Abstract
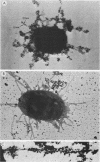
The distribution of type 1 and P pili on individual cells of an O6 uropathogenic Escherichia coli strain, 6260, was determined immunologically with pilus-specific monoclonal antibodies by indirect immunofluorescence and immunogold electron microscopy. Variations in pilus expression under different culture conditions were monitored with an indirect immunofluorescence assay; 63% of piliated cells expressed type 1 pili when grown on agar at 37 degrees C versus 14 to 38% when grown in broth at 37 degrees C. In contrast, generally fewer cells with P pili (18 to 44%) were detected on agar than when grown in broth (up to 86%). Both type 1 and P pili were absent from cells cultured at 20 degrees C. Immunogold and immunofluorescence double labeling techniques with monoclonal antibodies 11-2 and 91-1 were used to study subpopulations of cells with type 1 and P pili; 39 to 41% of the piliated cells demonstrated only type 1 pili, and 12 to 16% of the cells showed only P pili. The immunogold method proved more sensitive than the immunofluorescence technique for detecting subpopulations expressing both pili types simultaneously, 19 versus 7%. We observed variations between type 1 and P pili, both in expression on individual cells and in the distribution of subpopulations of cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINTON C. C., Jr Non-flagellar appendages of bacteria. Nature. 1959 Mar 21;183(4664):782–786. doi: 10.1038/183782a0. [DOI] [PubMed] [Google Scholar]
- Clegg S. Cloning of genes determining the production of mannose-resistant fimbriae in a uropathogenic strain of Escherichia coli belonging to serogroup O6. Infect Immun. 1982 Nov;38(2):739–744. doi: 10.1128/iai.38.2.739-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hansson H. A. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978 Jul;21(1):229–237. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977 Nov;18(2):330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gander R. M., Thomas V. L., Forland M. Mannose-resistant hemagglutination and P receptor recognition of uropathogenic Escherichia coli isolated from adult patients. J Infect Dis. 1985 Mar;151(3):508–513. doi: 10.1093/infdis/151.3.508. [DOI] [PubMed] [Google Scholar]
- Gander R. M., Thomas V. L. Utilization of anion-exchange chromatography and monoclonal antibodies to characterize multiple pilus types on a uropathogenic Escherichia coli O6 isolate. Infect Immun. 1986 Feb;51(2):385–393. doi: 10.1128/iai.51.2.385-393.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göransson M., Uhlin B. E. Environmental temperature regulates transcription of a virulence pili operon in E. coli. EMBO J. 1984 Dec 1;3(12):2885–2888. doi: 10.1002/j.1460-2075.1984.tb02225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Jodal U., Korhonen T. K., Lidin-Janson G., Lindberg U., Svanborg Edén C. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect Immun. 1981 Feb;31(2):564–570. doi: 10.1128/iai.31.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Leying H., Büscher K. H., Kroll H. P., Opferkuch W. Isolation and separation of physicochemically distinct fimbrial types expressed on a single culture of Escherichia coli O7:K1:H6. Infect Immun. 1985 Feb;47(2):549–554. doi: 10.1128/iai.47.2.549-554.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Klemm P., Orskov I., Orskov F. F7 and type 1-like fimbriae from three Escherichia coli strains isolated from urinary tract infections: protein chemical and immunological aspects. Infect Immun. 1982 May;36(2):462–468. doi: 10.1128/iai.36.2.462-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen V., Saxén H., Hultberg H., Svenson S. B. P-antigen-recognizing fimbriae from human uropathogenic Escherichia coli strains. Infect Immun. 1982 Jul;37(1):286–291. doi: 10.1128/iai.37.1.286-291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F. P., Lund B., Normark S. Genes of pyelonephritogenic E. coli required for digalactoside-specific agglutination of human cells. EMBO J. 1984 May;3(5):1167–1173. doi: 10.1002/j.1460-2075.1984.tb01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki B., Rhen M., Väisänen-Rhen V., Pere A., Korhonen T. K. Immunofluorescence study of fimbrial phase variation in Escherichia coli KS71. J Bacteriol. 1984 Nov;160(2):691–695. doi: 10.1128/jb.160.2.691-695.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J., Finne J., Achtman M., Väisänen V., Korhonen T. K. Escherichia coli strains binding neuraminyl alpha 2-3 galactosides. Biochem Biophys Res Commun. 1983 Mar 16;111(2):456–461. doi: 10.1016/0006-291x(83)90328-5. [DOI] [PubMed] [Google Scholar]
- Uhlin B. E., Norgren M., Båga M., Normark S. Adhesion to human cells by Escherichia coli lacking the major subunit of a digalactoside-specific pilus-adhesin. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1800–1804. doi: 10.1073/pnas.82.6.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]