Abstract
To maximize fitness, organisms must assess and select suitable habitat. Early research studying birds suggested that organisms consider primarily vegetation structural cues in their habitat choices. We show that experimental exposure to singing in the post-breeding period provides a social cue that is used for habitat selection the following year by a migrant songbird, the black-throated blue warbler (Dendroica caerulescens). Our experimental social cues coerced individuals to adopt territories in areas of very poor habitat quality where individuals typically do not occur. This indicates that social information can override typical associations with vegetation structure. We demonstrate that a strong settlement response was elicited because post-breeding song at a site is highly correlated with reproductive success. These results constitute a previously undocumented, but highly parsimonious mechanism for the inadvertent transfer of reproductive (public) information from successful breeders to dispersers. We hypothesize that post-breeding song is a pervasive and reliable cue for species that communicate vocally, inhabit temporally autocorrelated environments, produce young asynchronously and/or abandon territories after reproductive failure.
Keywords: habitat selection, location cues, social information, vegetation structure, dispersal, prospecting
1. Introduction
Habitat selection behaviour has consequences for speciation (Morris 2003), regulation of animal populations (Pulliam & Danielson 1991) and conservation, including a population's sensitivity to landscape fragmentation (Fletcher 2006) and other changing environmental conditions (Doligez et al. 2003). To maximize fitness, organisms must assess and select suitable habitat (Fretwell & Lucas 1970). Some important early research (Mayr 1926; Lack & Venables 1939) observed correlations between vegetation structure and density or productivity of animals. Subsequent influential studies of breeding-site selection formalized this idea under the assumption, usually implied through correlation, that organisms consider mainly vegetation structural cues in their habitat choices (MacArthur et al. 1962; Hilden 1965).
Recent research has demonstrated that organisms may also use social information acquired from observing other individuals as cues in habitat selection (Danchin et al. 2004). When sampling opportunities are limited and habitat quality is predictable over time, social information may provide the most efficient way for an individual to prospect as many candidate sites as possible (Boulinier & Danchin 1997; Valone & Templeton 2002).
Disentangling the relative roles of structural cues and social information in habitat selection is imperative if we are to identify conservation strategies for habitats and species, particularly in the face of global environmental change (Thomas et al. 2001). Socially transmitted information about a habitat may be gained more rapidly than through personal experience (Porneluzi 2003) or endogenous adaptations and thus could facilitate species response to rapid changes in habitat quality. However, no previous study has experimentally examined the relative importance of structural cues versus social information in habitat selection strategies. In this paper, we examine breeding-site selection of a Neotropical migrant songbird species, the black-throated blue warbler (Dendroica caerulescens), in relation to a strong gradient in vegetation structure and two types of conspecific social information.
Social information about habitat quality can be transmitted inadvertently to onlookers as either ‘location cues’ provided by the position of other individuals or ‘public information’ (PI) provided by other individuals' performance at a particular task (Valone 1989; Danchin et al. 2004). When it can be collected easily, PI should be the favoured cue guiding site selection because it is a more reliable indicator of fitness than location cues and can provide greater detail about habitat quality (Danchin et al. 2004; Parejo et al. 2007). Reducing costs associated with acquiring PI would be evolutionarily advantageous, and evidence exists that some animals employ shortcuts to acquire it (Valone 1996; van Alpen et al. 2003; Clark 2007). However, if collecting PI is difficult (Danchin et al. 2004; Parejo et al. 2007), location cues should be preferred because this type of information is abundant and readily available (Parejo et al. 2007). If reproductive performance is predictably correlated with vegetation structure, then vegetation itself should be a sufficient non-social cue of breeding habitat quality.
To determine the relative importance of these information types to animals selecting habitat, we experimentally investigated the degree to which individuals used (i) direct sampling of vegetation structure, (ii) location cues and (iii) PI. We predicted that if social information is the primary cue used in breeding-site selection, we should be able to elicit a settlement response even where appropriate vegetation structure is absent. To test this, we deployed decoys and/or playbacks (to simulate location cues and/or indicate reproductive success) across a gradient in vegetation structure that, under non-experimental conditions, seems to strongly influence warbler occurrence and abundance.
2. Material and methods
We chose black-throated blue warblers as our study species because their habitat requirements have been studied extensively (Holmes et al. 1996; Rodenhouse et al. 2003), they are specialized in their habitat use (Doran & Holmes 2005) and the structure of their mature hardwood forest habitat is relatively stable between years (Holmes et al. 1996). Also, there is correlative evidence that this species aggregates, which suggests that individuals may use others as cues to locate suitable habitat (Betts et al. 2006). Males arrive on the breeding grounds before females and choose sites from which to advertise to arriving females; therefore, in this study, we examined the settlement behaviour of each sex separately.
We conducted this study within the Pemigewasset river valley in the White Mountain National Forest in New Hampshire, USA. This landscape is dominated by contiguous second-growth forest consisting primarily of sugar maple (Acer saccharum), American beech (Fagus grandifolia) and yellow birch (Betula alleghaniensis) at the elevations studied (Doran & Holmes 2005).
(a) Social information experiment
We established 54 research sites across a vegetation structure gradient from early seral hardwood forest (less than 10-year-old clear-cuts) to mature (more than 60-year-old) hardwood and mixedwood forest which, under non-experimental conditions, seems to strongly influence warbler occurrence and abundance (low occurrence: few shrubs and mature trees, high occurrence: dense shrubs and mature trees; Doran & Holmes 2005; Betts et al. 2006). We stratified sites into three age-class categories that represented this broad habitat structure gradient: less than 10 years (n=15; shrub cover: 79.0±4.3 s.e. %, tree density: 0.1±0.1 s.e. ha−1); 10–30 years (n=21; shrub cover: 6.4±2.5 s.e. %, tree density: 3.1±2.6 s.e. ha−1); and more than 60 years (n=18; shrub cover: 52.9±6.1 s.e. %, tree density: 104.0±11.6 s.e. ha−1). We visually estimated shrub cover (%) within 25 m of sample points. We measured tree density (more than 10 cm diameter at breast height) using a two-basal area factor prism centred on each sample point. If black-throated blue warblers use only structural cues in habitat selection, gradients in habitat structure, but not experimental treatments, should influence settlement. To increase our confidence that settlement by this species was a response to our experimental treatments (and not some latent factor, such as previous experience), we chose sites where the species was unlikely to be present. We ensured that study sites were vacant during the breeding season of 2006 by conducting passive observation (10 min) and subsequent playback (for less than 5 min) of conspecific song. We deemed a site acceptable for inclusion in our study only if black-throated blue warblers were not detected within 50 m of the playback. All study sites were within forest stands of more than 10 ha and were greater than 250 m apart.
We assigned three experimental treatments to each study site using a balanced, randomized stratified design; stratification occurred within our vegetation structure classes (above). Treatments (each n=18) were: (i) location cues (playback of recorded black-throated blue warbler male song), (ii) public information (reproductive simulations: playback of recordings of fledglings begging for food, female calls and male song), or (iii) controls (silence). This randomization resulted in spatial interspersion of treatments. We detected no significant difference among experimental treatments in shrub cover (F=0.27, p=0.76) or tree density (F=0.25, p=0.78). We did not use playbacks of any sound at controls because birds can be influenced by anthropogenic and heterospecific sounds (Gess 2007; Seppanen et al. 2007), such that we could have unknowingly repelled or attracted warblers from or to these sites. We chose fledgling presence as the most likely public information cue, instead of nestling condition and abundance, because unlike cavity nesters (Doligez et al. 2002), nest failure rates are high in this species (approx. 0.50; Holmes et al. 1996), so nestling number and condition would probably be poorly correlated with reproductive success. Both calls and songs used in our playbacks were recorded locally in the same year as the experimental treatments. We accompanied each playback with hand-painted decoys (location cues: one male warbler; PI: one male, one female and two fledglings) to provide visual stimuli to prospectors. Treatments were applied for 10–12 days during two separate visits to each site from 10 July to 30 August 2006. We used automatic timers to activate the speaker systems for 10 hours of daily playback (from 05.00 to 15.00) over the entire 4- to 6-d treatment period. Sound tracks contained 15 s gaps between songs. To simulate bird movement or counter-singing, and to limit habituation to playbacks, songs and calls were alternated (every 15 min) between two speakers spaced 20 m apart centred on each sample point. Playback song frequency approximated that typical of natural conditions in the late breeding season.
(b) Prospecting and settlement
We checked for evidence of prospecting birds at all sites 4–7 days after the playback periods using 5 min point counts (a passive sampling technique; Hutto et al. 1986). We considered any birds (male or female) seen or heard within 50 m of the treatment site to be ‘present’ at a site. When possible, we visually sexed and aged male warblers as hatch year (HY; fledglings), second year (SY; first-year breeders) or after second year (ASY; experienced breeders) through binoculars by evaluating differences in plumage characteristics (Graves 1997).
In the year following our treatments (2007), we determined spring settlement by conducting 10 min point counts at each site five times from 5 to 25 May. We considered male black-throated blue warblers to be settled at a site if they exhibited territorial behaviour (singing, aggression with neighbouring males) within 50 m of the treatment location. Counts were separated by 1–4 days. After the fifth visit, we monitored the continued territorial presence of individuals at sites by observing reaction to 5 min playbacks of male song. Where possible, we aged male warblers.
To test for the presence of females, we observed response to the playback of 5 min recordings of black-capped chickadee (Poecile atricapilla) mobbing calls at all sites (Gunn et al. 2000); this vocalization usually elicits a strong response from female black-throated blue warblers. As anti-predator calls could have prevented settlement, we did not use mobbing playbacks until after settlement and territory establishment by males (after 28 May). Since black-throated blue warblers respond to mobbing calls only within territory boundaries (Betts et al. 2005), mobbing playbacks also allowed us to further verify that males had settled and were defending territories at sample sites.
We tested for differences in prospecting male settlement and female settlement among experimental treatments (location cues, PI, controls) using logistic regression. In all analyses, vegetation structures (shrub cover, tree density) were treated as continuous variables. We tested for pairwise differences among treatment classes using general linear hypothesis tests (Searle 1971) in the ‘Multcomp’ package (Hothorn et al. 2007) using R statistical software (R Development Core Team 2007). Independent deviance explained (DI) was calculated as
| (2.1) |
where Dv1 is the deviance explained by the variable(s) of interest; Ds is the explained deviance shared between Dv1 and other variables in the model set; and Dt is the total explained deviance (Chevan & Sutherland 1991).
We used generalized linear mixed models (GLMM) with binomial error structure (Broström 2007) to test the hypothesis that warbler settlement/absence depended on our treatments over repeated visits. We modelled ‘site’ as a cluster variable (random effect) and treatment as the fixed effect.
(c) Song rate experiment
The equally strong response of warblers to both experimental location cue and PI treatments (see §3) prompted us to ask whether conspecific song alone, in the post-breeding season, could be a reliable cue for breeding performance. To test the hypothesis that male song in the post-breeding season is correlated with reproductive success, we randomly selected 60 warbler territories from a concurrent study on the demography of black-throated blue warblers (Rodenhouse et al. 2003) that either successfully produced fledglings (n=30) or failed (n=30). We used 20 min point counts, instead of 10 min as in our previous sampling, to increase detection probability of infrequent songs. Counts were divided into six intervals of 3 min 20 s. Song frequency was calculated as the number of intervals (out of six) in which song occurred; this variable was thus a measure of the overall song output over time at a particular site. We conducted six rounds of song counts from 18 to 31 July 2007.
To test for differences in song rate between reproductively successful and unsuccessful sites, we used a GLMM with binomial error structure with site modelled as a cluster variable (random effect) and treatment (nest success, no nest success) and date as fixed effects. We tested the hypothesis that post-breeding song becomes more reliable over time by modelling the interaction: treatment×time.
3. Results
(a) Prospecting
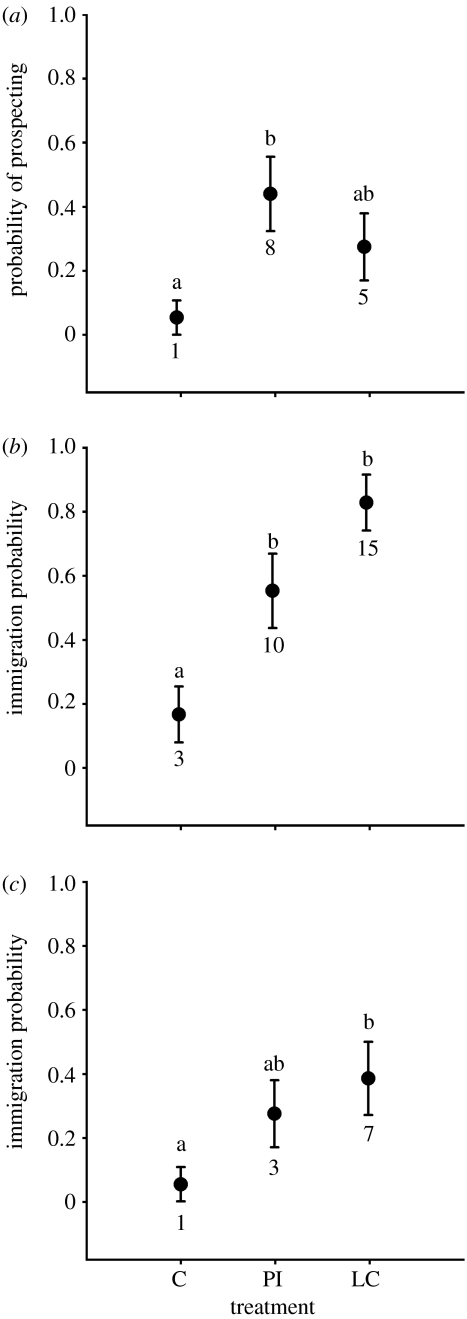
Warblers (both sexes combined) preferentially visited treatment sites where we experimentally provided social information during the post-breeding season; we observed more warblers at PI than control sites (figure 1a, logistic regression, Χ2=8.08, p=0.018) but detected no difference between location cue and PI treatments (location cue β=1.87 (95% CI: −0.67 to 4.43); PI β=2.61 (95% CI: 0.11–5.01)). Further, vegetation structures (especially shrub cover) that are normally strong predictors of warbler occurrence during the breeding season (Doran & Holmes 2005; Betts et al. 2006) were poor predictors of prospector occurrence (shrubs: Χ2=0.007, p=0.935, trees of more than 10 cm diameter at breast height: Χ2=0.894, p=0.344). Fledglings were present at 9 out of 13 (69%) locations where we observed warblers at treatment sites and were never observed at control sites. Warblers were present at treatment sites for up to 10 days following the cessation of playbacks.
Figure 1.
Relation between treatment (‘C’: control, ‘PI’: public information, ‘LC’: location cues) and (a) the proportion of sites where prospecting black-throated blue warblers were detected in the post-breeding season of the treatment year, (b) the proportion of sites settled by males in the following spring and (c) the proportion of sites settled by females in the following spring. Error bars represent±1 s.e. Lower case letters represent results of multiple comparisons tests. Values below error bars indicate number of sites occupied. n=18 for each treatment.
(b) Settlement
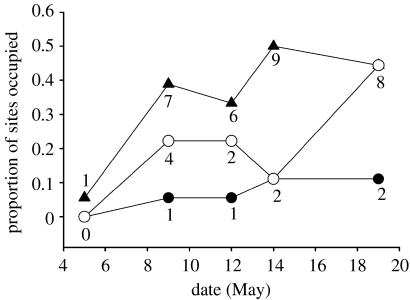
Male warblers were 4.1 times more likely to settle in location cue and PI sites than controls (figure 1b, Χ2=17.61, p=0.0001). We did not detect a difference in settlement between location cue and PI sites (location cue β=3.21 (95% CI: 1.17–5.27); PI β=1.83 (95% CI: 0.02–3.64)). Locations where we observed prospecting in the previous year were more likely to be settled the following spring (Χ2=4.96, p=0.026). The majority (68.7%) of sites were occupied for at least two visits (more than 3 days, see figure 2), and differences between treatments and controls were consistent throughout the settlement period (table 1, figure 2).
Figure 2.
Proportion of sites occupied by black-throated blue warbler males as a function of time during the settlement period (date in May) and treatment (triangles, location cues; open circles, PI; filled circles, controls). Values below data points indicate the number of sites occupied. n=18 for each treatment.
Table 1.
Results of GLMM testing the influence of treatment (control, public information, location cues) on the probability of male black-throated blue warbler settlement at the beginning of the breeding season (5–25 May). (In this analysis ‘site’ was included as a random effect.)
| β | z | LCI | UCI | p | |
|---|---|---|---|---|---|
| intercept (control) | −4.63 | −5.86 | −6.18 | −3.08 | |
| PI | 1.28 | 2.05 | 0.06 | 2.51 | 0.040 |
| location cues | 2.24 | 3.59 | 1.02 | 3.47 | <0.0001 |
| date | 0.46 | 3.37 | 0.19 | 0.73 | 0.001 |
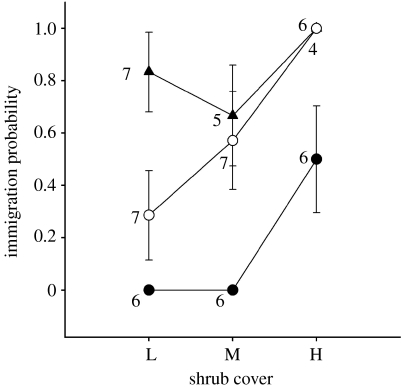
Despite a strong response by males to both types of social information (DI=71%), vegetation structures were still a useful predictor of warbler settlement (table 2, DI (vegetation structure)=29%, shrubs: Χ2=11.08, p=0.001, trees: Χ2=2.67, p=0.102). However, within the location cue treatment, neither shrub cover (Χ2=0.675, p=0.411) nor tree density (Χ2=0.030, p=0.803) predicted warbler occurrence; location cues overrode the use of vegetation cues (figure 3).
Table 2.
Results of logistic regression model including social information treatment (location cues, control, public information) and vegetation structure (tree density, shrub cover).
| β | Χ2 | LCI | UCI | p | |
|---|---|---|---|---|---|
| intercept | −4.14 | — | −1.76 | −0.69 | |
| treatment | |||||
| PI | 2.89 | 0.81 | 4.97 | ||
| location cues | 4.49 | 17.62 | 2.00 | 6.97 | <0.0001 |
| shrub cover | 0.03 | 11.08 | 0.01 | 0.06 | 0.01 |
| tree density | 0.01 | 2.67 | −0.01 | 0.03 | 0.12 |
Figure 3.
Relation between shrub abundance (L, low (0–5% cover); M, medium (greater than 5–80% cover; H, high (greater than 80% cover)) and the proportion of sites settled by black-throated blue warblers in the following spring (triangles, location cues; open circles, PI; filled circles, controls). Error bars show s.e. Values adjacent to error bars indicate sample size in each category.
Both experienced (after second year (ASY), 13/23: 57%) and first-time breeding males (second year (SY), 10/23: 43%) settled at treatment sites. However, only 17% (one out of six) of birds that settled in plots with typically unsuitable habitat (low shrub cover and tree density; 10- to 30-year-old stands) were ASY versus 83% that were SY. Also, SYs tended to settle in plots with lower shrub cover than ASYs (ASY=67.46±5.99, SY=35.17±6.63, t=2.52, p=0.019).
We found females at 27% (10 out of 36) of social information treatments versus 6% (1 out of 18) of control sites; female warblers were more likely to immigrate to sites where we experimentally provided location cues (versus control sites: Χ2=8.36, p=0.015), but did not occur more frequently at location cue sites than at PI sites (figure 1c, location cue β=2.38 (95% CI: 0.13–4.89); PI β=1.89 (95% CI: 0.68–4.43)). After controlling for the strong effect of male presence (Χ2=12.88, p=0.0003), social information treatments explained very little deviance in female settlement (DI=6.36%, Χ2=0.876, p=0.645). Females were not more likely to settle where we observed females at treatment sites in the previous year (Χ2=0.85, p=0.36), and we did not observe females at any sites where males did not settle.
(c) Post-breeding song
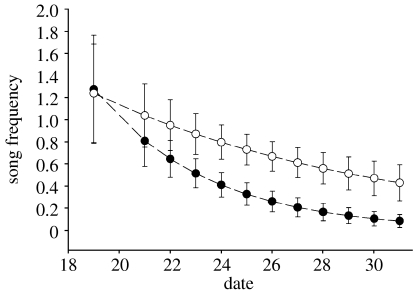
The equally strong response to location cue and PI treatments led us to hypothesize that male song alone, in the post-breeding season, should be correlated with seasonal reproductive success. Song frequency within territories was positively correlated with reproductive success, but only towards the end of the period observed (figure 4, GLMM: treatment×time interaction: Z=3.17, p=0.002; table 3). By late in the breeding season (31 July), singing was 5.1 times (95% CI: 1.89–22.28) more likely on territories that successfully fledged young than those that did not. Conspecific song in the post-breeding season was therefore a reliable indicator of breeding success.
Figure 4.
Singing frequency of male black-throated blue warblers as a function of time during the post-breeding season (19–31 July). Circles are fitted values (±95% CI) of the GLMM: song frequency=treatment (fledged (open circles) versus failed (filled circles))×time.
Table 3.
Summary of GLMM used to predict the song frequency of black-throated blue warblers as a function of whether fledglings survived (treatment) and time of breeding season (date). (Model fitted values are plotted in figure 4.)
| β | LCI | UCI | z | p | |
|---|---|---|---|---|---|
| intercept | 5.39 | 3.19 | 7.60 | 4.80 | <0.0001 |
| date | −0.30 | −0.39 | −0.21 | −6.22 | <0.0001 |
| treatment (fledglings) | −3.39 | −6.08 | −0.70 | −2.47 | 0.014 |
| treatment×date | 0.18 | 0.07 | 0.29 | 3.17 | 0.002 |
4. Discussion
These results reveal post-breeding song as a novel mechanism for the inadvertent transfer of information about reproduction from successful breeders to natal and breeding dispersers. Though previous studies have succeeded in manipulating immigration and emigration, the actual cues used in habitat selection have remained poorly known (Doligez et al. 2003; Nocera et al. 2006; Parejo et al. 2007). Fledgling condition (e.g. gape, skin and plumage colorations) and activity (Parejo et al. 2007) as well as parental feeding rates (Pärt & Doligez 2003) and number of nestlings (Doligez et al. 2002) have been proposed as possible proximate cues for settlement. Such detailed cues would presumably be time-consuming to collect, thus creating a trade-off between information quality and quantity (Dall et al. 2005; Stamps & Krishnan 2005). Our results reveal an alternative parsimonious solution; bird song alone may be a reliable indicator of reproductive performance. Song was common to both treatment types, and we observed prospecting warblers less often at control sites than at either treatment type, indicating that song may be the primary relevant factor for prospecting by warblers in the post-breeding period.
Both natal and breeding dispersers used social information collected during the post-breeding period in the previous year (via prospecting) and subsequently settled at these sites. However, older (ASY) birds may be more likely to use knowledge of structural cues gathered during past breeding experiences; young (SY) birds were more likely to immigrate to low-quality sites as a result of the provision of social information.
Females also tended to settle preferentially at treatment sites. Although this may indicate that females also prospected in the previous year, it is possible that females settled using the presence of males in the spring as a location cue for three reasons. First, females tend to arrive on the breeding grounds more than 4 days after males. Second, we did not observe females at any sites where males did not settle. If females based their settlement decisions on prospected information from the previous year, we would not have expected this to occur. Finally, we found no correspondence between the presence of females during the treatment year and female settlement the following spring. Previous research also suggests that female warblers settle at locations where there are high-quality males (Buchanan & Catchpole 1997; Kokko et al. 2006). Together, this evidence suggests that social information may generate a male-to-female informational cascade (Giraldeau et al. 2002)—a female may settle in an inappropriate habitat because a male made a faulty choice.
Importantly, the reliability of song as an indicator of site quality is strongly dependent upon temporal context. Because song is used during the early and peak breeding season to attract mates and defend territories (Morse 1970), it is an unreliable cue of territory quality during these periods; even nests of experienced breeders have approximately 50% chance of failure due to predation (Holmes et al. 1996). We have shown that song becomes a more reliable correlate of performance as the breeding season progresses. Reproductively successful birds may sing late in the season to influence song development in offspring (Beecher et al. 2007), thus simultaneously providing information about performance to eavesdroppers. Also, reproductively unsuccessful males are known to abandon territories during the breeding season (Hoover 2003), which would result in poor quality territories being devoid of late season song.
We hypothesize that post-breeding song may be a pervasive and reliable cue in habitat selection. However, several conditions probably apply. Young must be produced asynchronously or adults must abandon territories after reproductive failure during the breeding season. Both of these conditions supply a potential source of prospectors simultaneous to the provision of information by successful reproducers. Territory abandonment, or at least cessation in singing, at poor quality sites is necessary to prevent ‘false cues’. Finally, site quality must be autocorrelated across years.
Although vegetative structure contributed to the strength of the model describing settlement, our results do not support the long-held paradigm (e.g. Mayr 1926; Lack & Venables 1939) that organisms select habitats based primarily on structural cues. This has implications for how rapidly species are able to adjust breeding-site selection in response to the changing environmental conditions. Because vegetation structure is relatively slow to respond to changing climate, particularly in forest environments, reliance on structural cues would probably result in slow animal response to changes in underlying habitat quality. Similarly, for short-lived species, reliance on personally gathered information could be very costly (Stamps & Krishnan 2005). Conversely, by using vocal cues as PI, dispersers could collect information about large numbers of potential breeding sites at the end of a single breeding season. As we have shown, PI should be robust to severed correlations between vegetation structure and site quality; warblers settled in response to social information even at sites where apparently optimal vegetation structure (Doran & Holmes 2005; Betts et al. 2006) was absent. Such rapid transmission of information that has the capacity to trump typical species–vegetation associations should be a more efficient short-term mechanism of territory selection in the face of environmental change than personally acquired information, structural cues or genetic adaptation.
Acknowledgments
All field methods used in this research complied with the current regulations and animal ethics guidelines of the authors' respective institutions and countries.
The authors declare no conflict of interest.
The Hubbard Brook Ecosystem study, Northern Research Station, USDA Forest Service and many capable field assistants provided assistance with logistics and data collection. We thank L.-A. Giraldeau, R. T. Holmes, L. M. Ratcliffe, P. R. Martin, M. Mönkkönen, N. V. Siebrass and two anonymous reviewers for their helpful reviews of this manuscript. M.G.B. and J.J.N. were partly supported by postdoctoral fellowships from the Natural Sciences and Engineering Research Council of Canada.
Footnotes
This paper is dedicated to the memory of N. P. P. Simon—an exceptional scientist, educator and friend.
References
- Beecher M.D, Burt J.M, O'Loghlen A.L, Templeton C.N, Campbell S.E. Bird song learning in an eavesdropping context. Anim. Behav. 2007;73:929–935. doi:10.1016/j.anbehav.2006.10.013 [Google Scholar]
- Betts M.G, Hadley A.S, Doran P.J. Avian mobbing response is restricted by territory boundaries: experimental evidence from two species of forest warblers. Ethology. 2005;111:821–835. doi:10.1111/j.1439-0310.2005.01109.x [Google Scholar]
- Betts M.G, Diamond A.W, Forbes G.J, Villard M.-A, Gunn J.S. The importance of spatial autocorrelation, extent and resolution in predicting forest bird occurrence. Ecol. Model. 2006;191:197–224. doi:10.1016/j.ecolmodel.2005.04.027 [Google Scholar]
- Boulinier T, Danchin E. The use of conspecific reproductive success for breeding patch selection in terrestrial migratory species. Evol. Ecol. 1997;11:505–517. doi:10.1007/s10682-997-1507-0 [Google Scholar]
- Broström, G. 2007 TheGLMMMLpackage, v. 0.71-3. See http://cran.r-project.org/src/contrib/Descriptions/glmmML.html
- Buchanan K.L, Catchpole C.K. Female choice in the sedge warbler, Acrocephalus schoenobaenus: multiple cues from song and territory quality. Proc. R. Soc. B. 1997;264:521–526. doi:10.1098/rspb.1997.0074 [Google Scholar]
- Chevan A, Sutherland M. Hierarchical partitioning. Am. Stat. 1991;45:90–96. doi:10.2307/2684366 [Google Scholar]
- Clark R.W. Public information for solitary foragers: timber rattlesnakes use conspecific chemical cues to select ambush sites. Behav. Ecol. 2007;18:487–490. doi:10.1093/beheco/arm002 [Google Scholar]
- Dall S.R.X, Giraldeau L.-A, Olsson O, McNamara J.M, Stephens D.W. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 2005;20:187–193. doi: 10.1016/j.tree.2005.01.010. doi:10.1016/j.tree.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Danchin É, Giraldeau L.-A, Valone T.J, Wagner R.H. Public information: from nosy neighbors to cultural evolution. Science. 2004;305:487–491. doi: 10.1126/science.1098254. doi:10.1126/science.1098254 [DOI] [PubMed] [Google Scholar]
- Doligez B, Danchin E, Clobert J. Public information and breeding habitat selection in a wild bird population. Science. 2002;297:1168–1170. doi: 10.1126/science.1072838. doi:10.1126/science.1072838 [DOI] [PubMed] [Google Scholar]
- Doligez B, Cadet C, Danchin E, Boulinier T. When to use public information for breeding habitat selection? The role of environmental predictability and density dependence. Anim. Behav. 2003;66:973–988. doi:10.1006/anbe.2002.2270 [Google Scholar]
- Doran P.J, Holmes R.T. Habitat occupancy patterns of a forest dwelling songbird: causes and consequences. Can. J. Zool. 2005;83:1297–1305. doi:10.1139/z05-127 [Google Scholar]
- Fletcher R.J. Emergent properties of conspecific attraction in fragmented landscapes. Am. Nat. 2006;168:207–219. doi: 10.1086/505764. doi:10.1086/505764 [DOI] [PubMed] [Google Scholar]
- Fretwell S.D, Lucas H.L. On territorial behavior and other factors influencing habitat distribution in birds. 1. Theoretical development. Acta Biotheor. 1970;19:16–36. doi:10.1007/BF01601953 [Google Scholar]
- Gess A. Birds like music, too. Science. 2007;317:1864. doi: 10.1126/science.317.5846.1864b. doi:10.1126/science.317.5846.1864b [DOI] [PubMed] [Google Scholar]
- Giraldeau L.-A, Valone T.J, Templeton J.J. Potential disadvantages of using socially acquired information. Phil. Trans. R. Soc. B. 2002;357:1559–1566. doi: 10.1098/rstb.2002.1065. doi:10.1098/rstb.2002.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves G.R. Age determination of free-living male black-throated blue warblers during the breeding season. J. Field Ornithol. 1997;68:443–449. [Google Scholar]
- Gunn J.S, Desrochers A, Villard M.A, Bourque J, Ibarzabal J. Playbacks of mobbing calls of black-capped chickadees as a method to estimate reproductive activity of forest birds. J. Field Ornithol. 2000;71:472–483. [Google Scholar]
- Hilden O. Habitat selection in birds. Ann. Zool. Fenn. 1965;2:53–75. [Google Scholar]
- Holmes R.T, Marra P.P, Sherry T.W. Habitat-specific demography of breeding black-throated blue warblers (Dendroica caerulescens): implications for population dynamics. J. Anim. Ecol. 1996;65:183–195. doi:10.2307/5721 [Google Scholar]
- Hoover J.P. Decision rules for site fidelity in a migratory bird, the prothonotary warbler. Ecology. 2003;84:416–430. doi:10.1890/0012-9658(2003)084[0416:DRFSFI]2.0.CO;2 [Google Scholar]
- Hothorn, T., Bretz, F. & Westfall P. 2007 Themultcomppackage, v. 0.992-8. See http://cran.r-project.org/src/contrib/Descriptions/multcomp.html
- Hutto R.L, Pletschet S.M, Hendricks P. A fixed-radius point count method for nonbreeding and breeding season use. Auk. 1986;103:593–602. [Google Scholar]
- Kokko H, Gunnarsson T.G, Morrell L.J, Gill J.A. Why do female migratory birds arrive later than males? J. Anim. Ecol. 2006;75:1293–1303. doi: 10.1111/j.1365-2656.2006.01151.x. doi:10.1111/j.1365-2656.2006.01151.x [DOI] [PubMed] [Google Scholar]
- Lack D, Venables L.S.V. The habitat distribution of British woodland birds. J. Anim. Ecol. 1939;8:39–71. doi:10.2307/1252 [Google Scholar]
- MacArthur R.H, MacArthur J.W, Preer J. On bird species diversity II. Prediction of bird censuses from habitat measurements. Am. Nat. 1962;96:167–174. doi:10.1086/282219 [Google Scholar]
- Mayr E. Die ausbreitung des Girlitz (Serinus canaria serinus L.): Ein Beitrag zur Tiergeographie. J. Ornithol. 1926;74:571–671. doi:10.1007/BF01998227 [Google Scholar]
- Morris D.W. Toward an ecological synthesis: a case for habitat selection. Oecologia. 2003;136:1–13. doi: 10.1007/s00442-003-1241-4. doi:10.1007/s00442-003-1241-4 [DOI] [PubMed] [Google Scholar]
- Morse D.H. Differences between courtship and territorial songs. Nature. 1970;226:659–661. doi: 10.1038/226659a0. doi:10.1038/226659a0 [DOI] [PubMed] [Google Scholar]
- Nocera J.J, Forbes G.J, Giraldeau L.-A. Inadvertent social information in breeding site selection of natal dispersing birds. Proc. R. Soc. B. 2006;273:349–355. doi: 10.1098/rspb.2005.3318. doi:10.1098/rspb.2005.3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parejo D, White J, Clobert J, Dreiss A, Danchin E. Blue tits use fledgling quantity and quality as public information in breeding site choice. Ecology. 2007;88:2373–2382. doi: 10.1890/06-2000.1. doi:10.1890/06-2000.1 [DOI] [PubMed] [Google Scholar]
- Pärt T, Doligez B. Gathering public information for habitat selection: prospecting birds cue on parental activity. Proc. R. Soc. B. 2003;270:1809–1813. doi: 10.1098/rspb.2003.2419. doi:10.1098/rspb.2003.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porneluzi P.A. Prior breeding success affects return rates of territorial male ovenbirds. Condor. 2003;105:73–79. doi:10.1650/0010-5422(2003)105[73:PBSARR]2.0.CO;2 [Google Scholar]
- Pulliam H.R, Danielson B.J. Sources, sinks, and habitat selection—a landscape perspective on population dynamics. Am. Nat. 1991;137:S50–S66. doi:10.1086/285139 [Google Scholar]
- R Development Core Team 2007 R: a language environment for statistical computing, v. 2.6.1. Vienna, Austria: R Foundation for Statistical Computing.
- Rodenhouse N.L, Sillett T.S, Doran P.J, Holmes R.T. Multiple density-dependence mechanisms regulate a migratory bird population during the breeding season. Proc. R. Soc. B. 2003;270:2105–2110. doi: 10.1098/rspb.2003.2438. doi:10.1098/rspb.2003.2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle S.R. Wiley; New York, NY: 1971. Linear models. [Google Scholar]
- Seppanen J.T, Forsman J.T, Mönkkönen M, Thomson R.L. Social information use is a process across time, space, and ecology, reaching heterospecifics. Ecology. 2007;88:1622–1633. doi: 10.1890/06-1757.1. doi:10.1890/06-1757.1 [DOI] [PubMed] [Google Scholar]
- Stamps J, Krishnan V.V. Nonintuitive cue use in habitat selection. Ecology. 2005;86:2860–2867. doi:10.1890/05-0290 [Google Scholar]
- Thomas C.D, et al. Ecological and evolutionary processes at expanding range margins. Nature. 2001;411:577–581. doi: 10.1038/35079066. doi:10.1038/35079066 [DOI] [PubMed] [Google Scholar]
- Valone T.J. Group foraging, public information, and patch estimation. Oikos. 1989;56:357–363. doi:10.2307/3565621 [Google Scholar]
- Valone T.J. Food-associated calls as public information about patch quality. Oikos. 1996;77:153–157. doi:10.2307/3545595 [Google Scholar]
- Valone T.J, Templeton J.J. Public information for the assessment of quality: a widespread social phenomenon. Phil. Trans. R. Soc. B. 2002;357:1549–1557. doi: 10.1098/rstb.2002.1064. doi:10.1098/rstb.2002.1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alpen J.J.M, Bernstein C, Driessen G. Information acquisition and time allocation in insect parasitoids. Trends Ecol. Evol. 2003;18:81–88. doi:10.1016/S0169-5347(02)00035-6 [Google Scholar]