Abstract
The impacts of warming seas on the frequency and severity of bleaching events are well documented, but the potential for different Symbiodinium types to enhance the physiological tolerance of reef corals is not well understood. Here we compare the functionality and physiological properties of juvenile corals when experimentally infected with one of two homologous Symbiodinium types and exposed to combined heat and light stress. A suite of physiological indicators including chlorophyll a fluorescence, oxygen production and respiration, as well as pigment concentration consistently demonstrated lower metabolic costs and enhanced physiological tolerance of Acropora tenuis juveniles when hosting Symbiodinium type C1 compared with type D. In other studies, the same D-type has been shown to confer higher thermal tolerance than both C2 in adults and C1 in juveniles of the closely related species Acropora millepora. Our results challenge speculations that associations with type D are universally most robust to thermal stress. Although the heat tolerance of corals may be contingent on the Symbiodinium strain in hospite, our results highlight the complexity of interactions between symbiotic partners and a potential role for host factors in determining the physiological performance of reef corals.
Keywords: Symbiodinium, coral bleaching, heat stress, photosystem II, oxygen consumption and respiration, xanthophylls
1. Introduction
The obligate symbiosis between reef-building corals and dinoflagellates of the genus Symbiodinium has been fundamental to the evolution of reef corals. However, over the last few decades, this relationship has been disrupted on global scales by mass bleaching events, which render corals white through the loss of symbionts or pigments within them. The main triggers for these events are elevated sea surface temperatures acting synergistically with high irradiance levels (Brown 1997; Fitt et al. 2001; Lesser & Farrell 2004). Predicted increases in the frequency and severity of anomalously warm summers present a significant threat to coral reefs worldwide and to the goods and services they provide (Hoegh-Guldberg 1999; Hughes et al. 2003).
Recent studies demonstrating high genetic diversity within the genus Symbiodinium raise new possibilities regarding their potential role in the resilience of reef corals to climate stress. The genus consists of eight lineages or clades (A–H), each of which comprises multiple types (Baker 2003; Coffroth & Santos 2005). Although some coral colonies appear to harbour only a single symbiont type (Goulet 2006), others harbour two or more types simultaneously (Rowan & Knowlton 1995; Ulstrup & van Oppen 2003), which may include a dominant type and background levels of other types (Mieog et al. 2007).
It has been proposed that corals may adapt to warmer oceans by changing their symbiotic partners for new, heat-tolerant types (Buddemeier & Fautin 1993; Baker 2001), and form novel host–symbiont combinations, either by acquiring a new symbiont type (switching) or by increasing the relative abundance of a symbiont type already present within the host (shuffling; Baker 2001). Among coral endosymbionts, clade D Symbiodinium has been characterized as heat- or stress- tolerant based on increased prevalence of types within this clade in Caribbean and Indo-Pacific corals after bleaching events (Glynn et al. 2001; Toller et al. 2001; Baker et al. 2004; van Oppen et al. 2005; Jones et al. 2008), or in corals living in reef lagoons exposed to higher temperature regimes than surrounding waters (Fabricius et al. 2004). However, only a few published studies have tested and compared the physiological response with heat stress among corals hosting D types versus types in other Symbiodinium clades. In one study (Rowan 2004), adult corals hosting clade D Symbiodinium had higher rates of photochemical efficiency of photosystem II (PSII) and higher ratios of maximum net photosynthesis to respiration than corals hosting clade C. In a second study (Berkelmans & van Oppen 2006), adult corals that had shuffled their dominant endosymbiont from C2 to D (ITS1 defined types) following bleaching had higher photochemical efficiency and higher symbiont densities than C2-dominated colonies when subsequently tested in a heat stress experiment. These studies are consistent with field observations and support the notion that the upper thermal tolerance of corals is enhanced when hosting clade D Symbiodinium. Nevertheless, observations of both thermally robust and thermally sensitive types within Symbiodinium clades caution against making clade-wide generalizations (Tchernov et al. 2004) and highlight the need for comparative physiological studies of types within Symbiodinium clades. Moreover, because clade D is relatively uncommon in Indo-Pacific corals, in contrast to the ubiquity of clade C (LaJeunesse 2001; Baker 2003; LaJeunesse et al. 2003), knowledge of the influence of symbiont type on holobiont physiology has important implications for understanding the impact that warming oceans may have on coral communities.
Recent studies showing that symbiont stress responses differ between freshly isolated and in hospite cells suggest that the host may play a significant role in regulating the response of the holobiont (host–symbiont combination) to heat/light stress (Bhagooli & Hidaka 2003; Goulet et al. 2005). Host-driven protective mechanisms that could contribute to regulation of the holobiont's bleaching response include the production of anti-oxidant enzymes (Lesser et al. 1990), mycosporine-like amino acids (Dunlap & Shick 1998) and fluorescent pigments (Salih et al. 2000). Greater understanding of the host–symbiont interactions that govern holobiont physiology in intact coral–algal endosymbioses would provide fresh insights into the resilience of reef-building corals.
Here we use physiological indicators to compare bleaching tolerance between corals hosting Symbiodinium type C1 or D to test the hypothesis that Indo-Pacific corals achieve optimal bleaching tolerance when dominated by Symbiodinium clade D. Hereafter we use the term ‘clade’ to denote the sub-generic level of Symbiodinium classification; the term ‘type’ to denote genetic types within a clade and ‘C1’ and ‘D’ to denote specific ITS1 types (sensu van Oppen et al. 2001) when discussing our study species. Contrary to expectations, we show that C1-corals have higher thermal/light tolerance than D-corals in juveniles of the common Indo-Pacific coral Acropora tenuis. Our results challenge the view that clade D is universally associated with thermal robustness and provide evidence that the heat/light tolerance of Symbiodinium types differs with host species.
2. Material and methods
Three independent heat stress experiments were carried out using A. tenuis juveniles raised following spawning events between 2003 and 2005 at Magnetic Island (19°10′ S, 146°50′ E) in the central section of the Great Barrier Reef (GBR). This coral was selected for study because it naturally associates with types C1 and D during early ontogeny at our study site (Little et al. 2004), thus both types are homologous in juveniles of this species. The first experiment was a pilot study to identify appropriate temperature ranges and time-spans required to elicit a bleaching response in corals that had been experimentally inoculated with either ITS1 types C1 (GenBank accession no. AF380551) or D (GenBank accession no. EU024793) Symbiodinium. We refer to each association as C1- or D-corals. Two independent, full-scale stress experiments (experiments 1 and 2) were then completed to incorporate light as an additional stress factor and to evaluate the generality of the holobiont response at different juvenile ages. Experimental procedures used to develop and raise juveniles, experimentally infect them with different Symbiodinium types, and verification of clade type followed those of Little et al. (2004). Four temperatures were selected for the pilot study and experiment 1 (28, 30, 31 and 32°C) and three for experiment 2 (26, 29, and 32°C). Irradiance levels were 130 μmol photon m−2 s−1 for the pilot study, 160 and 360 μmol photon m−2 s−1 for experiment 1, and 250 μmol photon m−2 s−1 for experiment 2. Further details on experimental design and set-up for temperature and light treatments are given as supplementary material.
(a) Bleaching condition of corals: pilot study and experiment 1
Bleaching was quantified every other day by visual scoring of all experimental colonies. Colonies were scored as normal (normally pigmented), pale (including moderately bleached colonies), bleached (completely translucent tissue) or dead (bare skeleton in various stages of overgrowth by other organisms).
(b) Photochemistry of heat-stressed corals
The maximum quantum yield of PSII (Fv/Fm), a proxy for photochemical efficiency, was measured using a pulse amplitude modulated fluorometer (PAM). For the pilot study and experiment 1, we used a Mini PAM (Walz, Germany) fitted with a 2 mm diameter fibre optic probe. For experiment 2, we used an Imaging-PAM (I-PAM, Walz) that allowed us to haphazardly select three ‘areas of interest’ within each replicate colony using the Imaging-PAM software (ImagingWin v. 2.12a). Dark-adapted colonies were measured every morning before the lights went on.
To better characterize the physiological performance of the symbionts, we calculated the maximum excitation pressure over PSII(Qm) as described in Iglesias-Prieto et al. (2004). Excitation pressure was calculated using the equation,
| (2.1) |
where is the effective quantum yield of fluorescence in light-saturated conditions and Fv/Fm is the maximum quantum yield in a dark-adapted state. Excitation pressure was calculated based on a measurement after 1 hour of exposure to lights, following observations that Qm did not change significantly after 1, 4 and 7 hours of exposure to light in a pilot study preceding this experiment. Fluorescence measurements were taken every third day.
(c) O2 microelectrode characterization of photosynthesis and respiration
To further characterize the physiological impact of heat- and light stress on the juvenile holobiont, rates of gross and net photosynthesis as well as respiration were measured for four colonies per Symbiodinium type per temperature during the second experiment. Measurements were performed on days 1, 8 and 15 of heating using oxygen microelectrodes (approx. 50 μm in diameter; see the electronic supplementary material for set-up details).
Gross photosynthesis rate (Pg) was determined using the light–dark shift technique in units of nmol O2 cm−3 s−1 (Revsbech & Jorgensen 1983; see the electronic supplementary material). After attaining physiological steady state, oxygen microprofiles were measured through the diffusion boundary layer (DBL) in darkness and at 50, 150 and 250 μmol photons m−2 s−1. Oxygen flux measurements were then calculated from the oxygen concentration profiles as described in Kühl et al. (1995).
The ratio (Pn : RD) of the resulting flux in the dark (RD) and at 250 μmol photons m−2 s−1 (Pn) was calculated as a measure of metabolic cost incurred during stress. In order to estimate the irradiance above which the tissue exhibited net oxygen production, known as the compensation irradiance (Ec), oxygen flux estimates obtained at 0, 50, 150 and 250 μmol photons m−2 s−1 were linearly integrated and the Ec was estimated as the irradiance where the oxygen flux was zero.
(d) Chlorophyll a content and xanthophyll pigments
The concentrations of chlorophyll a (Chl a) and xanthophyll pigments in each coral juvenile were determined by reverse-phase high performance liquid chromatography (HPLC) using an integrated PC-interfaced Waters HPLC system in the second experiment. Small branch fragments (approx. 0.5 cm long) were sub-sampled on days 1, 8 and 15 of heating (n=3 per Symbiodinium type in each temperature treatment). After measurement of their reflectance spectra (see §2e), fragments were snap-frozen in liquid nitrogen and stored at −80°C. Following pigment extraction, the surface area of each fragment was calculated using photogrammetry and digital model construction as described in Jones et al. (2008).
Pigments were extracted in 1 ml of methanol for 2 hours in the dark at −20°C. Detailed extraction and analytical methods are provided in the electronic supplementary material. Chl a and xanthophylls (diadinoxanthin and diatoxanthin) were detected by photodiode array spectroscopy (350–750 nm) and by fluorescence (excitation: 440 nm, emission: 650 nm). Absorbance chromatograms were extracted at 440 nm. Pigment identity was confirmed by co-chromatography with authentic standards (Sigma Aldrich, and DHI, Denmark). The xanthophyll ratio was calculated as the ratio of diatoxanthin to the total xanthophyll pool [diadinoxanthin plus diatoxanthin] (Ambarsari et al. 1997). Due to the small size of fragments (38–66 mm2) and the limited number of colonies available for sub-sampling, it was not possible to collect samples for quantification of algal cells. While this restricted the interpretation of pigment data (i.e. whether differences were due to changes in the presence of pigment, or the number of algal cells), standardizing pigment concentrations to coral surface area allowed for comparison of general patterns among treatments.
(e) Reflectance spectra of corals and calculation of the chlorophyll a specific absorption coefficient (a*Chla)
To quantify changes in the light absorption efficiency of chlorophyll a in the symbionts, reflectance spectra of the corals and skeletons used in the second experiment were measured between 400 and 750 nm with 0.3 nm resolution using a USB2000 Fiber Optic Spectrometer (25 μm optical slit with grating #3 installed, Ocean Optics). The method was adapted from Enriquez et al. (2005). Coral colonies were sub-sampled by taking a small branch fragment and placing it on a black, non-reflecting surface in a small container filled with seawater. The fragment was positioned so that the side that received more downwelling light while attached to the colony was facing up. Illumination was provided by a metal halide lamp approximately 40 cm above the sample. Reflected light was collected with a 200 μm diameter waveguide attached to the spectrometer. The waveguide was placed underwater 0.5 cm away from the sample at a 45° angle. To avoid complications due to morphological variance, the waveguide was always pointed to the coenosarc (tissue that joins adjacent polyps). The field of view of the waveguide was approximately 0.1 cm2. Reflectance was calculated as the ratio of the radiance measured from the coral surface relative to the radiance obtained from a reference white diffusing surface. The specific absorption coefficient of chlorophyll a was calculated as described in Enriquez et al. (2005).
(f) Statistical analysis
Physiological parameters measured for C1- and D-corals, including Fv/Fm, Qm, Pg, Pn : RD, Ec, Chl a, and Dt/(Dt+Dn) were compared among temperature treatments at the end of the three experimental exposures. In addition, values for each parameter were compared between the first and last day of each experiment for each Symbiodinium type to examine changes within each association over the period of heat stress. Non-parametric tests (Mann–Whitney U) were used in all cases as transformation of the data did not satisfy the assumption of homogeneous variances required by ANOVA.
3. Results
(a) Bleaching condition of corals
Elevated temperatures had a much greater impact on juvenile corals of A. tenuis when they hosted ITS1 type D compared with type C1 Symbiodinium, both in terms of bleaching intensity and mortality. In the pilot study, all D-corals exposed to 32°C bleached or died after 29 days, whereas the proportion of C1-corals that bleached under the same conditions was only 5% (electronic supplementary material, figure 1a). Results from the two light level treatments in experiment 1 emphasize the importance of light dose and intensity on the bleaching response of corals. Corals exposed to elevated light levels bleached more rapidly than those exposed to lower light levels. The proportion of colonies that bleached or died in the 32°C treatment was 3–4 times greater for D-corals compared with C1-corals (70% at low light and 94% at high light for D-corals, compared with 13 and 33% for C1-corals, electronic supplementary material, figure 1a). In both the pilot study and experiment 1, the response was diminished at lower temperatures, but the pattern of bleaching by type association was consistent (electronic supplementary material, figure 1b).
(b) Photochemistry of heat-stressed coral juveniles
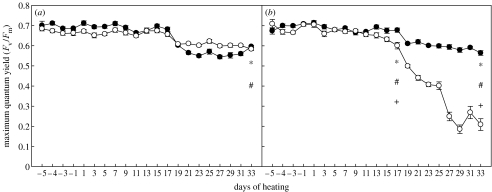
C1-corals had consistently greater maximum quantum yields (Fv/Fm) than D-corals in our three independent experiments (figures 1 and 2; electronic supplementary material, figure 2). At the end of the pilot study (33 days after heating began), Fv/Fm in C1-corals at 32°C was only 5% lower than the same association at 28°C (control temperature). In contrast, Fv/Fm for D-corals was 65% lower at 32°C than at the control temperature and almost threefold lower than in C1-corals (p<0.001, Mann–Whitney U, figure 1a,b). Both associations showed a significant decline in Fv/Fm over time at 32°C (p<0.001, Mann–Whitney U). However, the decline for D-corals was over 70% from initial values, compared with only 20.4% for C1-corals (figure 1b). At 31°C, the extent of the response was smaller, but Fv/Fm was still significantly higher for C1- than for D-corals (p<0.001, Mann–Whitney U, electronic supplementary material, figure 2b). Declines in Fv/Fm of both associations after day 17 (figure 1a,b; electronic supplementary material, figure 2a,b) coincided with an increase in photoperiod (from 7.5 to 10 hours) that was initiated on this day. The much more rapid decline of Fv/Fm for D-corals in the 32°C treatment after this point (figure 1b) emphasizes the additive and potentially synergistic interaction of light and temperature to substantially increase the sensitivity of this clade to heat stress.
Figure 1.
Maximum quantum yield (Fv/Fm) of corals hosting either Symbiodinium C1 (filled circles) or D (open circles) at (a) 28 and (b) 32°C. Values are means±s.e. for each Symbiodinium type (n=24). * and # notations refer to significant differences over time within C1- and D-corals, respectively. + denotes a difference between C1- and D-corals. Comparisons are by Mann–Whitney U-test.
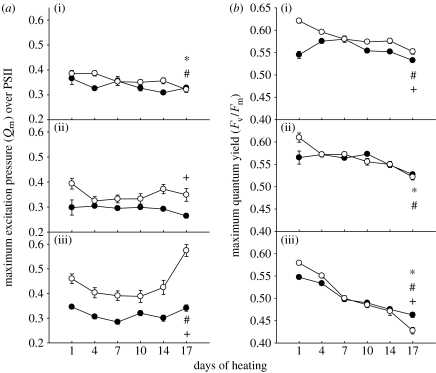
Figure 2.
(a) Maximum excitation pressure over PSII (Qm) of C1- (filled circles) or D-corals (open circles) at (a(i),b(i)) 26°C, (a(ii),b(ii)) 29°C and (a(iii),b(iii)) 32°C. (b) Maximum quantum yield (Fv/Fm) of the same corals. Values are means±s.e. for each Symbiodinium type (n=7–13). * and # notations refer to significant differences over time within C1- and D-corals, respectively. + denotes a difference between C1- and D-corals. Comparisons are by Mann–Whitney U-test.
In the first full-scale experiment, which incorporated two light levels, we again found that Fv/Fm of heat-stressed C1-corals was higher compared with D-corals. Fv/Fm declined significantly in all corals under high light in both the 31 and 32°C treatments (p<0.05, Mann–Whitney U, figure 3c–d in the electronic supplementary material). Changes in the photochemistry of corals were smaller in the low-light treatments and significant differences in Fv/Fm were only detected at 32°C (figure 3h in the electronic supplementary material).
In the second full-scale experiment, we monitored the maximum excitation pressure of PSII (Qm) in addition to Fv/Fm to further analyse the response of these coral–algal associations to heat stress. At 32°C, Qm was always significantly higher in D-corals than in C1-corals (p<0.001, Mann–Whitney U, figure 2a). In general, there was a significant increase in Qm by the end of heat exposure (day 17) for D-corals but not for C1-corals (p<0.001, Mann–Whitney U, figure 2a). At the intermediate temperature, Qm was always around 25% higher in D-corals but levels did not differ significantly between the start and end of the experiment for either association (figure 2a). Colonies at the control temperature showed a small but significant (p<0.05, Mann–Whitney U) decline in Qm for both associations throughout the experiment, but by the end there was no significant difference between them (figure 2a). As in the previous experiments, corals hosting C1-symbionts at 32°C had significantly higher Fv/Fm compared with D-corals (p<0.001, Mann–Whitney U, figure 2b). This was in spite of lower initial Fv/Fm in the C1-corals than in the D-corals. At the intermediate temperature (29°C), there was a small but non-significant drop in Fv/Fm for both associations (figure 2b). At the control temperature, Fv/Fm in C1-corals increased initially but levels did not differ significantly between the start and end of the experiment (figure 2b). In contrast, D-corals showed a sustained and significant decline in Fv/Fm in the control temperature treatment (p<0.05, Mann–Whitney U, figure 2b), but had slightly higher Fv/Fm than C1-corals by the end of the experiment.
(c) O2 microelectrode characterization of photosynthesis and respiration
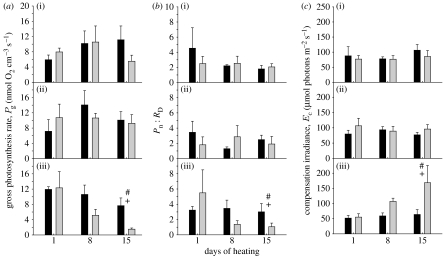
Comparisons of gross photosynthesis rate (Pg) confirm results of reduced photochemical efficiency of D-corals at elevated temperatures obtained using chlorophyll a fluorescence. In the highest temperature treatment (32°C), Pg in C1-corals was significantly higher (p<0.05, Mann–Whitney U, figure 3a) after 15 days of heating than in D-corals, which exhibited significant declines in Pg over time (p<0.05, Mann–Whitney U, figure 3a). Rates of gross photosynthesis of corals in the intermediate temperature treatment (29°C) were not significantly different from rates of corals in the control (26°C) treatment. Pg was similar for both coral–algal associations throughout the duration of the experiment in both the control and intermediate temperature treatments (figure 3a).
Figure 3.
O2 microelectrode measurement of photosynthesis in C1- (black columns) or D-corals (grey columns). (a) Gross photosynthesis rate, Pg (nmol O2 cm−3 coral surface s−1), (b) net photosynthesis rate versus dark respiration rate (Pn : RD) and (c) compensation irradiance, Ec, at (i) 26°C, (ii) 29°C and (iii) 32°C. Values are means±s.e. (n=4 for each Symbiodinium type). * and # notations refer to significant differences over time within C1- and D-corals, respectively. + denotes a difference between C1- and D-corals. Comparisons are by Mann–Whitney U-test.
The ratio between rates of net photosynthesis and dark respiration (Pn : RD) decreased significantly during heating in D-corals at 32°C, but not in C1-corals (p<0.05, Mann–Whitney U, figure 3b). There was no significant difference in Pn : RD between C1- and D-corals at the start of heating (day 1, figure 3b). However, at day 15, Pn : RD of C1-corals was significantly higher at 32°C (p<0.05, Mann–Whitney U, figure 3b). At day 15, Ec in D-corals was significantly higher than in C1-corals in the 32°C treatment (p<0.05, Mann–Whitney U, figure 3c).
(d) Chlorophyll a content, absorption coefficient (a*Chla) and xanthophyll pigments
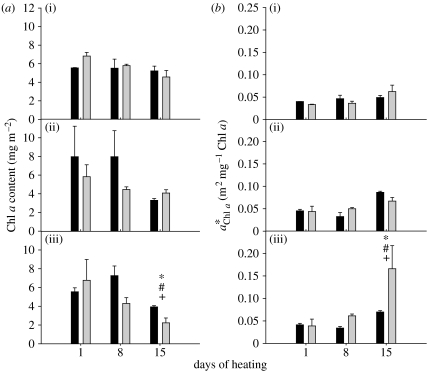
After 15 days of heating at 32°C, the Chl a content in both associations was significantly lower relative to initial levels (p<0.05, Mann–Whitney U). However, the Chl a content of C1-corals was 1.8 times higher (p<0.05, Mann–Whitney U) compared with that of D-corals, even though the latter had slightly higher initial Chl a concentrations (figure 4a). No significant differences were found within or between associations at the lower temperatures (26 and 29°C).
Figure 4.
(a) Chl a content in sub-samples of C1- (black bars) or D-corals (grey bars). (b) Specific absorption coefficient of Chl a in the same samples as in (a). (i) 26°C, (ii) 29°C and (iii) 32°C. Values are means±s.e. (n=3 for each Symbiodinium type). Where error bars are not visible, they are small and hidden by the columns. * and # notations refer to significant differences over time within C1- and D-corals, respectively. + denotes a difference between C1- and D-corals. Comparisons are by Mann–Whitney U-test.
The absorption coefficient of Chl a in D-corals was 2.4 times higher compared with C1-corals (p<0.05, Mann–Whitney U, figure 4b) after 15 days of heating at 32°C. Despite having similar initial levels, increased significantly over time in both associations, but in D-corals this increase was more than fourfold by day 15 (p<0.05, Mann–Whitney U, figure 4b). No significant differences were found within or between associations at the lower temperatures.
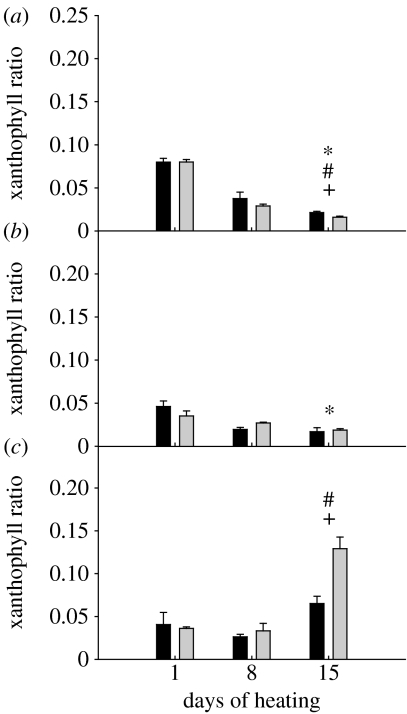
There was a significant decline in the total pool of xanthophylls in both associations at 29 and 32°C (p<0.05, Mann–Whitney U; data not shown). The change in the xanthophyll pool was driven by a significant decline in diadinoxanthin (p<0.05, Mann–Whitney U). However, when normalized to the amount of Chl a, the differences in xanthophyll pigments over time or between D and C1 types were not significant. The xanthophyll ratio of both associations at 32°C increased during the experiment; but by day 15, it was significantly higher in D-corals than in C1-corals (p<0.05, Mann–Whitney U, figure 5). Although changes in the ratio of xanthophyll pigments at 32°C suggested the activation of xanthophyll cycling as a photo-protective mechanism, there was no correlation between this elevated ratio and the bleaching response of the corals. The xanthophyll ratio of corals at 29°C was not significantly different between associations; but it showed a small but significant decline in C1-corals relative to initial levels (p<0.05, Mann–Whitney U, figure 5). At 26°C, the xanthophyll pool in C1-corals did not change significantly during the course of the experiment, whereas there was a significant drop in both pigments during the experiment for D-corals (p<0.05, Mann–Whitney U; data not shown). For both associations at this temperature, the xanthophyll ratio changed significantly from initial values and was significantly different between associations by the end of the experiment (p<0.05, Mann–Whitney U, figure 5).
Figure 5.
Changes in xanthophyll ratio (ratio of diatoxanthin to the sum of diatoxanthin and diadinoxanthin) of C1- (black bars) or D-corals (grey bars). (a) 26°C, (b) 29°C, (c) 32°C. Values are means±s.e. (n=3 for each Symbiodinium type). Where error bars are not visible, they are small and hidden by the columns. * and # notations refer to significant differences over time within C1- and D-corals, respectively. + denotes a difference between C1- and D-corals. Comparisons are by Mann–Whitney U-test.
4. Discussion
Our results demonstrate that the bleaching response of corals can vary dramatically depending on the Symbiodinium type with which they associate. Juveniles of the common coral A. tenuis, which naturally establish symbioses with both type C1- and D-Symbiodinium in field uptake studies (Little et al. 2004), were found to have much greater thermal tolerance when associated with type C1. The greater robustness of C1-juveniles to temperature and light stress was supported by all physiological parameters measured in each of three independent heat/light stress experiments. The only exception was the increased xanthophyll ratio in D-juveniles, which may have been induced to counter stress. However, it is possible that the xanthophyll pigments of these juveniles were still overwhelmed and therefore no improvement in the overall physiological state was observed. Moreover, the proportion of D-corals that bleached and/or died at 31 and 32°C was higher than C1-corals in all three experiments. Although a number of studies have suggested that corals associated with clade D have greater thermal tolerance (Glynn et al. 2001; Toller et al. 2001; Baker et al. 2004; Fabricius et al. 2004; Rowan 2004; Berkelmans & van Oppen 2006), our results demonstrate that enhanced bleaching tolerance of corals is not universally associated with Symbiodinium types within clade D. Moreover, our conclusion that type C1 is thermally robust is consistent with recent field observations showing a dramatic shift in the symbiont community of Acropora millepora from type C2 to types D, C1, or a mix of C1/D after bleaching (Jones et al. 2008). Although it is possible that clade D includes algal types that differ in thermal tolerance (see Tchernov et al. 2004), the specific D-type associated with lower bleaching tolerance in juveniles of A. tenuis in our study was shown to confer higher thermal tolerance in adults of A. millepora (Berkelmans & van Oppen 2006). Thus, caution must be exercised in making generalizations about the performance of Symbiodinium clades in hospite, and there is need for further studies to explore host–symbiont interactions and their impact on the physiology of the coral holobiont.
(a) Photochemical confirmation of enhanced thermal tolerance of C1-juveniles
In addition to macroscopic indicators of holobiont health, photochemical measures clearly demonstrate enhanced thermal tolerance of coral juveniles when associated with Symbiodinium type C1. The steady and up to threefold greater decline in photochemical efficiency of PSII in D-corals well into the period when they started to bleach suggests that these corals were experiencing chronic photo-inhibition (Brown et al. 1999; Gorbunov et al. 2001). In contrast, the smaller decline of Fv/Fm and lack of substantial bleaching in C1-corals is consistent with photo-acclimation (Robison & Warner 2006). Moreover, measurements of maximum excitation pressure over PSII (Qm) corroborated the reduced photochemical efficiency of clade D Symbiodinium when associated with A. tenuis. Qm takes into account the induction of photochemical and non-photochemical processes competing within the reaction centres of PSII for de-activation of chlorophyll a excited states (Maxwell et al. 1995; Iglesias-Prieto et al. 2004) and hence enables the distinction between photo-acclimation and photo-inhibition. Values close to 1 indicate photo-inhibition, whereas values close to 0 indicate light limitation. Our conclusion that the smaller decline of Fv/Fm and lack of bleaching in C1-corals in the high-temperature treatment represented photo-acclimation is supported by the lack of change in excitation pressures of PSII (Qm) at 32°C for C1-corals, in contrast to the significant increase found for D-corals. Under normal conditions, photosynthetic marine organisms can regulate Qm by changing the concentration of chlorophyll a, thereby modifying the light absorption efficiency of this pigment (; Enriquez 2005; Robison & Warner 2006), and thus decrease the probability of damage to PSII by chronic photo-inhibition. However, under thermal stress conditions, when the chain of degradation events leading to coral bleaching is activated by damage to PSII (Iglesias-Prieto & Trench 1994, 1997; Warner et al. 1996, 1999; Takahashi et al. 2004) or downstream from PSII (Jones et al. 1998), this photo-acclimation mechanism can break down. The higher values of Qm at 32°C in D-corals (figure 2), combined with lower amounts of chlorophyll a and higher absorption efficiency of this pigment (figure 4), provide strong evidence of decreased physiological performance of A. tenuis at high temperatures when associated with Symbiodinium D.
(b) The role of light in the bleaching response of heat-stressed corals
The rapid decline in photochemical efficiency in the pilot study when the photoperiod was increased highlights the importance of light in the bleaching response of corals. In D-corals in particular, rapid declines in photochemical efficiency correlated with rapid increases in the proportion of bleached colonies. Such inverse correlations may be explained by feedback loops that magnify photo-inhibition as coral tissues become more translucent. The highly reflective nature of coral skeletons (Kühl et al. 1995), particularly when the number of symbionts and/or amount of pigment is reduced during bleaching, results in a magnified light field within the host tissue and further exacerbates damage to remaining cells (Enriquez et al. 2005). Ironically, the rise in absorption efficiency of Chl a (a*) in the high-temperature treatment during the second experiment would imply that the efficiency of light capture increases with temperature. However, due to the loss of reaction centre integrity, this light becomes a liability and contributes to further degradation of PSII. In combination with the more rapid onset of bleaching in the first experiment in the high-light treatment, our results underscore the enormous role that light prehistory and dose can have on the bleaching response of heat-stressed corals (Brown et al. 2002).
(c) Contribution of symbiont to metabolic costs incurred during heat stress
Our oxygen microelectrode measurements add important insights into the photosynthetic performance of Symbiodinium types when associated with A. tenuis juveniles and further corroborate our conclusion that A. tenuis juveniles are more tolerant to combined heat- and light stress when associated with Symbiodinium C1. The reduced rate of photosynthesis (Pg) found for D-juveniles indicates a reduced capacity for carbon fixation (Li et al. 1984; Jones et al. 1998) that is consistent with photo-inhibition. Moreover, the decreased ratio of net photosynthesis to dark respiration (Pn : RD) for D-corals, but not C1-corals (figure 3), suggests that when associated with D Symbiodinium, the holobiont invests more heavily in maintenance and repair processes associated with metabolic costs incurred during heat stress (Warner et al. 1996; Takahashi et al. 2004). Such energetic costs are likely to impact other important parameters such as growth and reproduction of the holobiont (Michalek-Wagner & Willis 2001; Baird & Marshall 2002).
In addition, the lower efficiency of light utilization in photosynthesis found for heat- and light-stressed D-juveniles, as indicated by their increased compensation irradiance (Ec; Epping & Kühl 2000), suggests that greater energetic costs contributed to their poorer performance. The linear integration by which Ec was calculated may skew the irradiance intensity at which net energy acquisition occurs due to the normal shape of the photosynthesis–irradiance curve (Platt et al. 1980). However, the significant increase in Ec observed in D-corals at 32°C after 15 days (figure 3c) corresponds well with our other estimates of photosynthetic activity. Furthermore, due to the highly reflective nature of the coral skeleton, the light field around the symbionts was amplified as the experiment progressed and corals bleached (Enriquez et al. 2005). Therefore, the effects of underestimating Ec can be considered negligible. While every method has its limitations, the use of microsensors permits minimally invasive and accurate mapping of oxygen and photosynthesis activity at high spatial resolution. It has not been shown whether reactive oxygen species (ROS) could influence these measurements or by how much, however, the cleavage of ROS molecules to form free oxygen would be required to bias our results.
(d) Potential role of host factors in the heat stress response
Differences in the tolerances of C1-corals versus D-corals to heat and light stress between ours and previous studies may be explained partially by host factors, or interactions between hosts and symbionts that may modify the physiological response of the holobiont. For example, each partner in the symbioses is capable of producing protective enzymes involved in protein regeneration and/or anti-oxidant defence pathways (Shick et al. 1995; Downs et al. 2000; Brown et al. 2002). Synthesis of one or more of these enzymes in one partner may elicit a response in the other which differs according to its identity. Previous studies, which have shown that corals associated with type D are more thermally tolerant, have involved species in which type D is homologous, highlighting a potential role for host factors. Berkelmans & van Oppen (2006) showed that adult corals of A. millepora that had shuffled their dominant symbiont population after bleaching, from type C2 to D, were more thermally tolerant in a subsequent heat stress experiment. Similarly, juvenile A. millepora achieved superior thermal tolerance when associated with type D (J. C. Mieog 2007, personal communication), the type normally hosted by adults of this species at Magnetic Island. In both of these studies, the D-type was the same as those used in our study (GenBank accession no. EU024793). Although juveniles of A. tenuis host Symbiodinium type D at this location (Little et al. 2004), adult colonies do not (van Oppen et al. 2001), thus host factors required to maintain this association past an initial flexible stage may not have evolved.
Interestingly, A. tenuis juveniles initially establish a symbiosis with a mix of type D and C1 at this location, and although they rapidly become dominated by type D during early ontogeny (less than 1 year old), they grow much faster when hosting Symbiodinium type C1 (Little et al. 2004). Why juveniles of A. tenuis should establish and maintain a symbiosis with a Symbiodinium type not found in adults remains to be investigated. Possible explanations for the change from D to C1 dominance include (i) onset of as yet undescribed host factors in early ontogeny that may regulate the symbiosis and favour type C1-symbionts (Rodriguez-Lanetty et al. 2004), (ii) accumulation of deleterious impacts arising from associating with type D Symbiodinium that increases mortality of D-juveniles through time (Little et al. 2004), (iii) superior competitive ability of type C1-symbionts within host cells (Fitt 1985), (iv) changing physiological needs associated with life-history stage and/or (v) changing micro-environmental conditions associated with the growth of the host which differentially favour one type over the other through time.
In summary, juvenile A. tenuis achieved superior thermal tolerance when associated with Symbiodinium C1, the type normally hosted by adults at the study location. Type C1 is a very common and widespread symbiont in A. tenuis on the GBR, but the most common type found associated with this coral throughout the GBR is C2 (van Oppen et al. 2005). This and the fact that C is the most common and diverse clade in Indo-Pacific corals (LaJeunesse 2005) call for further exploration of how genetic diversity within clade C correlates to physiological diversity. Along with this, continued efforts to understand the cellular mechanisms underlying host–symbiont interactions will provide insights into how corals and the reefs they build may respond to environmental change.
Acknowledgments
We thank Andrew Negri for his time and technical help during analysis of pigments and protocol development for HPLC. Luke O'Donnell kindly provided us with the script used for estimating the surface area of coral fragments and valuable advice using the software for this purpose. We also thank the Orpheus Island Research Station staff and the many volunteers who provided assistance during fieldwork associated with coral spawning and raising of coral juveniles. Prof. Michael Kühl took part in valuable intellectual discussion, which improved earlier versions of this manuscript. This work was supported by the Australian Research Council, JCU and AIMS. D.A. received financial support from CONACYT (Mexico) and Brockmann/State of Jalisco Scholarship Foundation. K.E.U. was supported by an ISRS Fellowship.
Supplementary Material
Detailed materials and method section and supplementary figures 1 and 2
References
- Ambarsari I, Brown B.E, Barlow R.G, Britton G, Cummings D.G. Fluctuations in algal chlorophyll and carotenoid pigments during solar bleaching in the coral Goniastrea aspera at Phuket, Thailand. Mar. Ecol. Prog. Ser. 1997;159:303–307. doi:10.3354/meps159303 [Google Scholar]
- Baird A.H, Marshall P.A. Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2002;237:133–141. doi:10.3354/meps237133 [Google Scholar]
- Baker A.C. Reef corals bleach to survive change. Nature. 2001;411:765–766. doi: 10.1038/35081151. doi:10.1038/35081151 [DOI] [PubMed] [Google Scholar]
- Baker A.C. Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu. Rev. Ecol. Evol. Syst. 2003;34:661–689. doi:10.1146/annurev.ecolsys.34.011802.132417 [Google Scholar]
- Baker A.C, Starger C, McClanahan T, Glynn P.W. Coral's adaptive response to climate change. Nature. 2004;430:741. doi: 10.1038/430741a. doi:10.1038/430741a [DOI] [PubMed] [Google Scholar]
- Berkelmans R, van Oppen M.J.H. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc.R. Soc. B. 2006;273:2305–2312. doi: 10.1098/rspb.2006.3567. doi:10.1098/rspb.2006.3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagooli R, Hidaka M. Comparison of stress susceptibility of in hospite and isolated zooxanthellae among five coral species. J. Exp. Mar. Biol. Ecol. 2003;291:181–197. [Google Scholar]
- Brown B.E. Coral bleaching: causes and consequences. Coral Reefs. 1997;16:129–138. doi:10.1007/s003380050249 [Google Scholar]
- Brown B.E, Ambarsari I, Warner M.E, Fitt W.K, Dunne R.P, Gibb S.W, Cummings D.G. Diurnal changes in photochemical efficiency and xanthopyll concentrations in shallow water reef corals: evidence for photoinhibition and photoprotection. Coral Reefs. 1999;18:99–105. doi:10.1007/s003380050163 [Google Scholar]
- Brown B.E, Downs C.A, Dunne R.P, Gibb S.W. Exploring the basis of thermotolerance in the reef coral Goniastrea aspera. Mar. Ecol. Prog. Ser. 2002;242:119–129. doi:10.3354/meps242119 [Google Scholar]
- Buddemeier R.W, Fautin D.G. Coral bleaching as an adaptive mechanism. Bioscience. 1993;43:320–326. doi:10.2307/1312064 [Google Scholar]
- Coffroth M.A, Santos S.R. Genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist. 2005;156:19–34. doi: 10.1016/j.protis.2005.02.004. doi:10.1016/j.protis.2005.02.004 [DOI] [PubMed] [Google Scholar]
- Downs C.A, Mueller E, Phillips S, Fauth J, Woodley C. A molecular biomarker system for assessing the health of coral (Montastrea faveolata) during heat stress. Mar. Biotechnol. 2000;2:533–544. doi: 10.1007/s101260000038. doi:10.1007/s101260000038 [DOI] [PubMed] [Google Scholar]
- Dunlap W.C, Shick J.M. Ultraviolet radiation-absorbing mycosporine-like amino acids in coral reef organisms: a biochemical and environmental perspective. Coral Reefs. 1998;20:201–210. [Google Scholar]
- Enriquez S. Light absorption efficiency and the package effect in the leaves of the seagrass Thalassia testudinum. Mar. Ecol. Prog. Ser. 2005;289:141–150. doi:10.3354/meps289141 [Google Scholar]
- Enriquez S, Mendez E.R, Iglesias-Prieto R. Multiple scattering on coral skeletons enhances light absorption by symbiotic algae. Limnol. Oceanogr. 2005;50:1025–1032. [Google Scholar]
- Epping E, Kühl M. The responses of photosynthesis and oxygen consumption to short-term changes in temperature and irradiance in a cyanobacterial mat (Ebro Delta, Spain) Environ. Microbiol. 2000;2:465–474. doi: 10.1046/j.1462-2920.2000.00129.x. doi:10.1046/j.1462-2920.2000.00129.x [DOI] [PubMed] [Google Scholar]
- Fabricius K.E, Mieog J.C, Colin P.L, Idip D, van Oppen M.J.H. Identity and diversity of coral endosymbionts (zooxanthellae) from three Palauan reefs with contrasting bleaching, temperature and shading histories. Mol. Ecol. 2004;13:2445–2458. doi: 10.1111/j.1365-294X.2004.02230.x. doi:10.1111/j.1365-294X.2004.02230.x [DOI] [PubMed] [Google Scholar]
- Fitt, W. K. 1985 Effect of different strains of the zooxanthella Symbiodinium microadriaticum on growth and survival of their coelenterate and molluscan hosts. In Proc. 5th Int. Coral Reef Congress, Tahiti, French Polynesia, pp. 131–136.
- Fitt W.K, Brown B.E, Warner M.E, Dunne R.P. Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs. 2001;20:51–65. doi:10.1007/s003380100146 [Google Scholar]
- Glynn P.W, Mate J.L, Baker A, Calderon M.O. Coral bleaching and mortality in Panama and Ecuador during the 1997–1998 EL NINO-Southern Oscillation event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull. Mar. Sci. 2001;69:79–109. [Google Scholar]
- Gorbunov M.Y, Kolber Z, Lesser M.P, Falkowski P.G. Photosynthesis and photoprotection in symbiotic corals. Limnol. Oceanogr. 2001;46:75–85. [Google Scholar]
- Goulet T.L. Most corals may not change their symbionts. Mar. Ecol. Prog. Ser. 2006;321:1–7. doi:10.3354/meps321001 [Google Scholar]
- Goulet T.L, Cook C.B, Goulet D. Effect of short-term exposure to elevated temperatures and light levels on photosynthesis of different host–symbiont combinations in the Aiptasia pallidal Symbiodinium symbiosis. Limnol. Oceanogr. 2005;50:1490–1498. [Google Scholar]
- Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshw. Res. 1999;50:839–866. doi:10.1071/MF99078 [Google Scholar]
- Hughes T.P, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929. doi: 10.1126/science.1085046. doi:10.1126/science.1085046 [DOI] [PubMed] [Google Scholar]
- Iglesias-Prieto R, Trench R.K. Acclimation and adaptation to irradiance in symbiotic dinoflagellates. 1. Responses of the photosynthetic unit to changes in photon flux density. Mar. Ecol. Prog. Ser. 1994;113:163–175. doi:10.3354/meps113163 [Google Scholar]
- Iglesias-Prieto, R. & Trench, R. K. 1997 Photoadaptation, photoacclimation and niche diversification in invertebrate–dinoflagellate symbioses. In Proc. 8th Int. Coral Reef Symp pp. 1319–1324. Panama City, Panama: Smithsonian Tropical Research Institute.
- Iglesias-Prieto R, Beltra´n V.H, LaJeunesse T.C, Reyes-Bonilla H, Thome´ P.E. Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc. R. Soc. B. 2004;271:1757–1763. doi: 10.1098/rspb.2004.2757. doi:10.1098/rspb.2004.2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.J, Hoegh-Guldberg O, Larkum A.W.D, Schreiber U. Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ. 1998;21:1219–1230. doi:10.1046/j.1365-3040.1998.00345.x [Google Scholar]
- Jones A.M, Berkelmans R, van Oppen M.J.H, Mieog J.C, Sinclair W. A community shift in the symbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc. R. Soc. B. 2008;275:1359–1365. doi: 10.1098/rspb.2008.0069. doi:10.1098/rspb.2008.0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.M, Cantin N.E, Berkelmans R, Sinclair B, Negri A.P. A 3D modeling method to calculate the surface areas of coral branches. Coral Reefs. 2008;27:521–526. doi:10.1007/s00338-008-0354-y [Google Scholar]
- Kühl M, Cohen Y, Dalsgaard T, Jorgensen B, Revsbech N.P. Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, pH and light. Mar. Ecol. Prog. Ser. 1995;117:159–172. doi:10.3354/meps117159 [Google Scholar]
- LaJeunesse T.C. Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region: in search of a “species” level marker. J. Phycol. 2001;37:866–880. doi:10.1046/j.1529-8817.2001.01031.x [Google Scholar]
- LaJeunesse T.C. “Species” radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene–Pliocene transition. Mol. Biol. Evol. 2005;22:570–581. doi: 10.1093/molbev/msi042. doi:10.1093/molbev/msi042 [DOI] [PubMed] [Google Scholar]
- LaJeunesse T.C, Loh W.K, van Woesik R, Hoegh-Guldberg O, Schmidt G.W, Fitt W.K. Low symbiont diversity in southern Great Barrier Reef corals, relative to those of the Caribbean. Limnol. Oceanogr. 2003;48:2046–2054. [Google Scholar]
- Lesser M.P, Farrell J.H. Exposure to solar radiation increases damage to both host tissues and algal symbionts of corals during thermal stress. Coral Reefs. 2004;23:367–377. doi:10.1007/s00338-004-0392-z [Google Scholar]
- Lesser M.P, Stochaj W.R, Tapley D.W, Shick J.M. Bleaching in coral-reef anthozoans: effects of irradiance, ultraviolet-radiation, and temperature on the activities of protective enzymes against active oxygen. Coral Reefs. 1990;8:225–232. doi:10.1007/BF00265015 [Google Scholar]
- Li W.K.W, Smith J.C, Platt T. Temperature response of photosynthetic capacity and carboxylase activity in Arctic marine phytoplankton. Mar. Ecol. Prog. Ser. 1984;17:237–243. doi:10.3354/meps017237 [Google Scholar]
- Little A.F, van Oppen M.J.H, Willis B.L. Flexibility in algal endosymbioses shapes growth in reef corals. Science. 2004;304:1492–1494. doi: 10.1126/science.1095733. doi:10.1126/science.1095733 [DOI] [PubMed] [Google Scholar]
- Maxwell D.P, Falk S, Huner N. Photosystem II excitation pressure and development of resistance to photoinhibition (I. Light-harvesting complex II abundance and zeaxanthin content in Chlorella vulgaris) Plant Physiol. 1995;107:687–694. doi: 10.1104/pp.107.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek-Wagner K, Willis B.L. Impacts of bleaching on the soft coral Lobophytum compactum. I. Fecundity, fertilization and offspring viability. Coral Reefs. 2001;19:231–239. doi:10.1007/s003380170003 [Google Scholar]
- Mieog J, van Oppen M.J.H, Cantin N, Stam W.T, Olsen J.L. Real-time PCR reveals a high incidence of Symbiodinium clade D at low levels in four scleractinian corals across the Great Barrier Reef: implications for symbiont shuffling. Coral Reefs. 2007;26:449–457. doi:10.1007/s00338-007-0244-8 [Google Scholar]
- Platt T, Gallegos C.L, Harrison W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980;38:687–701. [Google Scholar]
- Revsbech N.P, Jorgensen B. Photosynthesis of benthic microflora measured with high spatial resolution by the oxygen microprofile method: capabilities and limitations of the method. Limnol. Oceanogr. 1983;28:749–756. [Google Scholar]
- Robison J.D, Warner M.E. Differential impacts of photoacclimation and thermal stress on the photobiology of four different phylotypes of Symbiodinium (Pyrrohphyta) J. Phycol. 2006;42:568–579. doi:10.1111/j.1529-8817.2006.00232.x [Google Scholar]
- Rodriguez-Lanetty M, Krupp D.A, Weis V.M. Distinct ITS types of Symbiodinium in clade C correlate with cnidarian/dinoflagellate specificity during onset of symbiosis. Mar. Ecol. Prog. Ser. 2004;275:97–102. doi:10.3354/meps275097 [Google Scholar]
- Rowan R. Thermal adaptations in reef coral symbionts. Nature. 2004;430:742. doi: 10.1038/430742a. doi:10.1038/430742a [DOI] [PubMed] [Google Scholar]
- Rowan R, Knowlton N. Intraspecific diversity and ecological zonation in coral–algal symbiosis. Proc. Natl Acad. Sci. USA. 1995;92:2850–2853. doi: 10.1073/pnas.92.7.2850. doi:10.1073/pnas.92.7.2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih A, Larkum A, Cox G, Kühl M, Hoegh-Guldberg O. Fluorescent pigments in corals are photoprotective. Nature. 2000;408:850–853. doi: 10.1038/35048564. doi:10.1038/35048564 [DOI] [PubMed] [Google Scholar]
- Shick J.M, Lesser M.P, Dunlap W.C, Stochaj W.R, Chalker B.E, Won J.W. Depth-dependent responses to solar ultraviolet-radiation and oxidative stress in the zooxanthellate coral Acropora microphthalma. Mar. Biol. 1995;122:41–51. doi:10.1007/BF00349276 [Google Scholar]
- Takahashi S, Takahashi N, Sakamizu M, van Woesik R, Yamasaki H. Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant Cell Environ. 2004;45:251–255. doi: 10.1093/pcp/pch028. [DOI] [PubMed] [Google Scholar]
- Tchernov D, Gorbunov M.Y, de Vargas C, Narayan Yadav S, Milligan A.J, Haggblom M, Falkowski P.G. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc. Natl Acad. Sci. USA. 2004;101:13 531–13 535. doi: 10.1073/pnas.0402907101. doi:10.1073/pnas.0402907101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toller W.W, Rowan R, Knowlton N. Repopulation of zooxanthellae in the Caribbean corals Montastraea annularis and M. faveolata following experimental and disease-associated bleaching. Biol. Bull. 2001;201:360. doi: 10.2307/1543614. doi:10.2307/1543614 [DOI] [PubMed] [Google Scholar]
- Ulstrup K.E, van Oppen M.J.H. Geographic and habitat partitioning of genetically distinct zooxanthellae (Symbiodinium) in Acropora corals on the Great Barrier Reef. Mol. Ecol. 2003;12:3477–3484. doi: 10.1046/j.1365-294x.2003.01988.x. doi:10.1046/j.1365-294X.2003.01988.x [DOI] [PubMed] [Google Scholar]
- van Oppen M.J.H, Palstra F.P, Piquet A.M.-T, Miller D.J. Patterns of coral–dinoflagellate associations in Acropora: significance of local availability and physiology of Symbiodinium strains and host–symbiont selectivity. Proc. R. Soc. B. 2001;268:1759–1767. doi: 10.1098/rspb.2001.1733. doi:10.1098/rspb.2001.1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oppen M.J.H, Mahiny A, Done T. Geographic distribution of zooxanthella types in three coral species on the Great Barrier Reef sampled after the 2002 bleaching event. Coral Reefs. 2005;24:482–487. doi:10.1007/s00338-005-0487-1 [Google Scholar]
- Warner M.E, Fitt W.K, Schmidt G.W. The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: a novel approach. Plant Cell Environ. 1996;19:291–299. doi:10.1111/j.1365-3040.1996.tb00251.x [Google Scholar]
- Warner M.E, Fitt W.K, Schmidt G.W. Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc. Natl Acad. Sci. USA. 1999;96:8007–8012. doi: 10.1073/pnas.96.14.8007. doi:10.1073/pnas.96.14.8007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed materials and method section and supplementary figures 1 and 2