Abstract
Niche expansion can lead to adaptive differentiation and speciation, but there are few examples from contemporary niche expansions about how this process is initiated. We assess the consequences of a niche expansion by Mexican jays (Aphelocoma ultramarina) along an elevation gradient. We predicted that jays at high elevation would have straighter bills adapted to feeding on pine cones, whereas jays at low elevation would have hooked bills adapted to feeding on acorns. We measured morphological and genetic variation of 95 adult jays and found significant differences in hook length between elevations in accordance with predictions, a pattern corroborated by analysis at the regional scale. Genetic results from microsatellite and mtDNA variation support phenotypic differentiation in the presence of gene flow coupled with weak, but detectable genetic differentiation between high- and low-elevation populations. These results demonstrate that niche expansion can lead to adaptive divergence despite gene flow between parapatric populations along an elevation gradient, providing information on a key precursor to ecological speciation.
Keywords: niche expansion, local adaptation, gene flow, sky islands, Aphelocoma ultramarina
1. Introduction
Niche expansion, where a species augments its realized niche when presented with ecological opportunity, is thought to play an important role in species formation (Simpson 1953; Schluter 2000), and its link to adaptive divergence has been the focus of study during colonization of islands (Losos et al. 1994; Grant 1998; Herrel et al. 2008) and postglacial lakes (Schluter & Rambaut 1996; Knudsen et al. 2006), and after host shifts in insect systems (Feder et al. 1988; Carroll & Boyd 1992). However, the ecological triggers of divergence during niche expansion are little studied compared with the ecological consequences of niche expansion (e.g. Diamond 1970; Blondel et al. 1988; Martin 1992). This is at least partly due to the inherent difficulty of reconstructing geographical ranges and habitat preferences on phylogenies of extant radiations, which are complicated by rapid lineage splitting (e.g. Jackman et al. 1999). Thus, investigations of contemporary niche expansions provide an opportunity to examine the ecological processes promoting divergence and the spatial context in which it can be initiated.
Our study investigates differentiation resulting from a recent niche expansion in a little-studied setting, a continental mountain range, the Sierra del Carmen of Coahuila, Mexico. The Sierra del Carmen is part of a regional network of sky islands—isolated mountain ranges harbouring high-elevation forest that are separated from one another by desert basins. Sky islands are thought to have formed since the last glacial maximum, as warming facilitated expansion of deserts in lowland basins, fragmenting highland forests (Betancourt et al. 1990). Probably due to its distance from colonizing sources, the Sierra del Carmen hosts an impoverished complement of resident bird species compared with other mountain ranges of similar size and latitude; as a result, several species have expanded their realized niches to include habitat in which they are normally absent (Miller 1955).
No species in the Sierra del Carmen has expanded its niche more conspicuously than the Mexican jay (Aphelocoma ultramarina; Miller 1955). Throughout most of its geographical range, the Mexican jay is found in a narrow belt of mid-elevation pine–oak–juniper woodland between 1350 and 1800 m, and is replaced at lower elevation by the Western scrub-jay (Aphelocoma californica) and at higher elevation by the Steller's jay (Cyanocitta stelleri; Brown & Brown 1985). In the Sierra del Carmen, Mexican jays have expanded their altitudinal range in the absence of these species and now occupy a dramatic habitat gradient that spans from oak scrub in low-elevation canyons at 1200 m to moist conifer forest of the highest peaks at 2700 m (Miller 1955).
Two aspects of the natural history of the Mexican jay make it particularly well suited to a study of adaptive differentiation along a habitat gradient. First, Mexican jays are highly sedentary (McCormack & Brown 2008), making differentiation at small spatial scales more likely. Second, Mexican jays live in flocks that defend territories with rigid borders year-round. Thus, survival depends on local resources, namely mast-seeding trees that comprise their main diet in winter when resources are otherwise scarce (McCormack & Brown 2008).
Different ecotypes of the Western scrub-jay (A. californica) show localized adaptation of the bill to feeding on pine seeds or acorns (Peterson 1993). The adaptive nature of the ecotypes has its basis in functional morphology: the acorn is grasped with the feet and pounded with the lower mandible to crack the husk, which is then removed using the hook (Peterson 1993). By contrast, jays insert their bills like tweezers between the scales of opened pine cones to extract seeds (Vander Wall & Balda 1981), an action that is hindered by the hook (Peterson 1993).
These bill differences in the Western scrub-jay occur between populations separated by large distances (more than 100 km) connected by minimal, if any, gene flow (Peterson 1991). The goal of our study was to determine whether niche expansion could promote similar adaptive differences at local scales (less than 10 km) along an elevation gradient where populations are presumably linked by gene flow. First, we established baseline ecological differences between high and low elevations and sampled jays at several sites throughout the mountain range to assess morphological and genetic variation. Following results from Peterson (1993), we predicted that jays in high-elevation conifer forest would have straighter bills, whereas jays in low-elevation oak woodland would have more hooked bills. We also measured bill morphology of jays in mountain ranges throughout the region, predicting a negative correlation between elevation and hook length. Such an independent, parallel pattern of morphological variation would strengthen the case that differences are adaptive. Finally, to bolster the case for niche expansion as the cause of adaptive divergence, we compared variation in bill traits between the Sierra del Carmen and a mountain range of similar size where Mexican jays have not undergone niche expansion. We predicted greater variation in hook length in the Sierra del Carmen, in accordance with the niche variation hypothesis, which states that greater habitat diversity resulting from niche expansion should allow for the formation of microgeographic structure and the coexistence of different ecotypes (van Valen 1965).
2. Material and methods
(a) Study site and field sampling
Mainly using mist nets, we sampled populations at three low-elevation and three high-elevation sites in the Sierra del Carmen and at six other sites in mountain ranges throughout the region where Mexican jays also experience some degree of niche expansion, though not to the extent as that seen in the Sierra del Carmen (figure 1). At one high-elevation site (Campo Dos), a small proportion (5 out of 30) of the sampled individuals were caught with a baited ground trap. As the trap was baited mainly with bread, the individuals captured were not expected to represent a biased sample of the population in terms of bill morphology. At each site, we sampled jays from 3 to 10 flocks to ensure that many closely related individuals were not included in the dataset. Jays captured in the field were aged and marked with a uniquely numbered aluminium band. After processing (see §2c), we released birds at their point of capture.
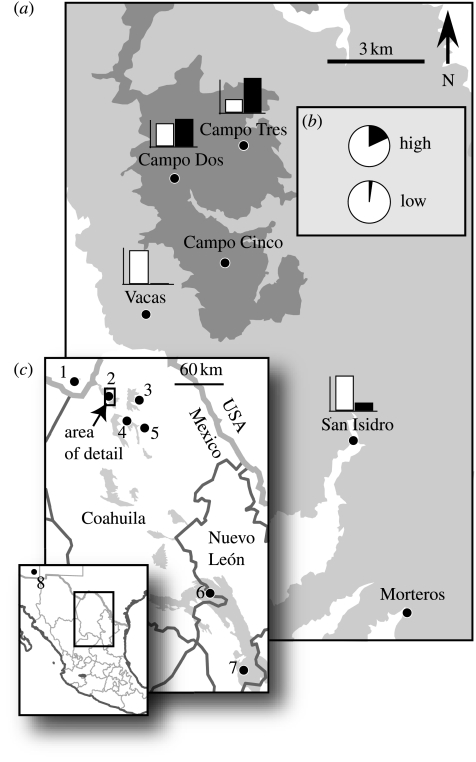
Figure 1.
(a) Sierra del Carmen showing six sampling sites at high elevation (above 2300 m, dark grey) and low elevation (1500–2300 m, light grey). Bar graphs above sites show relative basal area of oaks (white) and pines (black). (b) Relative number of acorns (white) to pine seeds (black) identified from feeding observations of jays at high and low elevations (d.f.=1, p=0.01). (c) Sampling locations in the United States (1, Chisos Mountains, Texas, n=10) and Mexico (2, Sierra del Carmen, n=96; 3, Rancho La Escondida, Serranías del Burro, n=20; 4, Rancho Las Cabras, Sierra Santa Rosa, n=5; 5, Rancho La Rosita, Sierra Santa Rosa, n=10; 6, El Taray, Sierra de Arteaga, n=10; 7, Ejido La Encantada, Sierra Peña Nevada, n=3). The Chiricahua Mountains are labelled site 8 in the small inset.
(b) Habitat and foraging analysis
We quantified habitat at four of the sampling sites using randomly located circular plots of 10 m radius (high elevation: Campo Dos, 12 plots; Campo Tres, 9 plots; low elevation: San Isidro, 20 plots; Vacas, 20 plots). We did not quantify vegetation at the other two sites (Campo Cinco and Morteros) because relatively few jays were caught there and vegetation at these sites was qualitatively similar to sites at similar elevation. We recorded species and diameter at breast height (DBH) for single-bole trees over 8 cm DBH and multiple-bole trees with combined DBH over 18 cm. Smaller trees were not commonly observed to produce mast. We used raw counts and DBH to calculate a total basal area for each tree species at each sampling site. We then calculated ratios of basal area of pines and oaks for each site.
Mexican jays are omnivorous during the spring and summer. In autumn, they start eating and storing seeds from mast-producing trees (McCormack & Brown 2008). We collected foraging data during two autumn field seasons (September–October of 2003–2004). After initially sighting a foraging jay, we recorded only the first eating observation whether or not the food item could be identified. We analysed only those observations for which the resource could be identified, testing for diet differences between high and low elevations with a one-tailed Fisher's exact probability test on the number of eating events on pine seeds and acorns.
(c) Morphological analysis
We measured unflattened wing length to the nearest 0.5 mm with a standard wing ruler and the following traits with digital calipers to the nearest 0.1 mm: tail length from the uropygial gland to the tip of the central rectrix; tarsus length from the tibiotarsus joint to the distal end of the tarsometatarsus; bill length from the anterior end of the nares; bill width and depth at the anterior end of the nares; and lower length from the tip of the lower mandible to the beginning of the depression where the rami meet. We measured each trait three times and recorded mean values, which were used in all subsequent analyses. All measurements were taken by J.E.M.
Hook length was measured using ImageJ software (Abramoff et al. 2004) from digital photos taken in the field using methods adapted from Bardwell et al. (2001). Photos were taken with a Canon Powershot A40 with macro lens attachment. To avoid parallax, we positioned the camera approximately 50 cm away from the subject, ensuring that the bill was perpendicular to the angle of the photograph. Each photo contained a size standard that was affixed to the bill for the photo and removed before the bird was released. We digitally measured hook length blind to individual origin by temporarily relabelling image files. We define hook length as the perpendicular distance from a line drawn tangent to the tomia at the base of the bill to the tip of the bill (see figure S1 in the electronic supplementary material). We estimated repeatability (Lessells & Boag 1987) of hook measurement from 10 repeated photos of 10 individuals. For each replicate photo, the bird was removed from the photo area and then brought back up to a perpendicular position.
We used data from adult (i.e. older than second year) birds because first-year birds often have shorter bills (Brown & Bhagabati 1998). Jays could be aged as adults with high accuracy by pigmentation of the inner upper mandible and moult limits (Pyle 1997). We subdivided data by sex and elevation and assessed traits for normality with Kolmogorov–Smirnov tests. We tested for elevational differences first using ANCOVA, controlling for effects of sex and season, and then, if differences were found, with Student's t-tests. Results are shown as ±1 s.e. We assessed differences in bill size and shape with principal components analysis (PCA) of the covariance matrix using bill length, lower mandible length, bill width, bill depth and hook length. To confirm an assumption of PCA, we assessed similarity of covariance matrices between populations at high and low elevations under a common principal components (CPC) framework (Flury 1988) using software available at www.uoregon.edu/∼pphil/programs/cpc/cpc.htm.
We performed a regression of hook length and elevation for jays captured throughout the region using robust standard errors for cluster-correlated data implemented with the cluster (variable) command in Stata Intercooled v. 8.2 (StataCorp 2003). This method controls for correlations within groups of data, in this case within sites, for calculation of p-values (Williams 2000). We ran the analysis with and without data from the Sierra del Carmen.
We tested for a difference in the coefficient of variation (CV=(standard deviation/mean)×100) of bill traits between jays in the Sierra del Carmen and the Chiricahua Mountains with an F-test of ln-transformed data (Lewontin 1966). Mexican jays in the Chiricahua Mountains belong to a different subspecies to those in the Sierra del Carmen and there is no current gene flow between these groups (McCormack et al. 2008). We measured bill morphology (bill length, width and depth) of 32 museum specimens from the Chiricahua Mountains and estimated hook length from digital photos following the methods described above. Chiricahua Mountain jays came from several locations in the mountain range: Pinery Canyon (4), Paradise (12), Cave Creek Canyon (1), unknown (15).
(d) Genetic analysis
Protocols for extraction, amplification and sequencing of mtDNA and microsatellites are described in McCormack et al. (2008). Briefly, we extracted genomic DNA from blood and/or feathers. Individuals were genetically sexed with a modification of Fridolfsson & Ellegren's (1999) polymerase chain reaction (PCR) technique, which employed a different reverse primer named MSZ1R (5′-ATCCATCAAGTCTCTAAAGAG-3′; designed by I. Lovette) to bind to the CHD1 gene on the Z chromosome. When run on an agarose gel, PCR products exhibit one band for males and two bands for females. We assessed variation at nine microsatellite loci (MJG1 and MJG8 from Li et al. (1997) and ApCo15, ApCo18, ApCo19, ApCo22, ApCo29, ApCo30 and ApCo40 from Stenzler & Fitzpatrick (2002)) and a 525 bp portion of hypervariable region I, the mitochondrial DNA (mtDNA) control region, using primers JCR03 (5′-CCCCCCCATGTTTTTACR-3′; Saunders & Edwards 2000) and H1248 (5′-CATCTTCAGTGTCATGCT-3′; Tarr 1995). All DNA sequences used in this study have been deposited in GenBank under accession numbers EU121315–EU121322.
We used Arlequin v. 2.0 (Schneider et al. 2000) to test microsatellite loci for linkage disequilibrium and deviations from Hardy–Weinberg equilibrium and to assess differentiation in microsatellites and mtDNA with exact tests of population differentiation (Raymond & Rousset 1995) using 10 000 Markov chain steps. Pairwise FST values (Weir & Cockerham 1984) between populations at high and low elevations were also calculated, and their significance from zero were tested by permuting haplotypes between populations 5000 times. For highly variable, multilocus datasets, pairwise FST values do not reach their maximum at 1, but are constrained by population-level homozygosity levels (Hedrick 1999). Therefore, for microsatellite data, we calculated maximum FST using Recode v. 0.1 (Meirmans 2006) and present differentiation as G'ST (Hedrick 2005), a standardized measure of genetic differentiation that we calculated by dividing obtained FST values by maximum FST.
We also looked for population structure using Structure v. 2.1 (Pritchard et al. 2000), a Bayesian clustering program that assigns individuals to detected clusters (K), yet does not require a priori information about the expected number of clusters or the geographical locations of individuals. We assumed admixture of populations and the potential for correlated allele frequencies among populations. We tested values of K from 1 to 8 with a burn-in of 200 000 followed by 1 000 000 iterations, with 10 runs for each K. We used the posterior probability (ln Pr[X|K]) to determine the optimal K. Differences between high and low elevations in cluster assignment were assessed for significance with a Kruskal–Wallis rank test.
3. Results
(a) Habitat, diet and morphology
Habitat differed between high and low elevations, with high-elevation sites having a higher ratio of basal area of pines to oaks (figure 1a). Diet also differed, with jays at high elevation eating a higher proportion of pine seeds compared with acorns (12 observations on pine seeds compared with 54 on acorns) than did low-elevation jays (1:42, respectively; Fisher's exact probability test: d.f.=1, p=0.01; figure 1b), although both populations ate more acorns than pine seeds.
Between 2002 and 2005, we captured 95 adult jays (60 males and 35 females). Traits were normally distributed (Kolmogorov–Smirnov tests: p>0.05) except for hook length, which was moderately left skewed at high elevation. Normalizing hook length with ln-transformation did not change the significance of our results, however, so we analysed data and present results in millimetres to facilitate their interpretation. After controlling for effects of sex and season, high- and low-elevation jays differed significantly in two traits: bill length and hook length (table 1). There was no significant interaction between sex and elevation or season and elevation for any trait.
Table 1.
ANCOVA results for eight morphological traits (Note: in traits where the effect of sex was significant, males were larger; where season was significant, autumn traits were larger.).
| trait | effect | F | p |
|---|---|---|---|
| wing | season | 4.88 | 0.03 |
| sex | 23.32 | <0.001 | |
| elevation | 0.1 | 0.75 | |
| tail | season | 12.28 | <0.001 |
| sex | 25.02 | <0.001 | |
| elevation | 0.6 | 0.44 | |
| tarsus | season | 0.99 | 0.32 |
| sex | 7.33 | 0.008 | |
| elevation | 1.13 | 0.29 | |
| bill length | season | 5.03 | 0.027 |
| sex | 10.52 | 0.002 | |
| elevation | 9.88 | 0.002 | |
| lower length | season | 7.51 | 0.007 |
| sex | 2.4 | 0.13 | |
| elevation | 0 | 0.98 | |
| bill width | season | 1.14 | 0.29 |
| sex | 0.06 | 0.81 | |
| elevation | 1.04 | 0.31 | |
| bill depth | season | 5.09 | 0.027 |
| sex | 18.6 | <0.001 | |
| elevation | 1.38 | 0.24 | |
| hook length | season | 0.26 | 0.61 |
| sex | 0.8 | 0.37 | |
| elevation | 7.06 | 0.009 |
Hook length was a highly repeatable measurement (intraclass correlation coefficient=0.85) and significantly longer in low-elevation jays (0.91±0.08 mm, n=48) than in high-elevation jays (0.64±0.06 mm, n=45; Student's t-test: t=−2.68, d.f.=91, p<0.01). This result remained significant when controlling for body size using the first axis of a PCA of all morphological traits as a covariate (ANCOVA: F=4.73, d.f.=74, p=0.03), and significance level increased when size was controlled for using tarsus length (ANCOVA: F=10.31, d.f.=90, p=0.002). Corroborating these results at the analysis level of sites within elevations, all low-elevation sites had larger mean hook sizes than all high-elevation sites (low elevation: Vacas (1.1 mm), Morteros (0.90 mm), and San Isidro (0.82 mm); high elevation: Campo Cinco (0.78 mm), Campo Tres (0.70 mm), and Campo Dos (0.59 mm)), although low sample sizes at some sites led to large variances and low statistical power to detect differences among sites (hence our use of pooled datasets).
In males, bill length was significantly shorter in high-elevation jays (19.22±0.21 mm, n=29) than in low-elevation jays (19.89±0.21 mm, n=31; Student's t-test: t=−2.27, d.f.=58, p=0.027). A concordant result was seen in females (high elevation: 18.56±0.21 mm, n=18; low elevation: 19.26±0.22 mm, n=17; Student's t-test: t=−2.31, d.f.=31, p=0.025). A large hook adds some length to the bill, so to explore whether differences in hook length could explain the differences in bill length we performed an ANCOVA with bill length as the dependent variable, elevation as the independent variable and hook length as a covariate. After controlling for hook length, bill length did not significantly differ by elevation for males (ANCOVA: F=1.01, d.f.=59, p=0.32) or females (ANCOVA: F=3.18, d.f.=32, p=0.08).
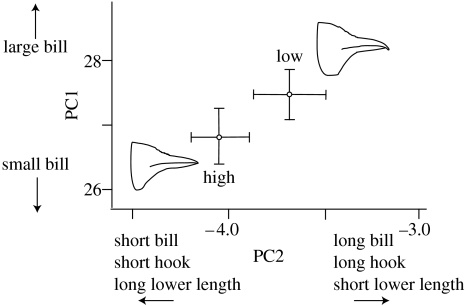
The CPC analysis showed concordance of covariance matrices between high- and low-elevation populations, validating an assumption of PCA. The PCA of five bill traits revealed variation on PC axes 1 and 2 explaining 72% (eigenvalue: 1.95) and 13% (eigenvalue: 0.35) of total variation, respectively. All traits had positive loadings on PC1, consistent with its interpretation as a general size axis. Bill length and hook length loaded positively and lower mandible length loaded negatively on PC2, indicating differences in shape. Jays at high and low elevations significantly differed in both PC1 and PC2 (figure 2).
Figure 2.
PCA of five bill traits. Jays at high and low elevations differ significantly in PC1 (two-tailed t-test: t=−2.24, d.f.=91, p=0.027) and PC2 (two-tailed t-test: t=−3.14, d.f.=91, p=0.002). Bars indicate 95% CI.
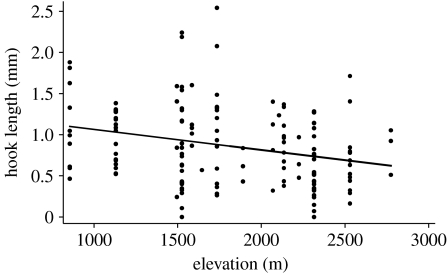
A regression of hook length on elevation for jays throughout the region revealed a significant negative correlation (figure 3). The relationship remained highly significant when jays from the Sierra del Carmen were excluded and the data reanalysed (regression: r2=0.059, F=30.85, d.f.=5, p=0.003, n=58).
Figure 3.
Regional correlation between hook length and elevation (regression: r2=0.075, F=23.32, d.f.=11, p=0.001, n=154). Dots represent individuals. Significance was assessed using robust variance to control for correlations within sites (see text).
A comparison of CVs indicated that male jays from the Sierra del Carmen were more variable in bill depth and hook length, in accordance with the niche variation hypothesis (table 2). After Bonferroni correction, female jays did not differ in any trait, and these data are not presented. Using pooled sex data, jays from the Sierra del Carmen had a mean hook length of 0.78 mm and a standard deviation of 0.52, whereas jays from the Chiricahua Mountains had a mean hook length of 1.00 mm and standard deviation of 0.39, leading to a significantly higher coefficient of variation for the Sierra del Carmen (66.4%) than the Chiricahua Mountains (39.3%; F=3.53, p<0.001).
Table 2.
Coefficients of variation for bill traits of male Mexican jays (*p<0.01, **p<0.001).
| trait | Sierra del Carmen | Chiricahua Mountains | F | ||
|---|---|---|---|---|---|
| n | CV | n | CV | ||
| bill length | 59 | 5.99 | 20 | 7.59 | 0.6 |
| lower length | 59 | 4.92 | 20 | 5.72 | 0.73 |
| bill width | 59 | 5.75 | 20 | 4.76 | 1.5 |
| bill depth | 59 | 4.29 | 18 | 2.72 | 4.79** |
| hook length | 60 | 63.27 | 18 | 43.01 | 3.82* |
(b) Genetic structure and gene flow
Genetic variation in microsatellites and mtDNA revealed a consistent pattern of weak but detectable differentiation. None of the nine microsatellite loci showed evidence of linkage disequilibrium or departures from Hardy–Weinberg equilibrium. An exact test for population differentiation of these loci between high and low elevations was highly significant (p<0.001). Maximum FST was calculated as 0.38, resulting in a significant pairwise G'ST (G'ST=0.021, p=0.02).
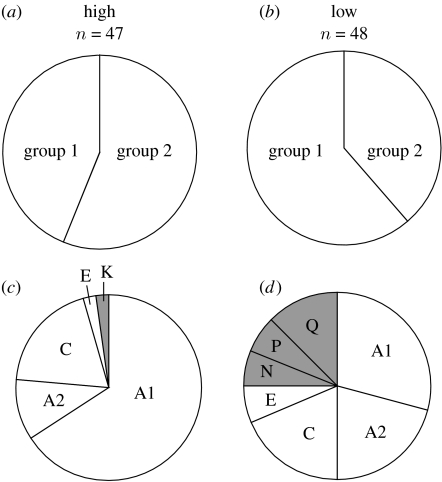
Results from Bayesian analysis of microsatellite variation complement these findings. Models with the number of genetic clusters K=1–3 (ln Pr(X|K)=−2985, −3008 and −3026, respectively) were substantially better than larger values of K (−3096, −3188, −3308, etc.). However, incremental increase of ln Pr(X|K) made distinguishing among K=1–3 problematic (Pritchard & Wen 2003). Visual inspection of individual cluster assignments at K=2 suggested elevational differences; therefore, we tested for a elevational difference in mean assignment to the different clusters. Mean assignment differed significantly between high and low elevations (Kruskal–Wallis test: d.f.=1, p=0.002; figure 4). Evaluation at K=3 did not add qualitatively to this assessment, with the first two clusters showing similar assignment as K=2, and the additional cluster showing equal assignment to both high and low elevations.
Figure 4.
Genetic differences between Mexican jay populations at high (n=47) and low (n=48) elevations. For (a,b) microsatellites, proportion of assignment to two groups as revealed with Bayesian cluster analysis differed significantly (Kruskal–Wallis test: d.f.=1, p=0.002). (c,d) MtDNA data showing shared (white) and private (grey) haplotypes.
Results from mtDNA reveal a similar pattern of differentiation. We found eight unique haplotypes: four were shared between high and low elevations, one was unique to high elevation and three were unique to low elevation (figure 4). High-elevation populations had lower haplotype diversity composed primarily of haplotypes also found at low elevation. Using mtDNA data, an exact test of population differentiation was highly significant (p<0.001), but FST was not (FST=0.023, p=0.15).
4. Discussion
(a) Niche expansion and adaptive divergence
The initial ecological setting and triggers of adaptive divergence resulting from niche expansion are difficult to document in action, and are often later obscured by range shifts, further diversification in non-ecological dimensions (Streelman & Danley 2003) or continuing phyletic evolution. This is well illustrated by adaptive radiations, whose preliminary stages are notoriously difficult to reconstruct (e.g. Jackman et al. 1999). For these reasons, it is desirable to study contemporary niche expansions to gain insight into what ecological and geographical settings promote differentiation. Well-described examples come from fishes in postglacial lakes (Schluter & Rambaut 1996; Knudsen et al. 2006; Roy et al. 2007), lizards on islands (Herrel et al. 2008) and host shifts in insect systems (Feder et al. 1988; Carroll & Boyd 1992), but little is known about the initial stages of niche expansion outside these settings.
Here we document adaptive divergence in bill morphology between parapatric populations of Mexican jays along an elevation gradient resulting from a relatively recent niche expansion (probably less than 18 000 years ago). Phenotypic divergence along elevation gradients has also been shown in other bird species (Price 1991; Soobramoney et al. 2005; Kleindorfer et al. 2006), though few studies have tested a priori adaptive hypotheses for trait differences. A common-garden experiment with a small passerine bird (Junco hyemalis) showed a heritable component to elevational differences in bill and body traits, although the traits in question also demonstrated some degree of plasticity (Bears et al. 2008). We do not know whether the morphological differences presented here represent a heritable or plastic change (see below), but the fact that they are adaptive seems well supported based on functional morphology and prior studies linking hooked and straight-billed jay ecotypes with oak and pine habitat, respectively (Peterson 1993). Furthermore, our finding that hook and bill length—both traits involved in feeding—differed between elevations, whereas other traits (e.g. tarsus and wing) did not (table 1) supports the adaptive hypothesis. A parallel regional pattern in bill morphology (figure 3) provides further support for adaptive differentiation and suggests that results from the Sierra del Carmen can be generalized to the larger geographical region where Mexican jays exist in the absence of scrub-jays and Steller's jays.
Whereas we predicted shorter hooks at high elevation, our finding that bill length was also shorter at high elevation was unexpected because pine-feeding scrub-jays have longer bills (Peterson 1993; Bardwell et al. 2001). However, the potential functional advantage of a long bill probably depends on feeding method and local pine cone morphology. Pine-specialized species such as Clark's nutcracker and the pinyon jay use their long and pointed bills similar to chisels to break open green pine cones, an action which scrub-jays and Mexican jays are unable to do (Vander Wall & Balda 1981). Thus, long bills are presumably only advantageous for the latter two species insofar as they help reach seeds deeply imbedded within open cones. Data from pine cones of the two most common pine species in the Sierra del Carmen indicate that average seed depth is shorter than the shortest bills (17.71±0.25 mm for Pinus ponderosa and 10.39±0.15 mm for Pinus strobiformis), suggesting that longer bills might not be advantageous for high-elevation Mexican jays (McCormack 2007). Moreover, differences in bill length are not significant when controlling for hook length, confirming the observation that a larger hook adds length to the bill and potentially drives differences in the latter.
Although the results discussed above provide evidence that adaptive divergence and niche expansion are coincident in the Sierra del Carmen, they need not imply a causal link between the two processes. For this reason, we compared variation in bill morphology in the Sierra del Carmen and Chiricahua Mountains, which share many ecological similarities except that Mexican jays have not expanded their niche in the Chiricahua Mountains due to the presence of other jay species at higher and lower elevations. Coefficients of variation for bill traits fall within the range of those found in other avian studies (van Valen 1965; Grant 1994) except for hook length, which had extremely high coefficients of variation, probably because hook length, unlike most morphological traits, can range between zero and its maximum. Greater variation in hook length (and bill depth) in the Sierra del Carmen conforms to the expectations of the niche variation hypothesis (van Valen 1965) and, although this analysis was limited to two mountain ranges and therefore conclusions based solely on these results should be treated cautiously, suggests a causal link between adaptive divergence and niche expansion. Higher variability in bill depth in the Sierra del Carmen was not predicted because no consistent differences between high and low elevations were found for this trait. However, variation in bill depth might be higher within populations in the Sierra del Carmen due to the wider resource base, which includes seeds and acorns of many different sizes. Intrapopulation variation in bill morphology and the importance of frequency-dependent selection are topics that await future research in this system.
(b) Evidence for divergent selection and the genetic basis of hook length
This study relies on two assumptions concerning the evolutionary mechanism responsible for generating morphological differences: that (i) divergent natural selection between elevations has led to trait differences; and (ii) differences in hook length are heritable. Further study is required to confirm both of these assumptions, but both seem probable given existing knowledge. First, strong differences in winter resources, specifically in density of pines versus oaks (figure 1), probably result in divergent selection pressures, especially for Mexican jays that exhibit no seasonal movement and are less inclined to wander than most species due to year-round flock territoriality (McCormack & Brown 2008). Although jays at both elevations were observed to eat more acorns than pine seeds (figure 1c), this could be because acorns were particularly abundant at high elevation during the 2 years when we assessed foraging (J. E. McCormack 2003–2004, personal observation). Also, foraging on acorns is easier to observe, as it occurs relatively low in the tree compared with pine seed foraging and involves a loud and obvious method of cracking open the seed. Differences in species diversity of pines and oaks potentially add another temporal layer to selection pressure. At the highest elevation site (Campo Tres), there is only one abundant oak species, whereas in the low-elevation canyons there is only one abundant pine species (at one of the low-elevation sites there are no pines at all). Because individuals of a mast-seeding species tend to fruit synchronously (Kelly & Sork 2002), these differences in species diversity will lead over the course of many years to periodic failures of the acorn crop at high elevation and the pine seed crop at low elevation. For example, the high-elevation oak species was not observed to produce acorns for five consecutive years during a recent drought (J. Delgadillo 1996–2007, personal communication). Erratic long-term resource dynamics could lead to intermittent bouts of divergent selection similar to how oscillations in seed production have led to short but intense bursts of selection on bill size and shape in Darwin's finches (Boag & Grant 1981). Study over a period of several years would be required to investigate this process in the Sierra del Carmen.
Second, for natural selection to have led to the observed differences, hook length must have a genetic basis; yet, adaptive plasticity is known to play a role in differentiation, especially during rapid adaptation (Losos et al. 2001). The genetic basis of hook length in jays is not known, but generally avian bill traits are highly heritable (Boag & van Noordwijk 1987). Peterson (1993) marshalled two lines of evidence to argue that hook length in the scrub-jay has a genetic basis. First, two groups of captive scrub-jays taken from the same clutch and fed on differing diets did not show changes in hook length after 1 year. Second, recent immigrants to pinyon woodland from oak woodland retained their hooks. In our study, the hook length of jays captured as adults multiple times (at least six months apart) was highly correlated (Pearson's r2=0.83, n=6). Although not possessing the same power as a common-garden experiment to separate genetic and environmental contributions to phenotype, this result does suggest that individual hook length is not extremely variable through seasons and years. In summary, we feel that this evidence argues for a genetic basis for variation in hook length, although we acknowledge that adaptive plasticity might play a role. To this end, moderate levels of adaptive plasticity might promote genetic assimilation and shifts to new adaptive peaks in bird populations experiencing niche expansion (Price et al. 2003).
(c) Neutral genetic divergence and gene flow
Considering the close proximity of our sampling sites in the Sierra del Carmen and the fact that there are no apparent barriers to dispersal, we expected to find little or no genetic structure in the Sierra del Carmen. However, results based on mtDNA and microsatellite variation support weak but detectable genetic differentiation between high and low elevations. Additionally, mtDNA haplotype frequencies showed lower diversity at high elevation and were composed of a subset of haplotypes found at low elevation (figure 4), supporting an expansion from low to high elevation. These results suggest one of the two possibilities: some measure of reproductive isolation has formed between high- and low-elevation jays, or there is isolation by distance at a much smaller scale than usually expected, due to the social structure and philopatry of Mexican jays (McCormack & Brown 2008). While further study, including denser sampling, is probably needed to discriminate between these hypotheses, in either case genetic structure at such a small geographical scale is noteworthy, and there are few examples in vertebrate systems at the scale described here (Garcia-Paris et al. 2000; Ogden & Thorpe 2002; Rico & Turner 2002). Fewer examples are known from passerine birds, mostly lekking species (Shorey et al. 2000) and cooperative breeders with high philopatry (Woxwold et al. 2006). Our genetic results are also consistent with an interpretation of divergence with gene flow (Rice & Hostert 1993). FST values calculated from mtDNA and microsatellites, though significant in the latter case, are low and result in estimates of migrants per generation that far exceed what is considered sufficient to homogenize populations in the absence of selection (Wright 1931). This study, therefore, also documents the ability of populations to diverge despite remarkably short geographical distances (3–15 km) and gene flow.
(d) Potential confounding factors
Known alternate hypotheses can either be rejected or do not provide as parsimonious an explanation for the observed differences in bill shape. Avian bill length can vary by season (Davis 1954) and due to wear (Borras et al. 2000). We found no evidence for an effect of season on hook length (table 1). Differential wear is unlikely to explain our pattern because jays with greater use of the hook (i.e. those in low-elevation oak woodland) are expected to suffer increased wear. Our results show the opposite pattern with low-elevation jays having longer hooks.
Hook length is known to be important for preening in some species, such as the rock dove (Columba livia; Clayton et al. 2005). Ectoparasite densities were found to be elevated in straight-billed scrub-jays (Moyer et al. 2002), possibly indicating a cost to straight bills and a role for selection for ectoparasite removal ability in driving differences in hook length. However, contrary to the ectoparasite hypothesis, data from the Sierra del Carmen indicate low levels of louse prevalence, and no significant differences in prevalence between elevations and low louse densities on high elevation (i.e. straight billed) jays (McCormack 2007). These data suggest that selection for preening ability is not the major factor influencing bill differentiation in the Sierra del Carmen, although this selective force could act as a constraint on evolution in response to selection for foraging ability.
(e) Pleistocene climatic fluctuations and implications for speciation
Palaeoecological data indicate that sky islands in northern Mexico were connected by woodland at the last glacial maximum and only fragmented in the last 10 000 years when Chihuahuan desert invaded lowland basins (Betancourt et al. 1990). Therefore, the elevation gradient in the Sierra del Carmen as it exists presently seems to be of relatively recent origin. Prodon et al. (2002) suggested that Pleistocene climatic fluctuations have been important in niche expansion of mid-elevation species, reasoning that as climate changes, habitats shift up and down elevation gradients, extirpating species at high and low elevations and thereby allowing mid-elevation species to expand their niches. This study lends support to this hypothesis and expands on its conclusions by showing that mid-elevation species might then be subject to adaptive differentiation.
Finally, niche expansion is an important precursor to microevolutionary processes such as adaptive divergence (Roy et al. 2007) that presumably lead to macroevolutionary events such as ecological speciation and adaptive radiation (Simpson 1953; Schluter 2000). Studies of intraspecific divergence in contemporary niche expansions, therefore, can elucidate the spatial and ecological context of incipient divergence in a way that is difficult to do after the fact. It is important to note that continuing divergence among the jay populations in this study is not assured, especially in the face of climate change, both natural and human influenced, that will undoubtedly result in shifting habitat distributions and homogenization of populations. So while differences in putative isolating traits (e.g. plumage and vocalizations) are as yet unstudied in this system, our work shows that the initial stages of a niche expansion can include adaptive divergence between parapatric populations connected by gene flow along an elevation gradient. Continuing research will be crucial for connecting incipient divergence in contemporary niche expansions to mechanisms of reproductive isolation to better understand how niche expansion leads from the type of microevolutionary divergence documented here to the macroevolutionary processes seen in other systems (e.g. Ryan et al. 2007).
Acknowledgments
Methods for sampling were approved by UCLA's Animal Research Protocol (ARC no. 2005-126-02).
We thank the McKinneys, Dan Roe and CEMEX who kindly permitted land use and provided logistical support in the Sierra del Carmen. Museo de las Aves de México and A. Navarro-Sigüenza and B. Hernández at the Museo de Zoología ‘Alfonso L. Herrera’ of Universidad Nacional Autónoma de México facilitated permits. C. Múzquiz, the Michaelis and Sellers families, Big Bend National Park and Ejido La Encantada provided access to field sites. J. Carrera, C. Sifuentes and SEMARNAT permitted the work in Mexico. B. Milá and G. Castañeda provided critical assistance for all aspects of the project. F. Heredia-Pineda, S. Gibert-Isern, J. Delgadillo-Villalobos, D. Doan-Crider, R. Skiles, G. Ferguson, J. Brown and N. Bhagabati provided information on the field site. A. Alvarado, O. Ballasteros, E. Berg, A. Byrd, M. Buck, T. Hanks, F. Hertel, E. Landay, B. Larison, G. Levandoski, E. López-Medrano, V. Rodríguez, L. Rubio, M. Starling and R. Tinajero provided field assistance. J. Pollinger, B. Starford and M. Starling assisted with laboratory work. We thank curators at the following museums for access to specimens: Donald R. Dickey Collection at UCLA, Los Angeles County Museum of Natural History, Harvard's Museum of Comparative Zoology and University of Kansas Museum of Natural History. The manuscript was improved with comments from C. Benkman, E. Berg, G. Grether, F. Hertel, J. Karubian, J. Pollinger, R. Wayne, M. Whitlock and a phalanx of anonymous reviewers. This research was funded by an American Ornithologists' Union research award, a UCLA Latin American Center Doctoral Student small grant, a University of California MEXUS programme Dissertation Research grant, and a National Science Foundation Graduate Research fellowship to J.E.M. and National Science Foundation grant IRCEB0236165 to T.B.S.
Supplementary Material
Hook length was measured as the distance (d) from the tip of the bill (A) to a line drawn tangent to the tomia at the base of the bill (C)
References
- Abramoff M.D, Magelhaes P.J, Ram S.J. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- Bardwell E, Benkman C.W, Gould W.R. Adaptive geographic variation in Western scrub-jays. Ecology. 2001;82:2617–2627. doi:10.2307/2679940 [Google Scholar]
- Bears H, Drever M.C, Martin K. Comparative morphology of dark-eyed juncos Junco hyemalis breeding at two elevations: a common aviary experiment. J. Avian Biol. 2008;39:152–162. doi:10.1111/j.2008.0908-8857.04191.x [Google Scholar]
- Betancourt J.L, van Devender T.R, Martin P.S. University of Arizona Press; Tucson, AZ: 1990. Packrat middens: the last 40,000 years of biotic change. [DOI] [PubMed] [Google Scholar]
- Blondel J, Chessel D, Frochot B. Bird species impoverishment, niche expansion, and density inflation in Mediterranean island habitats. Ecology. 1988;69:1899–1917. doi:10.2307/1941167 [Google Scholar]
- Boag P.T, Grant P.R. Intense natural selection in a population of Darwin's finches (Geospizinae) in the Galapagos. Science. 1981;214:82–85. doi: 10.1126/science.214.4516.82. doi:10.1126/science.214.4516.82 [DOI] [PubMed] [Google Scholar]
- Boag P.T, van Noordwijk A.J. Quantitative genetics. In: Cooke F, Buckley P.A, editors. Avian genetics: a population and ecological approach. Academic Press; London, UK: 1987. pp. 45–78. [Google Scholar]
- Borras A, Pascual J, Senar J.C. What do different bill measures measure and what is the best method to use in granivorous birds? J. Field Ornithol. 2000;71:606–611. doi:10.1648/0273-8570(2000)071[0606:WDDBMM]2.0.Co;2 [Google Scholar]
- Brown J.L, Bhagabati N. Variation in mass, wing, and culmen with age, sex, and season in the Mexican jay. J. Field Ornithol. 1998;69:18–29. [Google Scholar]
- Brown J.L, Brown E.R. Ecological correlates of group size in a communally breeding jay. Condor. 1985;87:309–315. doi:10.2307/1367209 [Google Scholar]
- Carroll S.P, Boyd C. Host race radiation in the soapberry bug: natural history with the history. Evolution. 1992;46:1053–1069. doi: 10.1111/j.1558-5646.1992.tb00619.x. doi:10.2307/2409756 [DOI] [PubMed] [Google Scholar]
- Clayton D.H, Moyer B.R, Bush S.E, Jones T.G, Gardiner D.W, Rhodes B.B, Goller F. Adaptive significance of avian beak morphology for ectoparasite control. Proc. R. Soc. B. 2005;272:811–817. doi: 10.1098/rspb.2004.3036. doi:10.1098/rspb.2004.3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. Seasonal changes in bill length of certain passerine birds. Condor. 1954;56:142–149. doi:10.2307/1364781 [Google Scholar]
- Diamond J.M. Ecological consequences of island colonization by southwest Pacific birds, 1. Types of niche shifts. Proc. Natl Acad. Sci. USA. 1970;67:529–536. doi: 10.1073/pnas.67.2.529. doi:10.1073/pnas.67.2.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder J.L, Chilcote C.A, Bush G.L. Genetic differentiation between sympatric host races of the apple maggot fly Rhagoletis pomonella. Nature. 1988;336:61–64. doi:10.1038/336061a0 [Google Scholar]
- Flury B. Wiley; New York, NY: 1988. Common principal components and related multivariate models. [Google Scholar]
- Fridolfsson A.K, Ellegren H. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 1999;30:116–121. doi:10.2307/3677252 [Google Scholar]
- Garcia-Paris M, Good D.A, Parra-Olea G, Wake D.B. Biodiversity of Costa Rican salamanders: implications of high levels of genetic differentiation and phylogeographic structure for species formation. Proc. Natl Acad. Sci. USA. 2000;97:1640–1647. doi: 10.1073/pnas.97.4.1640. doi:10.1073/pnas.97.4.1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P.R. Population variation and hybridization: comparison of finches from two archipelagos. Evol. Ecol. 1994;8:598–617. doi:10.1007/BF01237844 [Google Scholar]
- Grant, P. R. (ed.) 1998 Evolution on islands Oxford, UK: Oxford University Press.
- Hedrick P.W. Perspective: highly variable loci and their interpretation in evolution and conservation. Evolution. 1999;53:313–318. doi: 10.1111/j.1558-5646.1999.tb03767.x. doi:10.2307/2640768 [DOI] [PubMed] [Google Scholar]
- Hedrick P.W. A standardized genetic differentiation measure. Evolution. 2005;59:1633–1638. doi:10.1554/05-076.1 [PubMed] [Google Scholar]
- Herrel A, Huyghe K, Vanhooydonck B, Backeljau T, Breugelmans K, Grbac I, Van Damme R, Irschick D.J. Rapid large-scale evolutionary divergence in morphology and performance associated with exploitation of a different dietary resource. Proc. Natl Acad. Sci. USA. 2008;105:4792–4795. doi: 10.1073/pnas.0711998105. doi:10.1073/pnas.0711998105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman T.R, Larson A, Queiroz K.D, Losos J.B. Phylogenetic relationships and tempo of early diversification in Anolis lizards. Syst. Biol. 1999;48:254–285. doi:10.1080/106351599260283 [Google Scholar]
- Kelly D, Sork V.L. Mast seeding in plants: why, how, where? Annu. Rev. Ecol. Syst. 2002;33:427–447. doi:10.1146/annurev.ecolsys.33.020602.095433 [Google Scholar]
- Kleindorfer S, Chapman T.W, Winkler H, Sulloway F.J. Adaptive divergence in continuous populations of the Darwin's small ground finch (Geospiza fuliginosa) Evol. Ecol. Res. 2006;8:357–372. [Google Scholar]
- Knudsen R, Klemetsen A, Amundsen P, Hermansen B. Incipient speciation through niche expansion: an example from the Arctic charr in a subarctic lake. Proc. R. Soc. B. 2006;273:2291–2298. doi: 10.1098/rspb.2006.3582. doi:10.1098/rspb.2006.3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessells C.M, Boag P.T. Unrepeatable repeatabilities: a common mistake. Auk. 1987;104:116–121. [Google Scholar]
- Lewontin R.C. On the measurement of relative variability. Syst. Zool. 1966;15:141–142. doi:10.2307/2411632 [Google Scholar]
- Li S.-H, Huang Y.-J, Brown J.L. Isolation of tetranucleotide microsatellites from the Mexican jay Aphelocoma ultramarina. Mol. Ecol. 1997;6:499–501. doi: 10.1046/j.1365-294x.1997.00215.x. doi:10.1046/j.1365-294X.1997.00215.x [DOI] [PubMed] [Google Scholar]
- Losos J.B, Irschick D.J, Schoener T.W. Adaptation and constraint in the evolution of specialization of Bahamian Anolis lizards. Evolution. 1994;48:1786–1798. doi: 10.1111/j.1558-5646.1994.tb02214.x. doi:10.2307/2410508 [DOI] [PubMed] [Google Scholar]
- Losos J.B, Schoener T.W, Warheit K.I, Creer D. Experimental studies of adaptive differentiation in Bahamian Anolis lizards. Genetica. 2001;112:399–415. doi:10.1023/A:1013387705408 [PubMed] [Google Scholar]
- Martin J.-L. Niche expansion in an insular bird community: an autecological perspective. J. Biogeogr. 1992;19:375–381. doi:10.2307/2845565 [Google Scholar]
- McCormack, J. 2007 Evolutionary patterns and processes of divergence at multiple spatial and temporal scales in a New World corvid. PhD dissertation, University of California, Los Angeles.
- McCormack, J., & Brown, J. 2008 Mexican jay (Aphelocoma ultramarina). In The birds of North America Online (ed. A. Poole). Ithaca, NY: Cornell Laboratory of Ornithology. See http://bna.birds.cornell.edu/bna/species/118 (doi:10.2173/bna.118)
- McCormack J.E, Peterson A.T, Bonaccorso E, Smith T.B. Speciation in the highlands of Mexico: genetic and phenotypic divergence in the Mexican jay (Aphelocoma ultramarina) Mol. Ecol. 2008;17:2505–2521. doi: 10.1111/j.1365-294X.2008.03776.x. doi:10.1111/j.1365-294X.2008.03776.x [DOI] [PubMed] [Google Scholar]
- Meirmans P.G. Using the Amova framework to estimate a standardized genetic differentiation measure. Evolution. 2006;60:2399–2402. doi:10.1554/05-631.1 [PubMed] [Google Scholar]
- Miller A.H. Avifauna of the Sierra del Carmen, Coahuila, Mexico. Condor. 1955;57:154–178. doi:10.2307/1364864 [Google Scholar]
- Moyer B.R, Peterson A.T, Clayton D.H. Influence of bill shape on ectoparasite load in Western scrub-jays. Condor. 2002;104:675–678. doi:10.1650/0010-5422(2002)104[0675:IOBSOE]2.0.CO;2 [Google Scholar]
- Ogden R, Thorpe R.S. Molecular evidence for ecological speciation in tropical habitats. Proc. Natl Acad. Sci. USA. 2002;99:13 612–13 615. doi: 10.1073/pnas.212248499. doi:10.1073/pnas.212248499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A.T. Gene flow in scrub jays: frequency and direction of movement. Condor. 1991;93:926–934. doi:10.2307/3247727 [Google Scholar]
- Peterson A.T. Adaptive geographical variation in bill shape of scrub jays (Aphelocoma coerulescens) Am. Nat. 1993;142:508–527. doi:10.1086/285552 [Google Scholar]
- Price T. Morphology and ecology of breeding warblers along an altitudinal gradient in Kashmir, India. J. Anim. Ecol. 1991;60:643–664. doi:10.2307/5303 [Google Scholar]
- Price T.D, Qvarnstrom A, Irwin D.E. The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. B. 2003;270:1433–1440. doi: 10.1098/rspb.2003.2372. doi:10.1098/rspb.2003.2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard, J. K. & Wen, W. 2003 Documentation for Structure software: v. 2. See http://pritch.bsd.uchicago.edu
- Pritchard J.K, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodon R, Thibault J.-C, Dejaifve P.-A. Expansion vs. compression of bird altitudinal ranges on a Mediterranean island. Ecology. 2002;83:1294–1306. doi:10.2307/3071944 [Google Scholar]
- Pyle P. Slate Creek Press; Bolinas, CA: 1997. Identification guide to North American birds, part 1: Columbidae to Ploceidae. [Google Scholar]
- Raymond M, Rousset F. An exact test for population differentiation. Evolution. 1995;49:1280–1283. doi: 10.1111/j.1558-5646.1995.tb04456.x. doi:10.2307/2410454 [DOI] [PubMed] [Google Scholar]
- Rice W.R, Hostert E.E. Laboratory experiments on speciation: what have we learned in 40 years? Evolution. 1993;47:1637–1653. doi: 10.1111/j.1558-5646.1993.tb01257.x. doi:10.2307/2410209 [DOI] [PubMed] [Google Scholar]
- Rico C, Turner G.F. Extreme microallopatric divergence in a cichlid species from Lake Malawi. Mol. Ecol. 2002;11:1585–1590. doi: 10.1046/j.1365-294x.2002.01537.x. doi:10.1046/j.1365-294X.2002.01537.x [DOI] [PubMed] [Google Scholar]
- Roy D, Paterson G, Hamilton P.B, Heath D.D, Haffner G.D. Resource-based adaptive divergence in the freshwater fish Telmatherina from Lake Matano, Indonesia. Mol. Ecol. 2007;16:35–48. doi: 10.1111/j.1365-294X.2006.03106.x. doi:10.1111/j.1365-294X.2006.03106.x [DOI] [PubMed] [Google Scholar]
- Ryan P.G, Bloomer P, Moloney C.L, Grant T.J, Delport W. Ecological speciation in south Atlantic island finches. Science. 2007;315:1420–1423. doi: 10.1126/science.1138829. doi:10.1126/science.1138829 [DOI] [PubMed] [Google Scholar]
- Saunders M.A, Edwards S.V. Dynamics and phylogenetic implications of mtDNA control region sequences in New World jays (Aves: Corvidae) J. Mol. Evol. 2000;51:97–109. doi: 10.1007/s002390010070. [DOI] [PubMed] [Google Scholar]
- Schluter D. Oxford University Press; New York, NY: 2000. The ecology of adaptive radiation. [Google Scholar]
- Schluter D, Rambaut A. Ecological speciation in postglacial fishes. Phil. Trans. R. Soc. B. 1996;351:807–814. doi:10.1098/rstb.1996.0075 [Google Scholar]
- Schneider S, Roessli D, Excoffier L. Genetics and Biometry Laboratory; University of Geneva, Switzerland: 2000. Arlequin v. 2.000: a software for population genetics data analysis. [Google Scholar]
- Shorey L, Piertney S, Stone J, Hoglund J. Fine-scale genetic structuring on Manacus manacus leks. Nature. 2000;408:352–353. doi: 10.1038/35042562. doi:10.1038/35042562 [DOI] [PubMed] [Google Scholar]
- Simpson G.G. Columbia University Press; New York, NY: 1953. The major features of evolution. [Google Scholar]
- Soobramoney S, Downs C.T, Adams N.J. Morphological variation in the common fiscal (Lanius collaris) along an altitudinal gradient in southern Africa. Ostrich. 2005;76:130–141. [Google Scholar]
- StataCorp. StataCorp LP; College Station, TX: 2003. Stata statistical software, v.8. [Google Scholar]
- Stenzler L.M, Fitzpatrick J.W. Isolation of microsatellite loci in the Florida scrub-jay Aphelocoma coerulescens. Mol. Ecol. Notes. 2002;2:547–550. doi:10.1046/j.1471-8286.2002.00312.x [Google Scholar]
- Streelman J.T, Danley P.D. The stages of vertebrate evolutionary radiation. Trends Ecol. Evol. 2003;18:126–131. doi:10.1016/S0169-5347(02)00036-8 [Google Scholar]
- Tarr C.L. Primers for amplification and determination of mitochondrial control-region sequences in oscine passerines. Mol. Ecol. 1995;4:527–529. doi: 10.1111/j.1365-294x.1995.tb00251.x. doi:10.1111/j.1365-294X.1995.tb00251.x [DOI] [PubMed] [Google Scholar]
- van Valen L. Morphological variation and width of ecological niche. Am. Nat. 1965;99:377–390. doi:10.1086/282379 [Google Scholar]
- Vander Wall S.B, Balda R.P. Ecology and evolution of food-storage behavior in conifer-seed-caching corvids. Z. Tierpsychol. 1981;56:217–242. [Google Scholar]
- Weir B.S, Cockerham C.C. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. doi:10.2307/2408641 [DOI] [PubMed] [Google Scholar]
- Williams R.L. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. doi:10.1111/j.0006-341X.2000.00645.x [DOI] [PubMed] [Google Scholar]
- Woxwold I.A, Adcock G.J, Mulder R.A. Fine-scale genetic structure and dispersal in cooperatively breeding apostlebirds. Mol. Ecol. 2006;15:3139–3146. doi: 10.1111/j.1365-294X.2006.03009.x. doi:10.1111/j.1365-294X.2006.03009.x [DOI] [PubMed] [Google Scholar]
- Wright S. Evolution in Mendelian populations. Genetics. 1931;16:97–159. doi: 10.1093/genetics/16.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hook length was measured as the distance (d) from the tip of the bill (A) to a line drawn tangent to the tomia at the base of the bill (C)