Abstract
Some parasite cuckoo species lay eggs that, to the human eye, appear to mimic the appearance of the eggs of their favourite hosts, which hinders discrimination and removal of their eggs by host species. Hitherto, perception of cuckoo–host egg mimicry has been estimated based on human vision or spectrophotometry, which does not account for what the receivers' eye (i.e. hosts) actually discriminates. Using a discrimination model approach that reproduces host retinal functioning, and museum egg collections collected in the south of Finland, where at least six different races of the European cuckoo (Cuculus canorus) coexist, I first assess whether the colour design of cuckoo eggs of different races maximizes matching for two favourite avian hosts, viz. the redstart (Phoenicurus phoenicurus) and the pied wagtail (Motacilla alba). Second, I assess the role of nest luminosity on host perception of mimicry by the same two hosts. Phoenicurus-cuckoo eggs showed a better chromatic matching with the redstart-host eggs than other cuckoo races, and in most cases can not be discriminated. Sylvia-cuckoo eggs, however, showed better achromatic matching with redstart-host eggs than Phoenicurus-cuckoo eggs. Also, Motacilla-cuckoo eggs showed poorer chromatic and achromatic matching with pied wagtail-host eggs than Sylvia-cuckoo eggs. Nest luminosity affected chromatic and achromatic differences between cuckoo and host eggs, although only minimally affected the proportion of cuckoo eggs discriminated by chromatic signals. These results reveal that cuckoo races as assessed by humans do not entirely match with host perception of matching and that achromatic mechanisms could play a main role in the discrimination of cuckoo eggs at low-light levels.
Keywords: avian brood parasitism, cuckoo, discriminatory model, egg colour matching, host perception
1. Introduction
The interactions between the obligate avian brood parasite European cuckoo Cuculus canorus and its favourite hosts constitute some of the clearer textbook examples of animal coevolutionary interactions. Cuckoos lay their eggs in the nests of host species, and leave parental care of their offspring to unrelated foster parents (Davies 2000). Cuckoo parasitism is harmful for hosts, since once the cuckoo egg hatches the young cuckoo readily displaces all nest content (Wyllie 1981; Honza et al. 2007a), including eggs and nestmates. Consequently, the cuckoo chick grows up alone, getting rid of all host reproduction (Krüger 2007). Cuckoo parasitism is, therefore, a potent selective agent that has selected for effective anti-cuckoo defensive mechanisms in their hosts, which at the same time has selected for further elaborated counter-defences in the cuckoo to overcome host defences (Brooke & Davies 1988; Davies & Brooke 1988). In this coevolutionary arms race scenario, discrimination and removal of cuckoo eggs by hosts is the most widely extended host defensive mechanism that has selected for more acute host egg matching by the cuckoo.
The European cuckoo consists of different strains of females that belong to separate races, termed gentes (singular gens; Gibbs et al. 2000). Females of a gens specialize on specific host species (Marchetti et al. 1998) and lay eggs of a constant type that often mimic the eggs of the host (Chance 1940; Baker 1942). Long-term specialization of the cuckoo with one or a few favourite hosts with variable egg types, and a higher host proneness to reject cuckoo eggs which are dissimilar in appearance over those which are similar, has resulted in a striking host-specific egg polymorphism in the cuckoo (Davies 2000). So far, based on the visual inspection of their appearance, researchers have recognized at least 16 different cuckoo gentes throughout Europe (Wyllie 1981; Álvarez 1994; Moksnes & Røskaft 1995). Data collected from different cuckoo egg collections also revealed the paradox that eggs clearly adjudged by researchers as belonging to a same cuckoo gens were found parasitizing several host species with variable egg types, while eggs of the other gentes were mostly found in the nest of the particular host with which they correspond (Moksnes & Røskaft 1995; Honza et al. 2001a).
The validity of human vision for assessing cuckoo–host egg matching, however, has been questioned in recent years, with the demonstration that many bird species including cuckoo hosts can see UV wavelengths due to a fourth cone type in their retinas which is receptive to UV light (e.g. Bennett et al. 1996; Bowmaker et al. 1997; Cuthill et al. 1999, 2000). Indeed more recent application of spectrophotometry that accounts for UV wavelength has revealed the existence of differences hidden to humans in the coloration of cuckoo eggs laid in different hosts in the red-chested cuckoo Cuculus solitarius (Cherry & Bennett 2001), the common cuckoo (Avilés & Møller 2004) and the pallid cuckoo Cuculus pallidus (Starling et al. 2006). A limitation to these studies is that matching was measured as the difference in reflectance between cuckoo and host eggs, which does not account for what the host's eyes and its sensory systems actually process and discriminate (Vorobyev et al. 1998; Cuthill et al. 2000; Endler et al. 2005). Hitherto, no attempt has been made to assess cuckoo eggs in terms of signal efficacy theory. Efficacy of cuckoo eggs in terms of avoiding host discrimination (i.e. good matching) would be influenced by the colour of the cuckoo egg itself, the colour of the host eggs as a contrast element, the environment in which matching is perceived by the host and the perceptual abilities of the host (Endler 1990; Guilford & Dawkins 1991; Vorobyev et al. 1998; Théry 2006). Thus, a more thorough understanding of the evolution of cuckoo–host egg mimicry will require mimicry assessments from the perspective of the host.
Here, I used a visual model approach that is sensitive to the limits of avian perception to assess cuckoo–host egg matching from the perspective of a host. This approach integrates reflectance spectra of cuckoo and host eggs, and light regimes in the nests with the published information for photoreceptor sensitivities, photoreceptor noise, and the transmission properties of avian ocular media (Hart et al. 2000; Hart 2001) to calculate differences in matching as differences in host colour space (Vorobyev et al. 1998). The aims of this study were twofold. They were (i) to examine how two favourite hole-nesting hosts of the European cuckoo, viz. redstart Phoenicurus phoenicurus and pied wagtail Motacilla alba, would perceive matching with cuckoo eggs classified by humans as belonging to six different cuckoo gentes, and (ii) to examine the role of nest luminosity on host perception of cuckoo–host egg matching. A growing body of evidence suggests that perception of visual signals is influenced by environmental light conditions (Endler 1990; Endler & Théry 1996; Gómez & Théry 2004; Théry 2006). Indeed, under bright light conditions, chromatic differences between two spectrums are more perceptible than under poor light conditions (e.g. Schaefer et al. 2007). Therefore, because luminosity inside cavity nests is very low as compared with open-nests (Hunt et al. 2003; Avilés et al. 2008), mimicry estimations based solely on differences between host and cuckoo egg spectra may overestimate host's perception of matching, in particular for hole-nesting hosts.
2. Material and methods
(a) Data collection
I obtained reflectance values from host and cuckoo eggs from the egg collections at the Zoological Museum in Helsinki (Finland). Most clutches in this collection were personally collected by Ernst Wasenius in the first quarter of the twentieth century in the surroundings of Helsinki (southern Finland; Wasenius 1936; Avilés & Møller 2004). I selected for the analyses redstart (n=39 clutches and 161 eggs) and pied wagtail (n=22 clutches and 76 eggs) parasitized clutches, since these two hosts are the favourite ones in the south of Finland (Wasenius 1936; Avilés & Møller 2004). Based on visual inspection of matching with hosts eggs, Wasenius had identified six different cuckoo gentes in the region, which were well represented in his collection, viz. Phoenicurus, Fringilla, Motacilla, Anthus, Muscicapa and Sylvia gentes (Wasenius 1936). Wasenius' classification of cuckoo egg races was later corroborated by different researchers who inspected the same collection (e.g. Moksnes & Røskaft 1995; Avilés & Møller 2004). I collected average reflectance values for every cuckoo gens from 161 cuckoo eggs, which Wasenius had unquestionably attributed to the six gentes (electronic supplementary material, figure 1). The good matching to the human eye between cuckoo and host eggs may have resulted in some cases of cuckoo egg misidentification. However, cuckoo eggs are clearly larger than host eggs in Finland (Avilés & Møller 2004), and they have unusually great structural strength relative to host eggs (Honza et al. 2001b), which is easily perceptible by touching. Thus sampling biases due to misidentification of cuckoo eggs are presumably negligible in this study.
(b) Spectral measurements of eggs and nest luminosity
Spectral reflectance (300–700 nm) of eggs was recorded using an Ocean Optics equipment (S2000 spectrometer connected to a deuterium–halogen light (D2-W, mini) by a coaxial reflectance probe (QR-400-7-UV–vis) and the OOIBase32 operating software (Ocean Optics, Inc., Dunedin, FL, USA)). A stratified random sample of spectra from all regions of the eggs was obtained by dividing each egg into four bands around the long axis. Colour was measured in each of these four bands. Previous studies have shown that this technique provides highly repeatable measures of egg colour even for species with spotted eggs (Cherry & Bennett 2001; Langmore et al. 2003; Avilés et al. 2004, 2006a,b; Starling et al. 2006). Reflectance was measured with the probe placed at a constant distance at an angle of 45°. Measurements were relative and referred to a standard white (WS-2) and to the dark, which I calibrated before the measurement of each clutch. All the measurements were performed under standardized light conditions to avoid an effect of ambient light on spectromeasurements. The four measurements from each egg were averaged to give a mean spectrum per egg, and a mean host spectrum for each clutch was calculated (e.g. Cherry & Bennett 2001; Avilés et al. 2006a).
Perception of egg matching by cuckoo hosts is probably affected by the interaction between ambient light coloration in the nests and the reflectance spectra of the cuckoo and host eggs. Redstarts are strict hole-nesters, while pied wagtails nest in hole or crevices in a wide variety of natural and artificial sites (Cramp 1998). Thus, although a variety of nest light environments may be available to these two cuckoo hosts, the most common light environment in which discriminatory tasks of cuckoo eggs by redstart and pied wagtail hosts will occur is the cavity light from a hole-nest. Nest light spectra have been previously published and include standard nest light from hole-nesting and open-nesting bird species (see Avilés et al. 2008). Thus, I first used published irradiance spectra from hole-nesting birds to model calculations of matching between cuckoo and redstart and pied wagtail host eggs. Furthermore, because I was interested in studying the role of nest luminosity, I repeated model calculations considering the light in standard open-nests (Avilés et al. 2008). Thus, my analyses using two extreme nest light environments should be interpreted in the appropriate context: I am first assessing cuckoo–host egg matching in a nest light environment that is common to redstart and pied wagtail hosts, but also in a highly illuminated nest environment that will allow illustrating the role of variation in nest luminosity in cuckoo–host egg mimicry (e.g. Schaefer et al. 2007; Théry et al. 2008).
(c) Avian colour space modelling
I calculated discriminability of cuckoo eggs for each mean host egg spectra per clutch using the model of Vorobyev & Osorio (1998) as developed for the tetrachromatic visual system of birds in its long form (Vorobyev et al. 1998). This model provides a meaningful way of calculating the ability of a bird to distinguish between different colours while accounting for visual pigment absorbance, oil droplet transmittance and ocular media transmittance (Hart et al. 2000; Hart 2001). The model has been demonstrated to precisely describe visual discrimination in birds (Vorobyev & Osorio 1998; Goldsmith & Butler 2005) and has recently been successfully incorporated in the studies of perception of visual signals (e.g. Siddiqi et al. 2004; Håstad et al. 2005; Doucet et al. 2007; Gómez & Théry 2007; Avilés et al. 2008). The model establishes a colour distance ΔS that describes the colour contrasts between two eggs as follows:
| (2.1) |
where Δfi is the log ratio of the quantum catches for cone i, for egg H and C.
| (2.2) |
where RH(λ) represents the average reflectance of the host eggs in a clutch, RC(λ) is the average reflectance of cuckoo egg for a given cuckoo gens, I(λ) is the spectral irradiance at the nest, and S(λ) is the spectral sensitivity of the host i. Results of calculations using equation (2.1) provide the chromatic distance (ΔS) separating the perceptual value of two eggs in host receptor space. The units for ΔS are just noticeable differences (JNDs): values over 1 JND indicate that the cuckoo egg can be discriminated from the host eggs, while values below 1 JND would correspond to indistinguishable cuckoo eggs (Osorio & Vorobyev 1996). Spectral sensitivities have not been measured in the redstart and the pied wagtail. All previous studies, however, coincide that, except Corvidae and Tyrannidae (Ödeen & Håstad 2003), Passeridae are of the UV sensitive (UVS) type (Bowmaker et al. 1997; Hart et al. 1998, 2000). Thus, following recently published literature, I used spectral sensitivity data for the four single cones from the blue tit Cyanistes caeruleus as representative of a bird having a UVS cone (e.g. Håstad et al. 2005; Gómez & Théry 2007; Avilés et al. 2008). Further, following Håstad et al. (2005) for the noise calculations, I used cone proportions of 1, 1.92, 2.68 and 2.7 for a UVS species (Hart et al. 2000), and assumed that the signalling noise for each cone was independent of light intensity.
| (2.3) |
where ω is the Weber fraction (taken as 0.05) and ηi is the relative density of the cone class i on the retina.
Evidence suggests that birds use achromatic (brightness) contrasts in discriminatory tasks (reviewed in Kelber et al. 2003). Indeed theoretical physiological models of retinal sensitivity predict a higher relative relevance of achromatic over chromatic discrimination mechanisms at dim light conditions as those faced by hole-nester hosts with cuckoo eggs (Vorobyev & Osorio 1998). In birds double cones are assumed to be responsible for achromatic visual detection as compared with single cones that allow colour discrimination (e.g. Osorio et al. 1999a,b). Therefore, I calculated receptor signals for double cones using the formulae above and the spectral sensitivity data for double cones in blue tit (Hart et al. 2000).
For every sampled host clutch, I calculated chromatic and achromatic contrasts between the average host spectrum and the average spectrum for every cuckoo gens. Also, to illustrate the influence of nest luminosity on cuckoo–host egg matching, calculations were repeated for every sampled host clutch by using both the irradiance spectra of a typical open-nest and a hole-nest (see above).
(d) Statistical methods
Chromatic and achromatic contrasts between cuckoo and host eggs fitted to a normal distribution (Kolmogorov–Smirnov tests, p>0.05). I tested whether variation in chromatic and achromatic matching was explained by the different cuckoo gentes with generalized linear mixed models. Variables defining chromatic and achromatic contrasts were included as dependent variables while the cuckoo gens was entered as a fixed term in the model. Clutch identity was included in the analyses as a random factor to account for the non-independence of contrasts of the same host clutch with six different cuckoo eggs (i.e. one for each cuckoo gens). Finally, I used paired t-tests for dependent variables to explore the influence of the different light environments on the discriminability of cuckoo–host egg differences.
3. Results
(a) Host egg matching by different cuckoo gentes
(i) Redstart host
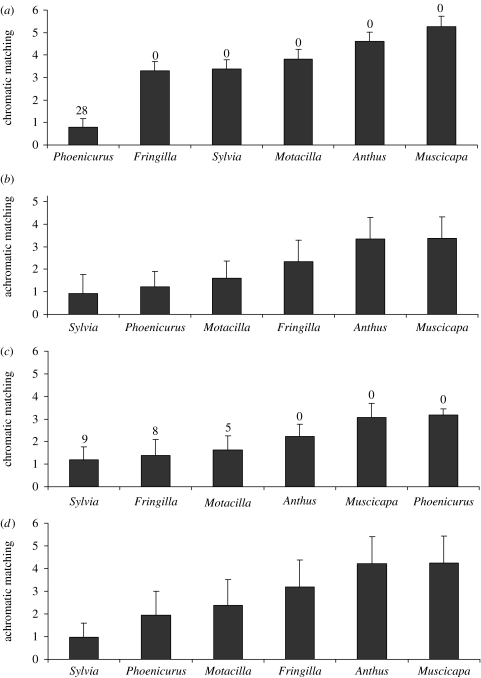
Chromatic matching with redstart eggs was affected by the cuckoo gentes (F5,190=1770.97, p<0.00001). Phoenicurus-cuckoo eggs largely showed better chromatic matching with the redstart-host eggs than the remaining cuckoo gentes (Scheffe tests: p<0.0001; figure 1). The colour discrimination model indicated that in 28 of 39 (71.8%) redstart clutches, the Phoenicurus-cuckoo egg was below the threshold value ΔS of 1.0 JND, while cuckoo eggs of the other gentes largely exceed the threshold value for discrimination of 1.0 JND (figure 1). The clutch identifier had a significant effect as a random term (F38,190=13.07, p<0.00001).
Figure 1.
Mean values of (a,c) chromatic and (b,d) achromatic matching between eggs assessed by humans as belonging to six different cuckoo gentes in southern Finland and average values per clutch of redstart (n=39 clutches) and pied wagtail (n=22 clutches) host eggs. Bars are standard deviations. Values of matching are sorted by size. Number of host clutches that had a value of chromatic contrast below the threshold value for discrimination ΔS of 1.0 JND are displayed above the bars. (a,b) Phoenicurus phoenicurus and (c,d) Motacilla alba.
Similarly, achromatic matching with redstart eggs showed a significant effect of the cuckoo gens (F5,190=219.96, p<0.00001). Unexpectedly, the degree of achromatic contrast with redstart-host eggs reached by Phoenicurus-cuckoo eggs was significantly poorer than that reached by Sylvia-cuckoo eggs (Scheffe tests: p<0.00001; figure 1). Phoenicurus-cuckoo eggs, however, shower a better achromatic matching with redstart eggs than those of the Fringilla-cuckoo, Anthus-cuckoo, Motacilla-cuckoo and Muscicapa-cuckoo eggs (Scheffe tests: p<0.001 in the four cases). The clutch identifier had a significant effect as a random term (F38,190=7.49, p=0.00006).
(ii) Pied wagtail host
Chromatic matching with pied wagtail eggs varied depending on the cuckoo gens (F5,105=177.16, p<0.00001). Surprisingly, Motacilla-cuckoo eggs showed larger levels of chromatic contrast with pied wagtail host eggs than Sylvia-cuckoo eggs (Scheffe test: p<0.00001, figure 1). The proportion of pied wagtail clutches in which the Motacilla-cuckoo and the Sylvia-cuckoo eggs were below the threshold value for discrimination did not differ significantly (5 of 22 (22.7%) Motacilla-cuckoo eggs versus 9 of 22 (41.0%) Sylvia-cuckoo eggs, Yates corrected Χ12=0.94, p=0.33). Anthus-cuckoo, Muscicapa-cuckoo and Phoenicurus-cuckoo eggs, however, showed significantly larger levels of chromatic contrast with the pied wagtail eggs than that by Motacilla-cuckoo eggs (Scheffe tests: p<0.001 in the three cases, figure 1). The clutch identifier had a significant effect as a random term (F21,105=16.85, p<0.00001).
Achromatic matching with pied wagtail eggs showed a significant effect of the cuckoo gens (F5,105=121.36, p<0.00001). The degree of achromatic matching with pied wagtail-host eggs reached by Sylvia-cuckoo eggs was significantly better than that reached by the remaining cuckoo gentes including Motacilla-cuckoo eggs (Scheffe tests: p<0.00001). Motacilla-cuckoo and Phoenicurus-cuckoo eggs showed a similar degree of achromatic matching with pied wagtail host eggs (Scheffe test: p=0.21, figure 1).
(b) Host egg matching and nest luminosity
(i) Redstart host
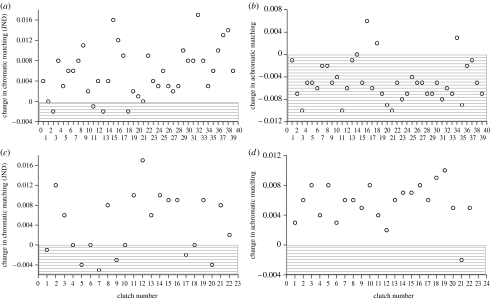
Nest luminosity had a significant effect on chromatic and achromatic matching between redstart host eggs and Phoenicurus-cuckoo host eggs (paired t-tests: chromatic matching: t=7.27, p<0.00001; achromatic matching: t=8.36, p<0.00001, n=39 clutches). Chromatic differences between Phoenicurus-cuckoo eggs and redstart host eggs were larger under high-lighted regimes, while achromatic differences were more evident under low-light regimes (figure 2). Nest luminosity, however, only minimally affected the proportion of redstart clutches in which the Phoenicurus-cuckoo egg was below the threshold value for discrimination ΔS of 1.0 JND (27 of 39 in high-light conditions versus 28 of 39 in low-light conditions).
Figure 2.
Efficiency in cuckoo egg detection in relation to nest luminosity. Points represent the value of the difference in (a,c) chromatic and (b,d) achromatic matching between cuckoo eggs of the Phoenicurus and Motacilla gentes and those of their corresponding hosts when viewed under high- and low-light regimes at the nests. Grey areas indicate where mismatching had greater values when nest luminosity was low (n=39 redstarts and 22 pied wagtail clutches).
(ii) Pied wagtail host
Nest light conditions also affected the perception of chromatic and achromatic matching between Motacilla-cuckoo eggs and pied wagtail eggs (paired t-tests: chromatic matching: t=4.53, p=0.0001; achromatic matching: t=8.93, p<0.00001, n=22 clutches). High-light regimes favoured larger differences in chromatic and achromatic matching between Motacilla-cuckoo eggs and pied wagtail eggs (figure 2). As for the redstart eggs, luminosity did not affect the proportion of pied wagtail host clutches in which the Motacilla-cuckoo egg was below the threshold value for discrimination ΔS of 1.0 JND (5 of 20 in high-light conditions versus 5 of 20 in low-light conditions).
4. Discussion
To my knowledge, this is the first study in which realistic models of the hosts' perceptual physiology have been applied to investigate the perception of cuckoo eggs according to host vision. Several interesting aspects of cuckoo egg design not visible to humans have been identified. First, cuckoo gentes as assessed by humans do not entirely match with host perception of matching. This conclusion follows from (i) cuckoo eggs of the Phoenicurus gens showing significantly larger achromatic mismatching with the eggs of the redstart host than cuckoo eggs of the Sylvia gens; and (ii) cuckoo eggs of the Motacilla gens showing significantly larger chromatic and achromatic mismatching with eggs of their corresponding pied wagtail host than cuckoo eggs of the Sylvia gens. Second, nest luminosity affects cuckoo–host matching as perceived by hosts. This conclusion follows from (i) the significantly larger chromatic contrasts of cuckoo eggs of the Phoenicurus and Motacilla gentes with the eggs of their corresponding hosts under bright than under dim nest environments; (ii) the significantly larger achromatic contrasts between Phoenicurus cuckoo and redstart host eggs under dim than under bright nest environments and (iii) the significantly larger achromatic matching between Motacilla cuckoo eggs and pied wagtail host eggs under bright than under dim nest environments.
Females of the Phoenicurus gens were comparatively more successful than females of the Motacilla gens in maximizing the mimicry of their eggs with those of their corresponding hosts under the light conditions prevailing in their nests. Visual models revealed that the vast majority of redstart hosts would not be able to discriminate a cuckoo egg of the Phoenicurus gens in their clutches by cueing in chromatic signals (i.e. chromatic differences exceeded the threshold value of 1 JND for discrimination), although redstarts would easily discriminate cuckoo eggs of other cuckoo gentes (figure 1). Most of pied wagtail hosts, however, may discriminate parasitism by Motacilla cuckoo females by cueing in chromatic signals. Even cuckoo eggs classified in the Sylvia and Fringilla gentes showed a better balance of matching with pied wagtail host eggs than cuckoo eggs classified as Motacilla cuckoo eggs (figure 1).
In Finland, cuckoo eggs found parasitizing redstart nests invariably correspond with the Phoenicurus gens (Wasenius 1936; Rutila et al. 2002), while pied wagtail nests have been frequently parasitized by Sylvia, Anthus and Fringilla cuckoo females besides Motacilla cuckoo females (Wasenius 1936; Avilés & Møller 2004). Furthermore, evidence suggests that redstarts may have problems in discriminating natural cuckoo eggs in their nests, even though they readily eject non-mimetic cuckoo eggs (Avilés et al. 2005). Actually, no ejection has been reported in naturally parasitized redstart nests, or in redstart nests artificially parasitized with natural cuckoo eggs (Rutila et al. 2002; Avilés et al. 2005), which supports my finding that Phoenicurus cuckoo eggs can not be discriminated by redstarts by cueing in chromatic signals. On the other hand, pied wagtail's discrimination abilities against cuckoo eggs have been assessed in Britain, where, paralleling the situation in the south of Finland, a cuckoo gens has specialized in pied wagtail hosts and evolved mimic eggs (Brooke & Davies 1988). In that study, pied wagtails rejected 50% of model cuckoo eggs of the Motacilla gens introduced in their nests, which corroborates my finding that Motacilla cuckoo eggs would mostly be discriminated by pied wagtail hosts. Interestingly, the rank order of frequency of rejection of model eggs resembling Motacilla (7 out of 14 nests), Anthus (12 out of 18 nests) and Phoenicurus (10 out of 13 nests) cuckoo eggs reported by Brooke & Davies (1988) coincides with the quality of chromatic, but not achromatic, matching as assessed by hosts in their nests in the south of Finland (figure 1). Therefore, differences in matching efficiency, as well as in host response to Phoenicurus and Motacilla cuckoo eggs, may suggest that these two cuckoo gentes show two distinct levels of specialization with their favourite hosts.
It is unknown to what degree cuckoo hosts rely on chromatic and/or achromatic cues when faced with egg discriminatory tasks. Indeed, spectral sensitivity may depend upon behavioural contexts (see review in Kelber et al. 2003). Recent studies in which egg appearance was estimated by spectrophotometry have demonstrated the involvement of particular wavelengths for egg discrimination either in presumably UV-sensitive (e.g. spotless starling, Sturnus unicolor: Avilés et al. 2006a; great reed warbler, Acrocephalus arundinaceus: Cherry et al. 2007; song thrush, Turdus philomelos: Honza et al. 2007b; blackcap, Sylvia atricapilla: Polaciková et al. 2007) or violet-sensitive birds (magpie, Pica pica: Avilés et al. 2004), which strongly suggests that colour vision is implicated in egg discrimination by birds. Some of these studies on cuckoo hosts simultaneously assessed the role of achromatic and chromatic components of egg coloration in host discriminatory tasks and results were contradictory. Cherry et al. (2007) found that great reed warbler hosts disregarded achromatic signals and rejected more those cuckoo eggs showing a poorer chromatic contrast with their eggs. Polaciková et al. (2007) reported that egg recognition in blackcaps is significantly influenced not only by intraclutch variation in chromatic aspect but also by the degree of achromatic matching between the blunt egg parts of parasite and host eggs. However, it is still unclear whether the relative use of achromatic and chromatic signals varies from one host species to another, and may be modulated by nest environmental luminosity.
Previous studies have provided indirect evidence of a role of nest luminosity in the evolution of egg discriminatory abilities. In a comparative study, Langmore et al. (2005) found a higher level of recognition of cuckoo parasitism in those host species with a higher luminosity at their nests. Here I have found some support for a role of nest luminosity in host discrimination, since host perception of cuckoo egg matching was affected by nest luminosity. Both redstart and pied wagtail hosts would perceive larger chromatic differences with cuckoo eggs in bright than in dim nest environments. The finding that chromatic discrimination of cuckoo eggs was favoured over achromatic discrimination as the light intensity at the nest increased agrees with empirical data for humans and other animals showing that photopic discrimination is based on predominantly colour-opponent channels (Vorobyev & Osorio 1998; Schaefer et al. 2007), and it follows logically from threshold spectral sensitivities calculated for a typical UVS bird eye (Vorobyev & Osorio 1998). Interestingly, I found that perception of the achromatic signal by the two hosts was significantly affected by nest luminosity, and favoured over chromatic discrimination in the low-light environment in which the strict hole-nester redstart host breeds. Vorobyev & Osorio (1998) had predicted a higher relevance of the achromatic over the chromatic discrimination mechanisms at dim light, and it is well known that many animals including birds use achromatic and chromatic signals for separate purposes (Kelber et al. 2003). Thus, my results may suggest a role for achromatic discrimination mechanisms at dim-light conditions among cuckoo hosts.
Interpretation of colour signal contrasts in different light environments relies on the proportion of signals in units greater than 1 just-noticeable-difference with increasingly poor visual conditions (Vorobyev & Osorio 1998). Therefore, even though I detected significant differences in chromatic contrasts, it cannot be concluded that luminosity affected perception of chromatic matching because chromatic differences were largely below the threshold value of 1 JND (figure 2). It must be noted, however, that this argument exclusively applies to colour (chromatic) discrimination for which thresholds have proved to successfully describe behavioural data on discrimination colour against a background (e.g. Kelber et al. 2003; Goldsmith & Butler 2005). However, for vertebrates the model often fails to predict thresholds in dim light because almost certainly the achromatic signal is used. Therefore, it may be that differences in achromatic contrasts between high- and dim-light nests were perceived by cuckoo hosts. In conclusion, my results suggest a negligible role of nest luminosity in host perception of chromatic differences between cuckoo and host eggs in hole-nests, since the bright light scenario modelled here corresponded with a highly illuminated nest environment (i.e. open-nests) that would largely exceed the luminosity levels at the highest illuminated host nests. However, my findings may suggest a role of discrimination mechanisms based on achromatic signals in cuckoo egg discrimination at dim-light conditions. Studies in which host discrimination of different degrees of chromatic and achromatic cuckoo egg mimesis were studied in open- and hole-nesting hosts may help in understanding the role of nest luminosity in the evolution of cuckoo–host egg mimicry.
The findings in this study emphasize the inaccuracy of human vision and simple comparison of reflectance spectra collected by spectrophotometry when assessing brood parasite mimicry of host eggs. The use of visual models allowed calculating cuckoo matching in relation to biologically relevant chromatic thresholds for egg detection. By using this approach, I detected variable levels of specialization among the different gentes of the common cuckoo, and the inability of redstart hosts to detect Phoenicurus cuckoo eggs at their nests. Furthermore, I reported a role for nest luminosity in host perception of cuckoo–host egg mimicry, which deserves further experimental study. Globally, these results highlighted the importance of incorporating realistic models of the host's perceptual physiology into the study of cuckoo–host egg mimicry, such as has been done for the study of perception of other visually mediated signals.
Acknowledgments
I thank J. J. Soler and D. Parejo for their helpful discussion on the manuscript. I am most grateful to T. Stjernberg for hospitality and help in searching for cuckoo clutches at the Natural History Museum in Helsinki. I also thank the referees and E. Adkins-Regan for their advice. During manuscript preparation, I was funded by a Ramon y Cajal Fellowship.
Supplementary Material
Mean reflectance spectra (300–700 nm) of different cuckoo gentes in which model calculations were based
References
- Álvarez F. A gens of cuckoo Cuculus canorus parasitizing rufous bush chat Cercotrichas galactotes. J. Avian Biol. 1994;25:239–243. doi:10.2307/3677081 [Google Scholar]
- Avilés J.M, Møller A.P. How is host egg mimicry maintained in the cuckoo Cuculus canorus? Biol. J. Linn. Soc. 2004;82:57–68. doi:10.1111/j.1095-8312.2004.00311.x [Google Scholar]
- Avilés J.M, Soler J.J, Soler M, Møller A.P. Egg appearance and rejection behaviour in magpies. Anim. Behav. 2004;67:951–958. doi:10.1016/j.anbehav.2003.08.022 [Google Scholar]
- Avilés J.M, Rutila J, Møller A.P. Should the redstart accept or reject cuckoo eggs? Behav. Ecol. Sociobiol. 2005;58:608–617. doi:10.1007/s00265-005-0941-7 [Google Scholar]
- Avilés J.M, Soler J.J, Pérez-Contreras T. Dark nests and egg colour in birds: a possible functional role of ultraviolet reflectance in egg detectability. Proc. R. Soc. B. 2006a;273:2821–2829. doi: 10.1098/rspb.2006.3674. doi:10.1098/rspb.2006.3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilés J.M, Stokke B.G, Moksnes A, Roskaft E, Asmul M, Møller A.P. Rapid increase in cuckoo egg matching in a recently parasitized reed warbler population. J. Evol. Biol. 2006b;19:1901–1910. doi: 10.1111/j.1420-9101.2006.01166.x. doi:10.1111/j.1420-9101.2006.01166.x [DOI] [PubMed] [Google Scholar]
- Avilés J.M, Soler J.J, Navarro C, Pérez-Contreras T. Dark nests and nestling conspicuousness in color patterns of altricial birds. Am. Nat. 2008;171:327–338. doi: 10.1086/527493. doi:10.1086/527493 [DOI] [PubMed] [Google Scholar]
- Baker E.C.S. Witherby; London, UK: 1942. Cuckoo problems. [Google Scholar]
- Bennett A.T.D, Cuthill I.C, Partridge J.C, Maier E.J. Ultraviolet vision and mate choice in zebra finches. Nature. 1996;380:433–435. doi:10.1038/380433a0 [Google Scholar]
- Bowmaker J.K, Heath L.A, Wilkie S.E, Hunt D.M. Visual pigments and oil droplets from six classes of photoreceptor in the retinas of birds. Vision Res. 1997;37:2183–2194. doi: 10.1016/s0042-6989(97)00026-6. doi:10.1016/S0042-6989(97)00026-6 [DOI] [PubMed] [Google Scholar]
- Brooke M. de L, Davies N.B. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature. 1988;335:630–632. doi:10.1038/335630a0 [Google Scholar]
- Chance E.P. Country Life; London, UK: 1940. The truth about the cuckoo. [Google Scholar]
- Cherry M.I, Bennett A.T.D. Egg colour matching in an African cuckoo, as revealed by ultraviolet–visible reflectance spectrophotometry. Proc. R. Soc. B. 2001;268:565–571. doi: 10.1098/rspb.2000.1414. doi:10.1098/rspb.2000.1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry M.I, Bennett A.T.D, Moskat C. Host intra-clutch variation, cuckoo egg matching and egg rejection by great reed warblers. Naturwissenschaften. 2007;94:441–447. doi: 10.1007/s00114-007-0216-4. doi:10.1007/s00114-007-0216-4 [DOI] [PubMed] [Google Scholar]
- Cramp S. Optimedia, Oxford University Press; Oxford, UK: 1998. Cramp's the complete birds of the Western Palearctic. [Google Scholar]
- Cuthill I.C, Bennett A.T.D, Partridge J.C, Maier E.J. Plumage reflectance and the objective assessment of avian sexual dichromatism. Am. Nat. 1999;153:183–200. doi: 10.1086/303160. doi:10.1086/303160 [DOI] [PubMed] [Google Scholar]
- Cuthill I.C, Partridge J.C, Bennett A.T.D, Church S.C, Hart N.S, Hunt S. Ultraviolet vision in birds. Adv. Study Behav. 2000;29:159–214. [Google Scholar]
- Davies N.B. T. A. D. Poyser; London, UK: 2000. Cuckoos, cowbirds and other cheats. [Google Scholar]
- Davies N.B, Brooke M. de L. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 1988;36:262–284. doi:10.1016/S0003-3472(88)80269-0 [Google Scholar]
- Doucet S.M, Mennill D.J, Hill G.E. The evolution of signal design in manakin plumage ornaments. Am. Nat. 2007;169:S62–S80. doi: 10.1086/510162. doi:10.1086/510162 [DOI] [PubMed] [Google Scholar]
- Endler J.A. On the measurement and classification of colour in studies of animal colour patterns. Biol. J. Linn. Soc. 1990;41:315–352. doi:10.1111/j.1095-8312.1990.tb00839.x [Google Scholar]
- Endler J.A, Théry M. Interacting effects of lek placement, display behavior, ambient light, and color patterns in three Neotropical forest-dwelling birds. Am. Nat. 1996;148:421–452. doi:10.1086/285934 [Google Scholar]
- Endler J.A, Westcott D.A, Madden J.R, Robson T. Animal visual systems and the evolution of color patterns: sensory processing illuminates signal evolution. Evolution. 2005;59:1795–1818. doi: 10.1554/04-669.1. doi:10.1554/04-669.1 [DOI] [PubMed] [Google Scholar]
- Gibbs H.L, Sorenson M.D, Marchetti K, Brooke M. de L, Davies N.B, Nakamura H. Genetic evidence for female host-specific races of the common cuckoo. Nature. 2000;407:183–186. doi: 10.1038/35025058. doi:10.1038/35025058 [DOI] [PubMed] [Google Scholar]
- Goldsmith T.H, Butler B.K. Color vision of the budgerigar (Melopsittacus undulatus): hue matches, tetrachromacy, and intensity discrimination. J. Comp. Physiol. A. 2005;191:933–951. doi: 10.1007/s00359-005-0024-2. doi:10.1007/s00359-005-0024-2 [DOI] [PubMed] [Google Scholar]
- Gómez D, Théry M. Influence of ambient light on the evolution of colour signals: comparative analysis of Neotropical rainforest bird community. Ecol. Lett. 2004;7:279–284. doi:10.1111/j.1461-0248.2004.00584.x [Google Scholar]
- Gómez D, Théry M. Simultaneous crypsis and conspicuousness in color patterns: comparative analysis of a Neotropical rainforest bird community. Am. Nat. 2007;169:S42–S61. doi: 10.1086/510138. doi:10.1086/510138 [DOI] [PubMed] [Google Scholar]
- Guilford T, Dawkins M.S. Receiver psychology and the evolution of animal signals. Anim. Behav. 1991;42:1–14. doi:10.1016/S0003-3472(05)80600-1 [Google Scholar]
- Hart N.S. The visual ecology of avian photoreceptors. Prog. Retin. Eye Res. 2001;20:675–703. doi: 10.1016/s1350-9462(01)00009-x. doi:10.1016/S1350-9462(01)00009-X [DOI] [PubMed] [Google Scholar]
- Hart N.S, Partridge J.C, Cuthill I.C. Visual pigments, oil droplets and cone photoreceptor distribution in the European starling (Sturnus vulgaris) J. Exp. Biol. 1998;201:1433–1446. doi: 10.1242/jeb.201.9.1433. [DOI] [PubMed] [Google Scholar]
- Hart N.S, Partridge J.C, Cuthill I.C, Bennett A.T.D. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.) J. Comp. Physiol. A. 2000;186:375–387. doi: 10.1007/s003590050437. doi:10.1007/s003590050437 [DOI] [PubMed] [Google Scholar]
- Håstad O, Victorsson J, Ödeen A. Differences in color vision make passerines less conspicuous in the eyes of their predators. Proc. Natl Acad. Sci. USA. 2005;102:6391–6394. doi: 10.1073/pnas.0409228102. doi:10.1073/pnas.0409228102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honza M, Moksnes A, Roskaft E, Stokke B.G. How are different common cuckoo Cuculus canorus egg morphs maintained? An evaluation of different hypotheses. Ardea. 2001a;89:341–352. [Google Scholar]
- Honza M, Picman J, Grim T, Novák V, Capek M, Mrlík V. How to hatch from an egg of great strength. A study of the common cuckoo. J. Avian Biol. 2001b;32:249–255. doi:10.1111/j.0908-8857.2001.320307.x [Google Scholar]
- Honza M, Voslajerová K, Moskát C. Eviction behaviour of the common cuckoo Cuculus canorus chicks. J. Avian Biol. 2007a;38:385–389. [Google Scholar]
- Honza M, Polacikova L, Prochazka P. Ultraviolet and green parts of the colour spectrum affect egg rejection in the song thrush (Turdus philomelos) Biol. J. Linn. Soc. 2007b;92:269–276. doi:10.1111/j.1095-8312.2007.00848.x [Google Scholar]
- Hunt S, Kilner R.M, Langmore N.E, Bennett A.T.D. Conspicuous, ultraviolet-rich mouth colours in begging chicks. Proc. R. Soc. B. 2003;270:S25–S28. doi: 10.1098/rsbl.2003.0009. doi:10.1098/rsbl.2003.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelber A, Vorobyev M, Osorio D. Animal colour vision—behavioural tests and physiological concepts. Biol. Rev. 2003;78:81–118. doi: 10.1017/s1464793102005985. doi:10.1017/S1464793102005985 [DOI] [PubMed] [Google Scholar]
- Krüger O. Cuckoos, cowbirds and hosts: adaptations, trade-offs and constraits. Phil. Trans. R. Soc. B. 2007;362:1873–1886. doi: 10.1098/rstb.2006.1849. doi:10.1098/rstb.2006.1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmore N.E, Hunt S, Kilner R.M. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature. 2003;122:157–160. doi: 10.1038/nature01460. doi:10.1038/nature01460 [DOI] [PubMed] [Google Scholar]
- Langmore N.E, et al. The evolution of egg rejection by cuckoo hosts in Australia and Europe. Behav. Ecol. 2005;16:686–692. doi:10.1093/beheco/ari041 [Google Scholar]
- Marchetti K, Nakamura H, Gibbs H.L. Host-race formation in the common cuckoo. Science. 1998;282:471–472. doi: 10.1126/science.282.5388.471. doi:10.1126/science.282.5388.471 [DOI] [PubMed] [Google Scholar]
- Moksnes A, Røskaft E. Egg-morphs and host preference in the common cuckoo (Cuculus canorus): an analysis of cuckoo and host eggs from European museum collections. J. Zool. Lond. 1995;236:625–648. [Google Scholar]
- Ödeen A, Håstad O. Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol. Biol. Evol. 2003;20:855–861. doi: 10.1093/molbev/msg108. doi:10.1093/molbev/msg108 [DOI] [PubMed] [Google Scholar]
- Osorio D, Vorobyev M. Colour vision as an adaptation to frugivory in primates. Proc. R. Soc. B. 1996;263:593–599. doi: 10.1098/rspb.1996.0089. doi:10.1098/rspb.1996.0089 [DOI] [PubMed] [Google Scholar]
- Osorio D, Miklosi A, Gonda Z. Visual ecology and perception of coloration patterns by domestic chicks. Evol. Ecol. 1999a;13:673–689. doi:10.1023/A:1011059715610 [Google Scholar]
- Osorio D, Vorobyev M, Jones C.D. Colour vision of domestic chicks. J. Exp. Biol. 1999b;202:2951–2959. doi: 10.1242/jeb.202.21.2951. [DOI] [PubMed] [Google Scholar]
- Polaciková L, Honza M, Procházka P, Topercer J, Stokke B.G. Colour characteristics of the blunt egg pole: cues for recognition of parasitic eggs as revealed by reflectance spectrophotometry. Anim. Behav. 2007;74:419–427. doi:10.1016/j.anbehav.2006.10.023 [Google Scholar]
- Rutila J, Latja R, Koskela K. The common cuckoo Cuculus canorus and its cavity nesting host, the redstart Phoenicurus phoenicurus: a peculiar cuckoo–host system? J. Avian Biol. 2002;33:414–419. doi:10.1034/j.1600-048X.2002.02937.x [Google Scholar]
- Schaefer M.H, Schaefer V, Vorobyev M. Are fruit colors adapted to consumer vision and birds equally efficient in detecting colorful signals? Am. Nat. 2007;169:S159–S169. doi: 10.1086/510097. doi:10.1086/510097 [DOI] [PubMed] [Google Scholar]
- Siddiqi A, Cronin T.W, Loew E.R, Vorobyev M, Summers K. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J. Exp. Biol. 2004;207:2471–2485. doi: 10.1242/jeb.01047. doi:10.1242/jeb.01047 [DOI] [PubMed] [Google Scholar]
- Starling M, Heinsohn R, Cockburn A, Langmore N.E. Cryptic gentes revealed in pallid cuckoos Cuculus pallidus using reflectance spectrophotometry. Proc. R. Soc. B. 2006;273:1929–1934. doi: 10.1098/rspb.2006.3490. doi:10.1098/rspb.2006.3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry M. Effects of light environment on color communication. In: Hill G.E, McGraw K.J, editors. Bird coloration. Mechanisms and measurements. vol. 1. Harvard University Press; Cambridge, MA: 2006. pp. 148–176. [Google Scholar]
- Théry M, Pincebourde S, Feer F. Dusk light environment optimizes visual perception of conspecifics in a crespuscular horned beetle. Behav. Ecol. 2008;19:627–634. doi:10.1093/beheco/arn024 [Google Scholar]
- Vorobyev M, Osorio D. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. B. 1998;265:351–358. doi: 10.1098/rspb.1998.0302. doi:10.1098/rspb.1998.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobyev M, Osorio D, Bennett A.T.D, Marshall N.J, Cuthill I.C. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A. 1998;183:621–633. doi: 10.1007/s003590050286. doi:10.1007/s003590050286 [DOI] [PubMed] [Google Scholar]
- Wasenius E. Om de i Finlands fauna typerna av gökägg och deras geografiska utbredning. Ornis Fenn. 1936;13:147–153. [Google Scholar]
- Wyllie I. Batsford; London, UK: 1981. The cuckoo. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean reflectance spectra (300–700 nm) of different cuckoo gentes in which model calculations were based