesting trend: bird species with high flight costs seem to fly much less than birds with low flight costs. Therefore, any study aiming at quantifying the impact of total flight cost on the energy budget of birds should measure both the flight time and the energy cost of flight for a single species, preferably on a long-term basis. One problem is that it is not possible to follow a wild bird for a sufficiently long period to quantify flight time accurately and continuously over several days.
Recent developments in data loggers (DLs) provide information on both flight behaviour and flight energetic costs in species performing flapping flight (Butler et al. 1998; Pelletier et al. 2007). Based on the fact that HR can be recorded continuously, Pelletier et al. (2007) developed a new technique using HR signature to quantify the frequency and duration of all flights performed daily (24 h) by a species. HR is also a good indicator of the MR in birds owing to the relationship expressed by the Fick principle (Owen 1969; Bevan et al. 1992, 1994; Bishop & Butler 1995; Butler et al. 2000; Ward et al. 2002). One method proposed by Bishop (1997) allows the use of HR to estimate the rate of oxygen consumption during flight from measured HR and estimates of stroke volume (V2) and oxygen extraction from the blood , where CaO2 is oxygen concentration in the arterial blood and is oxygen concentration in the mixed venous blood.
Because bird species using flapping flight mobilize their flight apparatus almost continuously, flight costs should be higher in these species (Butler 1991). This is especially true for volant diving birds characterized by short pointed wings enabling those species to move in two media of different densities, i.e. air and water (Storer 1971; Lovvorn & Jones 1994). Sea ducks have high wing loadings (i.e. the ratio of body mass to wing area; Greenewalt 1975) and as a consequence, they need to run across the water to take-off and become airborne (Norberg 1990). Common eider (Somateria mollissima, hereafter eider) is the largest sea duck (2 kg) in the Northern Hemisphere and dives for food (Guillemette et al. 2004). Throughout the year, they fly on a routine basis presumably to search for blue mussel beds (Pelletier et al. 2007) and escape from marine predators (Guillemette & Ouellet 2005b). Since take-off, acceleration and ascending components are fuelled by anaerobic metabolism (Guillemette & Ouellet 2005a; Ouellet et al. 2008), total flight costs (aerobic+anaerobic phases) should be high for this species. Thus, our objectives were to monitor and quantify daily flight budgets over an extensive period of time for a free-living bird and test the relationship between daily flight time and daily energy expenditure, at the intra- and inter-individual levels.
2. Material and methods
(a) Study site, capture and implantation procedures
Field work was carried out on Christians Island (55°19′ N, 15°12′ E) in the southern Baltic Sea, 18 km from the Danish island of Bornholm. The island is populated by approximately 2600 breeding pairs of eiders (Lyngs 2000). In 2003, 20 female eiders were equipped with DLs. All experimental females were captured in the second part of their incubation because they are more resistant to disturbance during this period (Bolduc & Guillemette 2003). Females were identified by a set of two colour bands on one tarsus and a numeric metallic band on the other tarsus.
We obtained a licence from Dyreforsøgtilsynet (Royal Veterinarian Corporation) in Denmark and all birds were cared for in accordance to the principles and guidelines of the Canadian Council on Animal Care. The 20 DLs used in the present study recorded HR (each 2 s) and hydrostatic pressure (diving depth, each 2 s). They were 36 mm long×28 mm wide×11 mm thick and weighed 21 g after encapsulation in paraffin wax for waterproofing and silicone coating for biocompatibility. The loggers accounted for 1.2% of the bird's body mass (1752±144 g, N=20) at implantation. All surgical procedures followed Guillemette et al. (2002) and were conducted indoors by a veterinary surgeon. Eighteen females returned to the colony (90%) to breed the next year, and of these, seventeen were caught and their DLs were removed.
(b) Flight behaviour variables
Only 11 loggers had their memory full (220–221 days), two were almost full (190–207 days) and four loggers failed to cover the period after the flightless period. Thus, the number of recorded days varied between females and ranged from 45 to 221 days spanning from May–June 2003 to mid-December 2003. Data from DLs with more than 190 recorded days were analysed (N=13). We used a purpose-designed software program to identify all flight events from the recorded HR flight signature described by Pelletier et al. (2007). Using a frequency distribution of flight duration, only local flights shorter than 30 min (99% of total flights) were considered in this study. Longer flights are considered as migratory movements and are considered elsewhere.
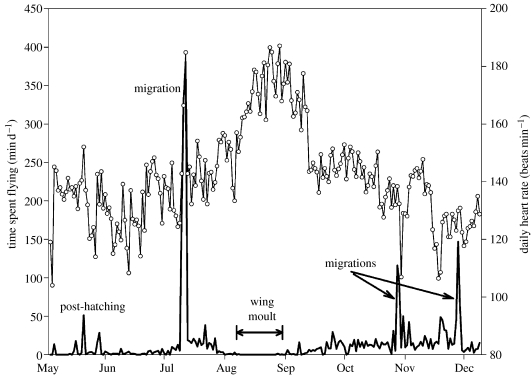
Flight behaviour was described using the following variables: the number of flights per day (NF); mean duration per flight (FD); and mean TSF per day. Thus, for each day, we calculated the number of flights and their duration (±2 s). The TSF per day, excluding migration and moult periods (figure 1), was determined by summing all flight events within a 24 h period. The duration of the flightless (wing moult) period was quantified simply by counting all days without flight (Guillemette et al. 2007).
Figure 1.
An example of time spent flying (thick solid line) per day and daily heart rate (circles) recordings for individual WB. The number of consecutive days without flight represents the wing moult period and days with more than 100 min spent flying per day correspond to migration periods. These both periods were removed from our analysis.
In order to compare with other species, the literature on avian time-budget studies was reviewed. Only studies dealing with the TSF per day (24 h) or the percentage of TSF per active period (from dawn to dusk) were used. No information was found on flight frequency and mean duration per flight in free-living birds. Results of this review are detailed in the electronic supplementary material. We present TSF per day (24 h) and percentage of TSF per active period when daylight duration was not given in studies. We examined the potential biases (in estimates of time spent flying per day) caused by sampling only during the daylight period (active period) by comparing our results (24 h) with the results obtained when excluding first, night flights and second twilight+night flights.
(c) Flight costs and daily MR
We used the average daily heart rate (DHR) as an index of daily metabolic rate (DMR), or daily energy expenditure, because it has been shown in many species that there is a close relationship between the rate of oxygen consumption and HR (Owen 1969; Lund & Folk 1976; Pauls 1980; Butler 1993; Boyd et al. 1999; Froget et al. 2001; Butler et al. 2004). However, because HRs do not translate completely the MR during flight (Butler et al. 2000; Ward et al. 2002), we calculated separately the non-flight metabolic rate (NFMR) and the flight metabolic rate (FMR) using our estimates of flight costs and TSF (see below). We thus calculated the non-flight heart rate (NFHR) for each day and female by summing the total number of heartbeats, excluding flight heart beats and dividing by the non-flight time (1440 min minus TSF). To estimate NFMR, we used the calibration study of Hawkins et al. (2000) to convert NFHR data into rate of oxygen consumption ( in ml O2 kg−1 min−1). The functional (reduced major axis) relationship (Ricker 1973) was =0.146 NFHR +9.677 (n=6, r2=0.75, p=0.023). We used the mean NFHR measured for eiders (148±18 beats min−1; table 1) and a body mass of 1.82 kg. Butler et al. (2004) reported that estimates of the mean of a number of individuals using the HR method are usually within less than 5% of the measured value. However, Butler et al. (2004) did not recommend converting HR into rate of oxygen consumption for an individual bird but instead using average values derived from a sample of experimental birds. One litre of oxygen consumed was multiplied by 20.083 kJ to obtain MR, assuming a respiratory quotient of 0.8. Thus, our estimate of DMR=NFMR+FMR.
Table 1.
Flight variables measured for 13 female common eiders during 175±17 days from reproduction to winter period (excluding moult and migration periods): mean time spent flying per day (TSF, min d−1), mean duration per flight (FD, min), mean number of flights per day (NF, d−1), mean daily heart rate (DHR, beats min−1; see §2) and mean non-flight heart rate excluding migrations (NFHR, beats min−1; see §2). Values are means±s.d. We observed any correlation at the inter-individual level between DHR and TSF (r=0.149, p=0.616) and between DHR and NF (r=0.399, p=0.178) and, between DHR and FD (r=− 0.526, p=0.069).
| individual | number of days | TSF (min d−1) | NF (d−1) | FD (min) | DHR (beats min−1) | NFHR (beats min−1) |
|---|---|---|---|---|---|---|
| BR | 164 | 12.8±12.6 | 4.7±3.5 | 2.7±2.6 | 159±31 | 158±31 |
| OO | 175 | 13.9±12.8 | 7.0±6.0 | 2.0±1.7 | 167±32 | 165±31 |
| OR | 171 | 9.3±11.1 | 3.2±3.5 | 2.9±2.4 | 136±13 | 135±13 |
| OY | 167 | 6.0±9.7 | 3.0±3.0 | 1.9±1.8 | 154±17 | 153±18 |
| RB | 217 | 5.5±9.3 | 2.5±3.6 | 2.2±2.0 | 139±25 | 138±25 |
| RR | 183 | 10.3±10.8 | 5.8±4.6 | 1.8±2.0 | 151±19 | 150±19 |
| RW | 164 | 11.1±10.5 | 3.9±3.2 | 2.9±2.4 | 127±14 | 126±14 |
| WB | 182 | 9.9±11.0 | 3.9±3.4 | 2.5±2.3 | 137±12 | 135±12 |
| WO | 189 | 11.7±11.2 | 5.2±4.6 | 2.2±2.1 | 180±29 | 179±29 |
| YB | 186 | 6.8±10.8 | 3.2±3.5 | 2.1±2.3 | 173±25 | 172±26 |
| YR | 176 | 10.0±11.3 | 4.0±3.2 | 2.5±2.4 | 116±11 | 115±12 |
| YW | 168 | 7.9±8.8 | 3.8±3.7 | 2.1±1.8 | 159±27 | 158±27 |
| YY | 185 | 7.7±10.2 | 3.1±3.4 | 2.4±2.8 | 140±21 | 139±20 |
| mean±s.e.m. | 179±14 | 9.6±2.6 | 4.1±1.3 | 2.3±0.4 | 149±19 | 148±18 |
As an indicator of maintenance cost in this species, Guillemette et al. (2007) used the lowest 5 min mean of HR per day (resting heart rate, RHR). In the present study, we measured an RHR of 86±15 (s.d.) beats min−1. Hawkins et al. (2000) also measured resting (in ml O2 kg−1 min−1) for birds fasting in air and the relationship was (n=6, r2=0.54, p=0.039) between HR and oxygen consumption. We used this regression to convert RHR into resting and then into resting metabolic rate (RMR).
Pelletier (2006) compared five models to estimate oxygen consumption during flight in common eider: two models based on the Fick equation (Bishop 1997), one aerodynamic model using the software Flight v. 1.11 (Pennycuick 1989) and two allometric models based on body mass and wing dimension (Masman & Klaassen 1987; Rayner 1995). From this comparison, he suggested that both models of Bishop (1997) are the most appropriate to estimate oxygen consumption during flight in eiders.
The first model of Bishop (1997) was based on the calculation of rate of oxygen uptake during flight with the Fick principle , where fH is the HR measured directly by the implanted DL (during ‘plateau’ phase of one flight; see Pelletier et al. 2007) and Vs is the stroke volume estimated from heart mass (MH) using an equation of heart mass-specific scaling of Vs during flight (Vs,flight=0.3 MH1.05; Bishop & Butler 1995). Unfortunately, as we did not know the heart mass of our experimental birds so we used a mean value obtained from a sample of six female eiders collected on their wintering ground (17.2±1.2 g). We estimated a Vs value of 6.20 ml. Moreover, is the difference in arteriovenous oxygen content. The arterial oxygen content, CaO2, was estimated, as prescribed by Bishop (1997), by multiplying haemoglobin concentration by 1.36 (to estimate saturated oxygen-carrying capacity) and then by 0.94 (assuming 94% saturation during maximal activity as measured in seven species of mammals running at ; Bishop 1997). We averaged haemoglobin concentration found for three species of diving ducks (Aythya sp.) ([Hb]=0.1593 g ml blood−1; Lovvorn & Jones 1994). Finally, Bishop (1997) assumed that the value of does not fall below 0.038 ml O2 ml blood−1 (it is again the mean value of seven species of mammals running at ; Bishop 1997).
The second model of Bishop (1997) is another form of the Fick principle: . He assumed that maximum cardiac output () was a function of MH and that there was no difference between birds and mammals during maximum cardiovascular performance (corroborated by Peters et al. 2005). Consequently, for both mammals and birds, and were estimated from haemoglobin concentration as in the first model.
(d) Relationship between TSF and DHR
We tested the hypothesis that a high level of flight activity was related to daily energy expenditure at both the inter- and intra-individual levels. To do this, we used TSF and DHR as a proxy of energy expenditure at the individual level. Because there is large variation of DHR at the inter-individual level, Green & Frappell (2007) used a factorial approach to perform such an analysis. We thus used both the absolute and factorial approaches in our analysis, and because the results were similar we presented only the analysis using the absolute approach.
Because we could not find any consistent relationships between DHR and TSF at the intra-individual level (see §3), we postulated that common eiders were compensating for a high level of flight activity by reducing other components of the energy budget. We test that hypothesis by correlating NFHR, as described above, with TSF.
(e) Statistical analysis
The null hypothesis was that there was no relationship between TSF variable and DHR. One salient feature of the HR data presented here is that they were recorded continuously, every 2 s for a long period of time. Thus, the method of data collection is exhaustive within each individual, which means that we have the whole population of observations for one specific bird during the recorded interval. This situation is unusual in the sense that there is no need for inferential statistics in our analysis at the intra-individual level. Although this eliminates the use of inferential analysis, it emphasizes the need to discuss the biological significance of the data and we are left with the challenging task of interpreting small variations in the data collected. As a rule, we considered a correlation to be valid when the sign of a relationship was consistent among individuals (e.g. all positive intra-individual correlations).
At the inter-individual level, all the variables were averaged for each individual (table 1) and Pearson's correlations were computed with the original observations between flight behaviour variables and daily HRs. Then, the permutation distributions of r were derived for each pair of variables. The p-value was determined from the position of the original correlations among the 10 000 resampling values (Good 2001, 2005). The statistical significance (alpha) was set at p<0.05. Resampling Stats v. 2 was used for statistical analyses. Values are means ± standard deviation (s.d.) unless otherwise stated.
3. Results
(a) Flight behaviour
Common Eiders fly very little throughout all phases of the annual cycle except for migrations (figure 1). Outside migrations, female eiders flew, on average, only 4.1±1.3 times d−1 and local flights lasted, on average, 2.3±0.4 min (table 1). Using the mean flight speed measured for eiders (17.5 m s−1; Kahlert et al. 2003), we estimated that they flew over relatively short distances during one flight, on average, 2.4 km. The distribution of all local flights showed that 95% of them lasted less than 6.0 min (equivalent to a distance of 6.3 km). The average TSF per day for all individuals was 9.6±2.6 min d−1 (table 1). With the same flight speed presented above, we estimated that, on average, eiders covered a mean distance of approximately 10 km d−1.
The number of flights per day was highly and significantly correlated with TSF per day (r=0.841, p<0.001) at the inter-individual level, whereas no correlation was found between the latter and mean duration per flight (r=0.240, p=0.308), indicating that the number of flights performed daily was the major determinant of the daily flight budget. Intra-individual variations in flight behaviour variables were more pronounced than inter-individual variations (table 1), indicating that the flying effort was substantial on some days whereas it was negligible for others.
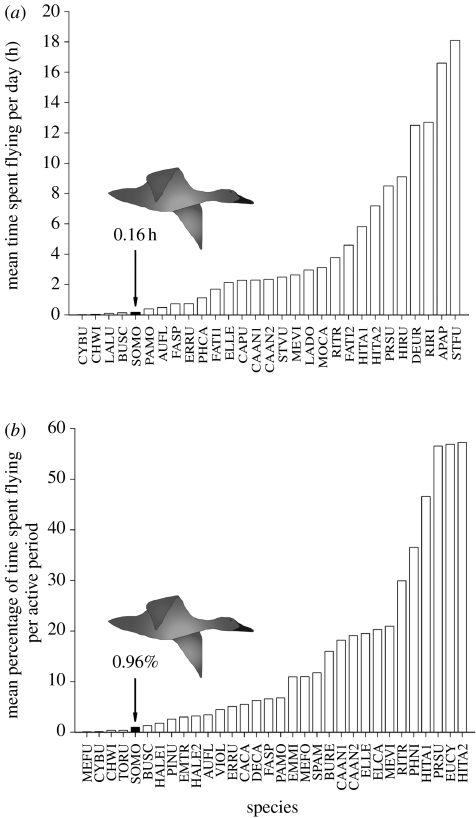
We compared the TSF with values for other species in absolute and relative (percentage of daylight) terms, and found that other species flew on average 4.1±5.1 h d−1 (figure 2a) or for 15.6±17.1% of daylight (figure 2b). In comparison, eiders in the present study spent 0.66% of their time flying per 24 h (or 0.16 h d−1). According to the interspecific comparison, 86% of species spent a higher percentage of time flying than the eiders (figure 2a,b). Some species, however, have similar or even lower flight times than eiders (see §4).
Figure 2.
Interspecific comparison of (a) mean time spent flying per day (mean=4.1±5.1 h d−1) and (b) mean percentage of time spent flying per active period (from dawn to dusk, mean=15.6±17.1% of daylight). Black bars are the mean result for common eiders (SOMO, Somateria mollissima) in this study: eiders spent 0.66% (or 0.16 h d−1) of their time flying per 24 h and, to be consistent with other daylight studies, eiders spent 0.96% of their time flying per active period. (APAP (Apus apus), AUFL (Auriparus flaviceps), BURE (Buteo regalis), BUSC (Bubo scandiacus), CAAN1 (Calypte anna), CAAN2 (C. anna), CACA (Cardinalis cardinalis), CAPU (Calidris pusilla), CHWI (Charadrius wilsonia), CYBU (Cygnus buccinator), DECA (Dendroica caerulescens), DEUR (Delichon urbica), ELCA (Elanus caeruleus), ELLE (Elanus leucurus), EMMI (Empidonax minimus), EMTR (Empidonax traillii), ERRU (Erithacus rubecula), EUCY (Euphagus cyanocephalus), FASP (Falco sparverius), FATI1 (Falco tinnunculus), FATI2 (F. tinnunculus), HALE1 (Haliaeetus leucocephalus), HALE2 (H. leucocephalus), HIRU (Hirundo rustica), HITA1 (Hirundo tahitica), HITA2 (H. tahitica), LADO (Larus dominicanus), LALU (Lanius ludovicianus), MEFO (Melanerpes formicivorus), MEFU (Melanitta fusca), MEVI (Merops viridis), MOCA (Morus capensis), PAMO (Parus montanus), PHCA (Phalacrocorax carbo), PHNI (Phainopepla nitens), PINU (Pica nuttalli), PRSU (Progne subis), RIRI (Riparia riparia), RITR (Rissa tridactyla), SPAM (Spiza americana), STFU (Sterna fuscata), STVU (Sturnus vulgaris), TORU (Toxostoma rufum), VIOL (Vireo olivaceus) (see appendix in the electronic supplementary material for details)).
(b) Estimates of flight costs and energy expenditure
From the mean NFHR measured for eiders (148±18 beats min−1; table 1) and the metabolic cost of flight (see below), we estimated that on average they expend 1726 kJ per day on a daily routine, which is equivalent to 19.98 W. Moreover, from the mean RHR (86±15 beats min−1), we obtained a RMR of 7.50 W. From the mean HR recorded during flight (359±21 beats min−1), we estimated flight costs to be between 123 and 149 W (according to one of other of the methods used and including only the forward flapping flight phase of flight activity). As a result, flight costs are high compared with RMR (16–20×RMR). Using 16–20×RMR as flight costs, the proportion of the daily energy budget associated with flight was therefore 4.3–5.2% or six to eight times the portion of daily flight time budget (0.66% TSF per day).
(c) Relationship between TSF and DHR
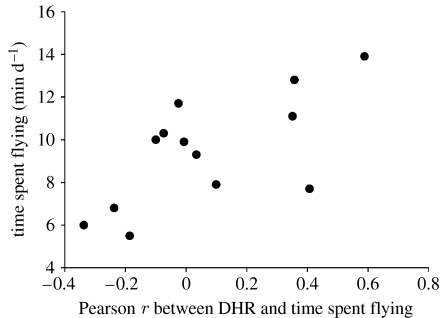
At the inter-individual level, we found no relationship between TSF and DHR (r=0.149, p=0.616). At the intra-individual level, the relationship between DHR and TSF is not consistent among individuals (table 2). Out of 13 individuals, seven had negative correlations between these two variables and six exhibited positive correlations. Interestingly, there is a positive and significant relationship (r=0.678, p=0.012) between the time devoted to flight and the level of correlation between DHR and the TSF (figure 3). In other words, the intra-individual correlation between DHR and the TSF is more likely to be positive when an individual invests more time in flying. By contrast, the presence of negative correlations at the intra-individual level suggests that some female Common Eider were reducing their energy expenditure somehow when their level of flight activity was high.
Table 2.
Pearson's r correlation calculated between time spent flying per day and daily heart rate (DHR), non-flight heart rate (NFHR) or NFHR including migrations at intra-individual level in 13 common eiders.
| individual | DHR (excluding migrations) | NFHR (excluding migrations) | NFHR (including migrations) |
|---|---|---|---|
| BR | 0.357 | 0.314 | 0.275 |
| OO | 0.588 | 0.546 | 0.327 |
| OR | 0.034 | −0.010 | −0.222 |
| OY | −0.337 | −0.452 | −0.421 |
| RB | −0.186 | −0.232 | −0.140 |
| RR | −0.074 | −0.147 | −0.193 |
| RW | 0.351 | 0.170 | −0.247 |
| WB | −0.007 | −0.145 | −0.251 |
| WO | −0.025 | −0.071 | −0.099 |
| YB | −0.237 | −0.301 | −0.271 |
| YR | −0.100 | −0.274 | −0.211 |
| YW | 0.099 | 0.052 | 0.029 |
| YY | 0.407 | 0.333 | 0.103 |
Figure 3.
Relationship between time spent flying (TSF) per day and the correlations relating daily heart rate (DHR) and TSF at the intra-individual level in 13 common eiders (r=0.678, p=0.012).
To test that hypothesis further, we initially removed all the heart beats associated with flight from DHR for each day and each female to get the NFHR and calculated a new correlation coefficient. We then performed a similar calculation with the migration period included. We found that most intra-individual correlations between TSF and NFHR were negatives, which were even more pronounced when migration periods were included (table 2).
4. Discussion
We present the first long-term measurements, to our knowledge, of flight time for a wild bird based on an exhaustive recording of flight activity. Results indicate that the Common Eider uses flight very little throughout the annual cycle and, in spite of being energetically costly, we did not detect any substantial influence of eiders' flight activity on their energy budget. In the following, we discuss how these findings could be the outcome of energy budget limitations or simply because food supplies do not require frequent foraging movements.
(a) Flight time
The common eider is one of the thriftiest volant birds, with less than 10 min spent flying per day. Most time-budget studies of other species (44 species in 36 studies) were conducted during a single phase of the annual cycle (mainly breeding, 48%, or wintering, 30%), during a few days of the annual cycle (one study) or during an unspecified phase (18%). Moreover, most studies were conducted during daylight (98%) and/or in semi-artificial environments such as large aviaries (39%). All these potential biases most probably illustrate the logistical difficulty in following flying birds. We show here the possible biases by subsampling the TSF of common eiders using only the daylight period. We found that if we had monitored flight only during the daylight period, we would have introduced an error for the estimation of TSF of −12% when including twilight (8.5 min d−1 on average) or −30% when excluding twilight (6.7 min d−1). This is related to the fact that eiders prefer to fly around dawn (Pelletier et al. 2007).
(b) Flight costs and the energy budget
Flight costs were estimated to be between 123 and 149 W, or 16–20×RMR, depending on the method used. Although these flight costs are high, which is consistent with their peculiar wing morphology (Guillemette & Ouellet 2005a), they most probably represent an underestimation. Take-offs, ascents, descents and landings make up a large part of every short flight and are more costly than cruising flights (Nudds & Bryant 2000; Hambly et al. 2002, 2004). For short flights performed by small birds (duration of a few seconds for passerines), high costs can be explained in part as a result of the large induced power requirement to generate lift, work against gravity and fly at speeds below minimum power speed (Pennycuick 1989). However, large birds cannot generate enough lift at low speeds and the short-burst flight performance required for take-off is dominated mainly by the anaerobic metabolism (Marden 1994; Guillemette & Ouellet 2005a). Larger birds must generate more power to achieve the same lift and, as a consequence, they increase their flight muscle power output over the typical aerobic limit (100 W kg−1; Marden 1994). Nevertheless, take-offs and landings represent only a small proportion (less than 10%) of an average flight (2.3 min).
A notable result of our study is that there is little evidence for a positive relationship between TSF and DHR despite the fact that common eider use flapping flight continuously while flying. We suggest this result to be the consequence of the low proportion of the energy budget that flight cost represents. Birt-Friesen et al. (1989) observed that field MR was higher for seabirds using flapping flight. By contrast, our estimate of DMR for the eider (1726 kJ d−1) is 22% below the values predicted by a recent allometric model pertaining to 36 species of marine birds (Ellis & Gabrielsen 2002). At the inter-individual level, our results also contrast with many reports stating that time spent in high-cost activities like flight is positively related to daily energy expenditure (Flint & Nagy 1984; Masman & Klaassen 1987; Birt-Friesen et al. 1989; Bryant & Tatner 1991; Carlson & Moreno 1992; Nudds & Bryant 2000). However, these authors mentioned that inaccurate predictions of flight times could yield errors in correlation tests and that it should be a priority in future studies to ensure that the flight component be accurately sampled. Since we exhaustively quantified all local flights performed during several months, we can exclude this error source in our study.
We were unable to find consistent relationships between TSF and DHR at the intra-individual level (table 2). This is because the correlations were positive in some cases and negative in others. However, a clearer picture emerges when heart beats associated with flight are removed from DHR (=NFHR; see §2). Indeed, we found a negative relationship between NFHR and TSF (table 2) for a majority of individuals, indicating that a high level of flight activity was associated with a reduction of the rest of the energy budget. How exactly this is achieved is unknown, but we are left with the conclusion that expensive flight costs do not lead to an expensive lifestyle for the eider duck.
(c) Why fly?
An interesting question arising from our study is whether the little flight activity performed by an eider is the result of energy budget limitations or the lack of any requirement to move rapidly to better foraging habitats? In the first case, an eider would voluntarily reduce the TSF per day in order to save energy for other activity to restrict the energy budget within certain limits. Time allocated to expensive activities like flight is finely tuned and any increase of that component would interfere with other components of the energy budget. However, the physiological (energy budget) hypothesis competes with the ecological one: the little flight activity observed in this study may be connected to the fact that common eiders exploit an abundant and low depleting food resource composed of sessile prey (e.g. molluscs; Guillemette & Himmelman 1996; Larsen & Guillemette 2000). Evidence that flight is not a prerequisite of finding food in this species is provided by the fact that they forage regularly during wing moult when they are completely flightless for several weeks (Guillemette et al. 2007). Further evidence is emerging from studies of other species of sea ducks with similar food habits of the genus Tachyeres, found in southern South America, which includes three permanently flightless species (Livezey & Humphrey 1982). Thus, we may wonder why common eiders did not abandon the ability to fly altogether during the course of evolution.
There are three possible answers to that question. First, although finding food may not be an obligatory function of flight in this species, it may allow an increase in foraging opportunities and permit a higher degree of prey selection. Like other sea ducks, Common Eiders swallow their prey whole with their exoskeletons and intake of inorganic material can be largely reduced by seeking prey of small size, as it reduces shell intake (Guillemette 1998). In support of this, Pelletier et al. (2007) showed a strong and positive correlation between flight and dive frequencies in Common Eiders, suggesting that flight was an integral part of the foraging strategy in this species. Second, flight can be of paramount importance to escape from harsh environmental conditions. This is especially true for a bird species inhabiting habitats dominated by extensive ice formations in winter that move back and forth with currents and winds (Guillemette & Himmelman 1996). Third, flight may be the only way to escape predators, especially because both aerial (eagles) and aquatic (seals) predators can catch and kill diving birds (Guillemette & Ouellet 2005b). In his review, Pomeroy (1990) observed that survival probability of flightless birds was less than volant species' and concluded that predator evasion was the main function of flight. In conclusion, we suggest that the (fitness) benefits of retaining the ability to fly in this species exceed the costs of such an expensive mode of locomotion. Our results suggest that maintenance of this aptitude in this species is made possible by minimizing flight activity with no or little influence on their energy budget, although we do not know yet if such a low flight activity is physiologically or ecologically determined.
Acknowledgments
We thank Dr Annette Flagstad for the surgeries, Peter Lyngs and Yves Rigou for their help in the field, and Torben and Emma Jørgensen for their collaboration with the implantations. We thank Joël Bêty, Jacques Larochelle and Julien Lambrey de Souza and two anonymous reviewers for their constructive comments on the manuscript. This study was conducted in collaboration with the National Environmental Research Institute of Denmark and was funded through the Canadian Natural Sciences and Engineering Research Council (NSERC) discovery and equipment grants to M. Guillemette and a graduate fellowship to D. Pelletier.
Supplementary Material
Litterature review of bird studies presenting measurements of time spent flying per day
References
- Bevan R.M, Keijer E, Butler P.J. A method for controlling the feeding behaviour of aquatic birds: heart rate and oxygen consumption during dives of different duration. J. Exp. Biol. 1992;162:91–106. [Google Scholar]
- Bevan R.M, Woakes A.J, Butler P.J. The use of heart rate to estimate oxygen consumption of free-ranging black-browed albatrosses Diomedea melanophrys. J. Exp. Biol. 1994;193:119–137. doi: 10.1242/jeb.193.1.119. [DOI] [PubMed] [Google Scholar]
- Birt-Friesen V.L, Montevecchi D.K, Cairns D.K, Macko S.A. Activity-specific metabolic rates of free-living northern gannets and other seabirds. Ecology. 1989;70:357–367. doi:10.2307/1937540 [Google Scholar]
- Bishop C.M. Heart mass and the maximum cardiac output of birds and mammals: implications for estimating the maximum aerobic power input of flying animals. Phil. Trans. R. Soc. B. 1997;352:447–456. doi:10.1098/rstb.1997.0032 [Google Scholar]
- Bishop C.M, Butler P.J. Physiological modelling of oxygen consumption in birds during flight. J. Exp. Biol. 1995;198:2153–2163. doi: 10.1242/jeb.198.10.2153. [DOI] [PubMed] [Google Scholar]
- Bolduc F, Guillemette M. Human disturbance and nesting success of Common Eiders: interaction between visitors and gulls. Biol. Conserv. 2003;110:77–83. doi:10.1016/S0006-3207(02)00178-7 [Google Scholar]
- Boyd I.L, Bevan R.M, Woakes A.J, Butler P.J. Heart rate and behaviour of fur seals, implications for measurement of field energetics. Am. J. Physiol. 1999;276:H844–H857. doi: 10.1152/ajpheart.1999.276.3.H844. [DOI] [PubMed] [Google Scholar]
- Bryant D.M, Tatner P. Intraspecies variation in avian energy expenditure: correlates and constraints. Ibis. 1991;133:236–245. doi:10.1111/j.1474-919X.1991.tb04565.x [Google Scholar]
- Butler P.J. Exercise in birds. J. Exp. Biol. 1991;160:233–262. [Google Scholar]
- Butler P.J. To what extent can heart rate be used as an indicator of metabolic rate in free-living marine mammals? Symp. Zool. Soc. Lond. 1993;66:317–332. [Google Scholar]
- Butler, P. J. & Bishop, C. M. 2000 Flight. In Sturkie's avian physiology (ed. G. C. Whittow), 5th edn. New York, NY: Academic Press.
- Butler P.J, Woakes A.J, Bishop C.M. Behaviour and physiology of Svalbard Barnacle Geese Branta leucopsis during their autumn migration. J. Avian Biol. 1998;29:536–545. doi:10.2307/3677173 [Google Scholar]
- Butler P.J, Woakes A.J, Bevan R.M, Stephenson R. Heart rate and rate of oxygen consumption during flight of the barnacle goose, Branta leucopsis. Comp. Biochem. Physiol. A. 2000;126:379–385. doi: 10.1016/s1095-6433(00)00221-x. doi:10.1016/S1095-6433(00)00221-X [DOI] [PubMed] [Google Scholar]
- Butler P.J, Green J.A, Boyd I.L, Speakman J.R. Measuring metabolic rate in the field: the pros and cons of the doubly labelled water and heart rate methods. Funct. Ecol. 2004;18:168–183. doi:10.1111/j.0269-8463.2004.00821.x [Google Scholar]
- Carlson A, Moreno J. Cost of short flights in the Willow Tit measured with doubly-labeled water. Auk. 1992;109:389–393. [Google Scholar]
- Ellis, H. I. 1984 Energetics of free-ranging seabirds. In Seabird Energetics (eds G. C. Whittow & H. Rahn), pp. 203–234. New York, NY: Plenum Press.
- Ellis H.I, Gabrielsen G.W. Energetics of free-ranging seabirds. In: Schreiber E.A, Burger J, editors. Biology of marine birds. CRC Press; Boca Raton, FL: 2002. pp. 359–407. [Google Scholar]
- Flint E.N, Nagy K.A. Flight energetics of free-living sooty terns. Auk. 1984;101:288–294. [Google Scholar]
- Froget G, Butler P.J, Handrich Y, Woakes A.J. Heart rate as an indicator of oxygen consumption; influence of body condition in the king penguin. J. Exp. Biol. 2001;204:2133–2144. doi: 10.1242/jeb.204.12.2133. [DOI] [PubMed] [Google Scholar]
- Good P.I. 2nd edn. Birkhauser; Boston, MA: 2001. Resampling methods. [Google Scholar]
- Good P.I. Wiley; New York, NY: 2005. Introduction to statistics through resampling methods and Microsoft Office Excel. [Google Scholar]
- Green J.A, Frappell P.B. Improving the precision and accuracy of estimates made using the heart rate method. Physiol. Biochem. Zool. 2007;80:551–555. doi: 10.1086/519961. doi:10.1086/519961 [DOI] [PubMed] [Google Scholar]
- Greenewalt C.H. The flight of birds. Trans. Am. Phil. Soc. 1975;65:4. [Google Scholar]
- Guillemette M. The effect of time and digestion constraints in common eiders while feeding and diving over blue mussel beds. Funct. Ecol. 1998;12:123–131. doi:10.1046/j.1365-2435.1998.00164.x [Google Scholar]
- Guillemette M, Himmelman J. H Distribution of common eiders over mussel beds in winter: does the ideal-free distribution apply? Oikos. 1996;76:435–442. doi:10.2307/3546337 [Google Scholar]
- Guillemette M, Ouellet J.-F. Temporary flightlessness in pre-laying common eiders Somateria mollissima: are females constrained by excessive wing-loading or by minimal flight muscle ratio? Ibis. 2005a;147:293–300. doi:10.1111/j.1474-919x.2005.00401.x [Google Scholar]
- Guillemette M, Ouellet J.-F. Temporary flightlessness as a potential cost of reproduction in pre-laying common eiders Somateria mollissima. Ibis. 2005b;147:301–306. doi:10.1111/j.1474-919x.2005.00402.x [Google Scholar]
- Guillemette M, Woakes A.J, Flagstad A, Butler P.J. Effects of data-loggers implanted for a full year in female common eiders. Condor. 2002;104:448–452. doi:10.1650/0010-5422(2002)104[0448:EODLIF]2.0.CO;2 [Google Scholar]
- Guillemette M, Woakes A.J, Hénaux V, Grandbois J.-M, Butler P.J. The effect of depth on the diving behaviour of common eiders. Can. J. Zool. 2004;82:1818–1826. doi:10.1139/z04-180 [Google Scholar]
- Guillemette M, Pelletier D, Grandbois J.-M, Butler P.J. Flightlessness and the energetic cost of wing molt in a large sea duck. Ecology. 2007;88:2936–2945. doi: 10.1890/06-1751.1. doi:10.1890/06-1751.1 [DOI] [PubMed] [Google Scholar]
- Hambly C, Harper E.J, Speakman J.R. Cost of flight in the zebra finch (Taenopygia guttata): a novel approach based on elimination of 13C labelled bicarbonate. J. Comp. Physiol. B. 2002;172:529–539. doi: 10.1007/s00360-002-0279-7. doi:10.1007/s00360-002-0279-7 [DOI] [PubMed] [Google Scholar]
- Hambly C, Pinshow B, Wiersma P, Verhulst S, Piertney S.B, Harper E.J, Speakman J.R. Comparison of the cost of short flights in a nectarivorous and a non-nectarivorous bird. J. Exp. Biol. 2004;207:3959–3968. doi: 10.1242/jeb.01233. doi:10.1242/jeb.01233 [DOI] [PubMed] [Google Scholar]
- Hawkins P.A.J, Butler P.J, Woakes A.J, Speakman J.R. Estimation of the rate of oxygen consumption of the common eider duck (Somateria mollissima), with some measurements of heart rate during voluntary dives. J. Exp. Biol. 2000;203:2819–2832. doi: 10.1242/jeb.203.18.2819. [DOI] [PubMed] [Google Scholar]
- Kahlert, J., Petersen, I. K., Fox, A. D., Desholm, M. & Clausager, I. 2003 Investigations of birds during construction and operation of Nysted offshore wind farm at Rødsand. NERI report, p. 78.
- Larsen J.K, Guillemette M. Influence of annual variation in food supply on abundance of wintering common eiders Somateria mollissima. Mar. Ecol. Prog. Ser. 2000;201:301–309. doi:10.3354/meps201301 [Google Scholar]
- Livezey B.C, Humphrey P.S. Escape behaviour of steamer ducks. Wildfowl. 1982;33:12–16. [Google Scholar]
- Lovvorn J.R, Jones D.R. Biomechanical conflicts between adaptations for diving and aerial flight in estuarine birds. Estuaries. 1994;17:62–75. doi:10.2307/1352335 [Google Scholar]
- Lund G.F, Folk G.E. Simultaneous measurements of heart rate and oxygen consumption in black-tailed prairie dogs (Cynomys ludovicianus) Biochem. Physiol. 1976;55A:201–206. doi: 10.1016/0300-9629(76)90131-6. doi:10.1016/0300-9629(76)90131-6 [DOI] [PubMed] [Google Scholar]
- Lyngs P. Status of the Danish Breeding population of Eiders Somateria mollissima 1988–93. Dansk Ornitologisk Forenings Tidsskrift. 2000;94:12–18. [Google Scholar]
- Marden J.H. From damselflies to pterosaurs: how burst and sustainable flight performance scale with size. Am. J. Physiol. 1994;266:R1077–R1084. doi: 10.1152/ajpregu.1994.266.4.R1077. [DOI] [PubMed] [Google Scholar]
- Masman D, Klaassen M. Energy expenditure during free flight in trained and free-living Eurasian kestrels (Falco tinnunculus) Auk. 1987;104:603–616. [Google Scholar]
- McNab B.K. Energy conservation and the evolution of flightlessness in birds. Am. Nat. 1994;144:628–642. doi:10.1086/285697 [Google Scholar]
- McNab B.K. Comstock Publishing Associates, Cornell University Press; Ithaca, NY: 2002. The physiological ecology of vertebrates, a view from energetics. [Google Scholar]
- Norberg U.M. Springer; Berlin/Heidelberg, Germany: 1990. Vertebrate flight. Mechanics, physiology, morphology, ecology and evolution. [Google Scholar]
- Nudds R.L, Bryant D.M. The energetic cost of short flights in birds. J. Exp. Biol. 2000;203:1561–1572. doi: 10.1242/jeb.203.10.1561. [DOI] [PubMed] [Google Scholar]
- Ouellet J.-F, Guillemette M, Blier P.U. Morphological and physiological aspects of takeoff aptitudes of female common eiders (Somateria mollissima) during the pre-laying period. Can. J. Zool. 2008;86:462–469. [Google Scholar]
- Owen R.B., Jr Heart rate, a measure of metabolism in blue-winged teal. Comp. Biochem. Physiol. 1969;31:431–436. doi: 10.1016/0010-406x(69)90024-3. doi:10.1016/0010-406X(69)90024-3 [DOI] [PubMed] [Google Scholar]
- Pauls R.W. Heart rate as an index of energy expenditure in red squirrels (Tamiasciurus hudsonicus) Comp. Biochem. Physiol. 1980;67A:409–418. doi:10.1016/S0300-9629(80)80017-X [Google Scholar]
- Pelletier, D. 2006 Étude à long terme du comportement et de l'énergétique du vol chez l'Eider à duvet (Somateria mollissima) en milieu naturel. MSc thesis, Université du Québec à Rimouski, Québec, Canada, 147 p.
- Pelletier D, Guillemette M, Grandbois J.-M, Butler P.J. It is time to move: linking flight and foraging behaviour in a diving bird. Biol. Lett. 2007;3:357–359. doi: 10.1098/rsbl.2007.0088. doi:10.1098/rsbl.2007.0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennycuick C.J. Oxford University Press; Oxford, UK: 1989. Bird flight performance, a practical calculation manual. [Google Scholar]
- Peters G.W, Steiner D.A, Rigoni J.A, Mascilli A.D, Schnepp R.W, Thomas S.P. Cardiorespiratory adjustments of homing pigeons to steady wind tunnel flight. J. Exp. Biol. 2005;208:3019–3120. doi: 10.1242/jeb.01751. doi:10.1242/jeb.01751 [DOI] [PubMed] [Google Scholar]
- Pomeroy D. Why fly? The possible benefits for lower mortality. Biol. J. Linn. Soc. 1990;40:53–65. doi:10.1111/j.1095-8312.1990.tb00534.x [Google Scholar]
- Rayner J.M.V. Flight mechanics and constraints on flight performance. Isr. J. Zool. 1995;41:321–342. [Google Scholar]
- Ricker W.E. Linear regressions in fishery research. J. Fish. Res. Board Can. 1973;30:409–434. [Google Scholar]
- Storer R.W. Adaptive radiation in birds. In: Farner D.S, King J.R, editors. Avian biology 1. Academic Press; London, UK: 1971. pp. 149–188. [Google Scholar]
- Tatner P, Bryant D.M. Flight cost of a small passerine measured using doubly labeled water: implications for energetics studies. Auk. 1986;103:169–180. [Google Scholar]
- Ward S, Bishop C.M, Woakes A.J, Butler P.J. Heart rate and the rate of oxygen consumption of flying and walking barnacle geese (Branta leucopsis) and bar-headed geese (Anser indicus) J. Exp. Biol. 2002;205:3347–3356. doi: 10.1242/jeb.205.21.3347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Litterature review of bird studies presenting measurements of time spent flying per day