Abstract
Trace fossils of insect feeding have contributed substantially to our understanding of the evolution of insect–plant interactions. The most complex phenotypes of herbivory are galls, whose diagnostic morphologies often allow the identification of the gall inducer. Although fossil insect-induced galls over 300 Myr old are known, most are two-dimensional impressions lacking adequate morphological detail either for the precise identification of the causer or for detection of the communities of specialist parasitoids and inquilines inhabiting modern plant galls. Here, we describe the first evidence for such multitrophic associations in Pleistocene fossil galls from the Eemian interglacial (130 000–115 000 years ago) of The Netherlands. The exceptionally well-preserved fossils can be attributed to extant species of Andricus gallwasps (Hymenoptera: Cynipidae) galling oaks (Quercus), and provide the first fossil evidence of gall attack by herbivorous inquiline gallwasps. Furthermore, phylogenetic placement of one fossil in a lineage showing obligate host plant alternation implies the presence of a second oak species, Quercus cerris, currently unknown from Eemian fossils in northwestern Europe. This contrasts with the southern European native range of Q. cerris in the current interglacial and suggests that gallwasp invasions following human planting of Q. cerris in northern Europe may represent a return to preglacial distribution limits.
Keywords: cynipid, fossil, Pleistocene, multitrophic, gall, oak
1. Introduction
Trace fossils made by feeding insects have played a major role in our understanding of the evolution of insect–plant interactions (Labandeira & Phillips 1996; Wilf et al. 2001, 2006; Currano et al. 2008). The most structurally complex signs of insect activity in plant tissues are galls, plant tissues whose development is controlled by the gall-inducing organism (the galler) and which provide the galler with nutrition and protection (Cornell 1983; Price et al. 1987; Stone & Schönrogge 2003). The insect control of gall morphology is such that galls are the extended phenotypes of galler genes (Crespi & Worobey 1998; Stone & Cook 1998), and many gallers can be identified to species on the basis of gall morphology alone (Raman et al. 2005). Although three-dimensionally preserved insect-induced galls, dated at ca 305 Myr, are known, most are two-dimensional impressions whose lack of morphological characters precludes more specific identification of the causer (e.g. Scott et al. 1994; Labandeira & Phillips 1996). However, the inducers of well-preserved and structurally complex galls can sometimes be identified with high taxonomic resolution (e.g. Dieguez et al. 1996; Waggoner 1999; Erwin & Schick 2007).
In addition to the causer, many galls support communities of specialist herbivorous inquilines and natural enemies. The herbivorous inquilines are obligate inhabitants of galls induced by specific host galler species, and though they do not feed on the galler, their activities can cause its death either directly or indirectly (Shorthouse 1980; Washburn & Cornell 1981; Askew 1984; Wiebes-Rijks & Shorthouse 1992; Ronquist 1994). The inquilines are often closely related to the gall inducers whose galls they attack (Ronquist 1994; Crespi & Abbot 1999; Miller & Crespi 2003), and this association (termed agastoparasitism by Ronquist 1994), closely parallels true parasitism. Both gall inducers and inquilines are attacked by natural enemies, particularly parasitoid wasps, and all three trophic groups commonly leave characteristic signatures in gall tissues (Stone et al. 2002; Raman et al. 2005). Fossil galls thus have the potential to provide direct evidence of within-gall multispecies associations. Here, we describe the first evidence for such associations, in Pleistocene fossil galls from the Eemian interglacial (130 000–115 000 years ago) whose excellent three-dimensional preservation not only allows precise identification of the causers but also provides the first fossil evidence of gall attack by specialist inquilines. Furthermore, phylogenetic placement of one of the Eemian gall causers in a lineage showing highly conserved host plant associations allows new palaeobotanical inferences for northwestern Europe that provide a striking contrast with the current interglacial.
2. Fossil material studied
The fossil galls (figures 1 and 2) were discovered in a gravel pit near Raalte, Overijssel Province, The Netherlands (see van der Ham et al. (2008) for a description of the site and associated biological material). They were preserved in Late Eemian (ca 125 000 BP) sediments of the Kreftenheye Formation (de Mulder et al. 2003) laid down by the ancient Rhine in the valley of the present river IJssel (van der Ham et al. 2008). Eemian vegetation at the site comprised temperate woodland including wetland trees (e.g. Alnus, Populus and Salix) and upland forest species (e.g. Abies alba, Acer, Carpinus betulus, Ilex aquifolium and Quercus). The fossils are of two types.
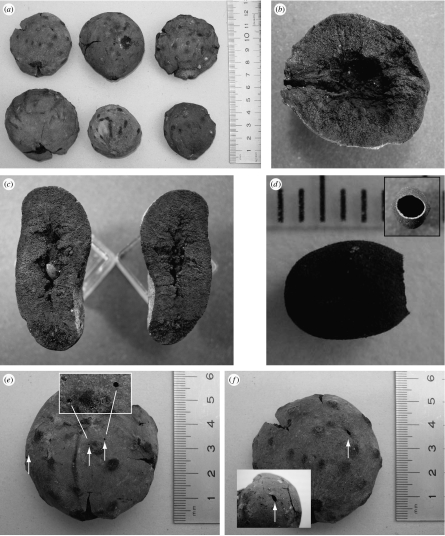
Type 1. The most abundant type (n=47; figure 1) is preserved as a slightly (figure 1b) to moderately (figure 1c) compressed structure, originally approximately spherical and 37–57 mm in diameter. The original outer gall surface is exceptionally well preserved and marked with small regularly spaced tubercles or parallel ridges (figure 1a). The fossils have a hollow interior (figure 1b,c) and, when complete, contain a single thin-walled chamber 5–6 mm long (figure 1d) that in some cases is attached to the interior wall on each side of its long axis (figure 1c). In all cases, this chamber has a smooth-edged hole 2 mm in diameter at one end. Three fossils have holes in the exterior (arrowed in figure 1e,f); each has a single larger slightly lenticular hole (figure 1f; dimensions 1.9×0.8 mm, 3×1.2 mm and 1.9×1.0 mm), while two galls have 1–3 smaller circular holes 0.6–0.8 mm in diameter (figure 1e,f).
Type 2. A single example (figure 2a,b) was found of a second type. One surface lacks obvious structure but bears several small holes approximately 1 mm in diameter (arrowed, figure 2a). The opposite surface comprises an aggregation of thin-walled chambers (figure 2b) approximately 5 mm long, joined in one case by a smoothly rounded hole 2 mm in diameter (arrowed, figure 2b).
Figure 1.
Type 1 gall fossils. (a) External views (scale in cm). (b) Longitudinal section showing internal airspace, with inner larval chamber missing. The gall's point of attachment is to the left. (c) Two halves of a sectioned compressed gall, showing the larval chamber in the left of the section. (d) A larval chamber, with the adult emergence hole to the right and (inset) in end-on view (scale in mm). (e,f) Two views of the same fossil gall, showing multiple emergence holes (arrowed). In (e), two of the small emergence holes are shown in enlarged view (boxed).
Figure 2.
(a) Type 2 fossil external view, showing small emergence holes (arrowed, scale bar in mm). (b) Internal view of the same specimen, showing aggregated larval chambers (scale bar in mm). (c) A sectioned asexual generation gall of the modern oak gallwasp A. quercusradicis (scale bar, 1 cm). (d) Mature asexual generation gall of the modern oak gallwasp Andricus hungaricus. (e) The same in cross-section, showing the suspended inner larval chamber (c–e, courtesy of Dr György Csóka).
3. Diagnosis
Both fossil types can be unambiguously identified as galls induced by oak gallwasps (Hymenoptera: Cynipidae) on the basis of striking similarity to modern forms. No other gall inducers produce galls of this size and complexity in the Western Palaearctic (Docters van Leeuwen 1957; Buhr 1964–1965). Oak cynipid galls are diagnostic not only of the gallwasp species but also of alternating sexual and asexual generations in a parthenogenetic life cycle (Stone & Cook 1998; Stone et al. 2002, 2008). Phylogenetic relationships, oak host associations and associated gall communities of the Western Palaearctic gallwasp fauna have been studied in depth (Stone & Cook 1998; Cook et al. 2002; Rokas et al. 2003b), allowing extensive inference of biology and associated communities from gall structures. Studies of within-species genetic diversity show that all modern Western Palaearctic oak gallwasp species so far studied are at least 1–2 Myr old, and so long predate the Eemian (Rokas et al. 2001, 2003a; Stone et al. 2002, 2007; Challis et al. 2007). The Raalte fossils are thus certainly young enough to be attributable in principle to extant species.
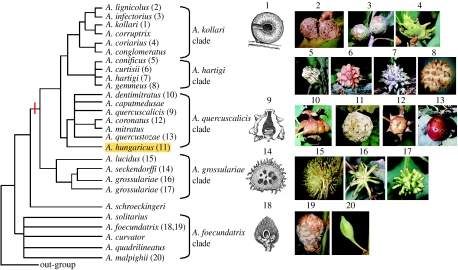
Type 1. Only oak cynipid gallwasps induce galls of this size and complexity and the presence of a single larval chamber within an internal airspace places the galler with certainty within the quercuscalicis clade of the genus Andricus (figure 3). More significantly, these fossils are identical in size, external structure (figure 2d) and the suspension of the larval chamber within an internal airspace (figure 2e) to the asexual generation galls of extant Andricus hungaricus (Hartig 1843). These are the first fossil galls ever to be so clearly attributable to an extant gall inducer. The asexual generation galls of A. hungaricus are among the largest in the Western Palaearctic, and develop on shoot buds only of Quercus robur. They comprise up to 20% tannin dry weight (Ambrus 1974) and are resistant enough to decay that gallwasps diapause up to 6 years (Stone et al. 2002)—properties that may predispose these galls to fossilization.
Type 2. The clustered cells in this fossil most closely resemble the asexual generation galls of Andricus quercusradicis (figure 2c). These galls comprise multiple aggregated larval chambers within a woody outer layer lacking distinctive surface sculpture, reaching a total diameter of 80 mm (Docters van Leeuwen 1957; Ambrus 1974). We interpret the type 2 fossil as a fragment of such a gall, whose exterior bears the small holes in figure 2a. The quality of preservation of the type 1 galls suggests that remnants at least of any more distinctive surface structures would be visible. Other woody, multichambered Western Palaearctic cynipid galls contain smaller larval chambers (approx. 2 mm long: some Plagiotrochus and Pseudoneuroterus), comprise larval chambers with no surrounding woody material (Callirhytis asexual generation galls in acorns) or have an outer surface ornamented with spines (the Andricus grossulariae clade in Andricus; figure 3; Docters van Leeuwen 1957; Ambrus 1974; Stone & Cook 1998; Melika et al. 2000; Nieves-Aldrey 2001).
Figure 3.
A phylogeny of the oak gallwasp genus Andricus, generated from DNA sequence data and showing the main species groups found in the Western Palaearctic. The gall structures illustrated are for the asexual generation. The only group to contain a separate larval chamber within an internal airspace is the Andricus quercuscalicis clade. The red bar shows the common ancestor of the host-alternating Andricus clade. Phylogeny from Stone & Cook (1998) and Cook et al. (2002). 1, Andricus kollari; 2, Andricus lignicolus; 3, Andricus infectorius; 4, Andricus coriarius; 5, Andricus conificus; 6, Andricus curtisii; 7, Andricus hartigi; 8, Andricus gemmeus; 9, A. quercuscalicis (shown in cross-section); 10, Andricus dentimitratus; 11, A. hungaricus; 12, Andricus coronatus; 13, Andricus quercustozae; 14, Andricus seckendorffi; 15, Andricus lucidus; 16, 17, Andricus grossulariae; 18,19, Andricus foecundatrix; 20, Andricus malpighii.
(a) Multispecies interactions
Both gall types show holes in the gall surface and, for the type 2 fossil, between internal larval chambers. These holes are characteristic of those made by emerging adult members of the gall community. The single larger surface holes on type 1 galls match the diameter of the aperture in the single inner larval chamber (figure 1d), and were chewed by an emerging gallwasp. Gallwasps emerging from multichambered galls commonly chew their way through vacated surrounding chambers, explaining the larger internal hole in the type 2 fossil. The smaller surface openings are too small to have been made by the galler in either case. Oak cynipid galls are commonly occupied by inquiline cynipids (Cynipidae: Synergini) that cannot induce their own galls but inhabit those induced by other causers, predominantly gallwasps (Stone et al. 2002). The inquilines are always smaller than the gall inducer and induce their larval chambers either within the host larval chamber (in which case the galler is always killed) or in the outer gall tissues (Washburn & Cornell 1981; Askew 1984; Wiebes-Rijks & Shorthouse 1992; Ronquist 1994; Stone et al. 2002). The presence of both large and small emergence holes in a single type 1 gall can only be attributed to attack by outer gall inquilines. All outer gall inquilines in modern oak cynipid galls are species in the genus Synergus, and the small emergence holes must lead to chambers induced by Eemian Synergus. Synergus inquilines are themselves attacked by chalcid parasitoids (Askew 1961, 1984; Schönrogge et al. 1995, 1996a,b) and the smaller emergence holes could thus have been made either by emerging adult inquilines or parasitoids. Both of these trophic groups are associated with modern A. hungaricus galls (Melika et al. 1997). The many-chambered structure of the type 2 fossil makes the identification of causers of the smaller surface emergence holes less certain: though too small to have been made by the galler, they could have been made by emerging inquilines or by parasitoids of either inquilines or gallers.
4. Discussion
(a) Multispecies interactions
To our knowledge, this is the first concrete evidence for multiple trophic groups in a fossil gall. Though fossil inquiline cynipids are known as far back as the Middle Eocene (45 Myr ago; Ronquist 1999), this is the first direct fossil evidence of their presence in cynipid gall tissues. Inquilines in the outer tissues of cynipid galls support rich communities of chalcid parasitoids that are often distinct from those attacking the inducer in the same gall (Askew 1961, 1984; Schönrogge et al. 1995, 1996a,b). Though we cannot distinguish emergence holes made by these two trophic groups, it is highly probable that some of the emergence holes in the Raalte fossils were made by chalcid parasitoids. The precise identification of the gall inducer from the gall phenotype, and hence a prior expectation of the relative sizes of the gall inducer and other gall inhabitants, was central to our interpretation of the trace fossils. It is possible that other fossil galls showing apparent emergence holes that are small relative to the size of the gall may represent evidence of inquiline or parasitoid attack: candidates include galls DT83 and DT84 in the web-based Guide to Insect (and Other) Damage Types on Compressed Plant Fossils at http://paleobiology.si.edu/insects/index.html (Dr C. Labandeira 2008, personal communication). However, many large galls house multiple small gall inducers (see Raman et al. (2005) for examples in many arthropod groups), and the relative size of galls and emergence holes may be an unreliable guide to the presence of non-galling inhabitants. Nevertheless, we expect that the examination of other fossil galls attributable to extant galler taxa, whose associated communities are well known, will yield further evidence of dependent trophic interactions.
(b) Oak gallwasps as indicators of past floras
Andricus hungaricus lies within a clade of Andricus species whose lifecycles require obligate alternation between two different taxonomic sections of the genus Quercus (Cook et al. 2002; Stone et al. 2008). The asexual generation always galls section Quercus sensu stricto oaks (such as Q. robur and Quercus petraea), while the sexual generation always galls section Cerris oaks (particularly Turkey oak, Quercus cerris). The most recent common ancestor of this host-alternating clade long predates the Pleistocene (Cook et al. 2002), implying that the Eemian causer of gall type 1 was also a host alternator. This allows a novel palaeobotanical inference, because while the presence of section Quercus oaks at the Raalte site is confirmed by pollen and macrofossils (van der Ham et al. 2008), there is no direct evidence for section Cerris oaks. The type 1 fossils imply that during the relatively short (ca 15 000 years) Eemian interglacial, both oak sections and their associated insects escaped their southern European glacial refugia (Petit et al. 2002) to colonize northern Europe. The identification of the type 2 fossil provides additional (though weaker) support for this conclusion, for although both generations of A. quercusradicis can be found on section Cerris and section Quercus, the sexual generation is most commonly associated with section Cerris (Ambrus 1974; Melika et al. 2000).
(c) Implications for modern gallwasp distributions
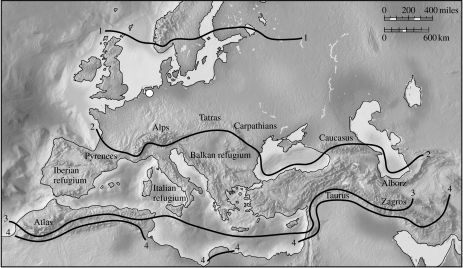
The presence of section Cerris oaks inferred from the Raalte fossils contrasts with the oak flora native to the same region in the current interglacial. Only two section Quercus oaks, Q. robur and Q. petraea, are native to northern Europe, while section Cerris oaks (and hence associated gallwasps) are restricted to southern Europe and predominantly to regions south of the Pyrenees, Alps and Carpathians (figure 4). More specifically, A. hungaricus is now restricted to a region far to the south and east of its Eemian distribution, in Hungary and the Balkan refugium (figure 4; Ambrus 1974; Melika et al. 2000). However, over the last 500 years, human planting has extended the distribution of Q. cerris far beyond its natural distribution, and it is now naturalized as far north and west as Ireland and Scotland (Walker et al. 2002). Northwards range expansion by Q. cerris was thus prevented not by physiological limitations, but by inability to escape its Pleistocene glacial refugia (Svenning & Skov 2004). The introduction of Q. cerris has in turn triggered invasion of northern Europe by multiple host-alternating gallwasps and their natural enemies (Stone et al. 2002; Hayward & Stone 2006), with potential for major direct (competition, parasitoid attack) and indirect (apparent competition mediated by shared enemies) impacts on native communities (Schönrogge et al. 1995, 1996a,b, 1999, 2000; Stone et al. 1995; Atkinson et al. 2002). At least four host-alternating species are now established in The Netherlands (Andricus corruptrix, Andricus kollari (figure 3, gall 1), Andricus lignicolus (figure 3, gall 2), Andricus quercuscalicis (figure 3, gall 9): Docters van Leeuwen 1957; Stone & Sunnucks 1993; Stone et al. 2007). The Raalte fossils imply that these gallwasps, though anthropogenic invaders in the current interglacial, were native to northwestern Europe in the previous interglacial. Their current range expansion should thus perhaps be considered as a return to preglacial distribution limits and ecology.
Figure 4.
Location of the Raalte sampling location (white circle) relative to modern natural distributions of the oak sections Quercus sensu stricto and Cerris. Line 1 represents the northern limit of oaks in the section Quercus and of all oaks in the Western Palaearctic. Only section Quercus oaks are native between lines 1 and 2. Line 2 represents the northern limit of the natural distribution of section Cerris oaks. Oaks in sections Quercus and Cerris are naturally found together between lines 2 and 3. Line 3 represents the southern limit of oaks in the section Quercus. Only section Cerris oaks are found between lines 3 and 4. Line 4 represents the southern limit of section Cerris oaks. Distributions are based on regional floras as explained in Stone et al. (2007).
Acknowledgments
We thank the REKO Grondverzet en Wegenbouw bedrijf Raalte B.V. for access to the Hooge Broek site, and George Melika (Pest Diagnostic Laboratory, Plant Protection & Soil Conservation Directorate of County Vas) for initial contacts between G.N.S. and R.W.J.M.v.d.H. We thank Conrad C. Labandeira and three anonymous referees for their comments that improved the manuscript.
References
- Ambrus, B. 1974 Cynipida-Gubacsok-Cecidia Cynipidarum Fauna Hungariae, XII, 1/a. Budapest, Hungary: Academic Press.
- Askew R.R. On the biology of the inhabitants of oak galls of Cynipidae (Hymenoptera) in Britain. Trans. Soc. Brit. Entomol. 1961;14:237–268. [Google Scholar]
- Askew R.R. The biology of gall wasps. In: Ananthakrischan T.N, editor. Biology of gall insects. Oxford and IBH Publishing Co.; Calcutta, India: 1984. pp. 223–271. [Google Scholar]
- Atkinson R.J, McVean G.A.T, Stone G.N. Use of population genetic data to infer oviposition behaviour: species-specific patterns in four oak gallwasps (Hymenoptera: Cynipidae) Proc. R. Soc. B. 2002;269:383–390. doi: 10.1098/rspb.2001.1820. doi:10.1098/rspb.2001.1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr, H. 1964–1965 Bestimmungstabellen der Gallen (Zoo- und Phytocecidien) an Pflanzen Mittel- und Nordeuropas, vol. 2. Jena, Germany: Gustav Fischer Verlag.
- Challis R.J, Mutun S, Nieves-Aldrey J.-L, Preuss S, Rokas A, Aebi A, Sadeghi E, Tavakoli M, Stone G.N. Longitudinal range expansion and cryptic eastern species in the western Palaearctic oak gallwasp Andricus coriarius. Mol. Ecol. 2007;16:2103–2114. doi: 10.1111/j.1365-294X.2006.03210.x. doi:10.1111/j.1365-294X.2006.03210.x [DOI] [PubMed] [Google Scholar]
- Cook J.M, Rokas A, Pagel M, Stone G.N. Evolutionary shifts between host oak sections and host plant organs in Andricus gallwasps. Evolution. 2002;56:1821–1830. doi: 10.1111/j.0014-3820.2002.tb00196.x. doi:10.1111/j.0014-3820.2002.tb00196.x [DOI] [PubMed] [Google Scholar]
- Cornell H.V. The secondary chemistry and complex morphology of galls formed by the Cynipinae (Hymenoptera): why and how? Am. Midl. Nat. 1983;110:225–234. doi:10.2307/2425263 [Google Scholar]
- Crespi B, Abbot P. The behavioral ecology and evolution of kleptoparasitism in Australian gall thrips. Fla. Entomol. 1999;82:147–164. doi:10.2307/3496568 [Google Scholar]
- Crespi B, Worobey M. Comparative analysis of gall morphology in Australian gall thrips: the evolution of extended phenotypes. Evolution. 1998;52:1686–1696. doi: 10.1111/j.1558-5646.1998.tb02248.x. doi:10.2307/2411341 [DOI] [PubMed] [Google Scholar]
- Currano E.D, Wilf P, Wing S.L, Labandeira C.C, Lovelock E.C, Royer D.L. Sharply increased insect herbivory during the Paleocene–Eocene thermal maximum. Proc. Natl Acad. Sci. USA. 2008;105:1960–1964. doi: 10.1073/pnas.0708646105. doi:10.1073/pnas.0708646105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mulder E.F.J, Geluk M.C, Ritsema I, Westerhoff W.E, Wong T.E. Wolters-Noordhoff; Groningen, The Netherlands: 2003. De ondergrond van Nederland. [Google Scholar]
- Dieguez C, Nieves-Aldrey J.L, Barron E. Fossil galls (zoocecids) from the Upper Miocene of La Cerdana (Lerida, Spain) Rev. Palaeobot. Palynol. 1996;94:329–343. doi:10.1016/S0034-6667(96)00004-8 [Google Scholar]
- Docters van Leeuwen W.M. W.J. Thieme; Zutphen, The Netherlands: 1957. Gallenboek. [Google Scholar]
- Erwin D.M, Schick K.N. New Miocene oak galls (Cynipini) and their bearing on the history of cynipid wasps in western North America. J. Palaeontol. 2007;81:568–580. doi:10.1666/05031.1 [Google Scholar]
- Hayward A, Stone G.N. Comparative phylogeography across two trophic levels: the oak gall wasp Andricus kollari and its chalcid parasitoid Megastigmus stigmatizans. Mol. Ecol. 2006;15:479–489. doi: 10.1111/j.1365-294X.2005.02811.x. doi:10.1111/j.1365-294X.2005.02811.x [DOI] [PubMed] [Google Scholar]
- Labandeira C.C, Phillips T.L.A. Carboniferous insect gall: insight into early ecologic history of the Holometabola. Proc. Natl Acad. Sci. USA. 1996;93:8470–8474. doi: 10.1073/pnas.93.16.8470. doi:10.1073/pnas.93.16.8470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melika, G., Thuróczy, C. & Csóka, G. 1997 Parasitoid insects reared from unisexual galls of Andricus hungaricus Hartig (Hymenoptera: Cynipidae). In Proc. Forest Research Institute (Erdészeti Kutatások), vol. 86–87, pp. 201–205. [In Hungarian, with English summary.]
- Melika G, Csóka G, Pujade-Villar J. Check-list of oak gall wasp of Hungary, with some taxonomic notes (Hymenoptera: Cynipidae, Cynipinae, Cynipini) Annales Historico-Naturales Musei Nationalis Hungarici. 2000;92:265–296. [Google Scholar]
- Miller D, Crespi B. The evolution of inquilinism, host-plant use and mitochodrial substitution rates in Tamalia gall aphids. J. Evol. Biol. 2003;16:731–743. doi: 10.1046/j.1420-9101.2003.00567.x. doi:10.1046/j.1420-9101.2003.00567.x [DOI] [PubMed] [Google Scholar]
- Nieves-Aldrey, J. L. 2001 Hymenoptera, Cynipidae. In Fauna iberica, vol. 16 (ed. M. A. Ramos), pp. 1–636. Madrid, Spain: Museo Nacional de Ciencias Naturales.
- Petit R.J, et al. Identification of refugia and post-glacial colonisation routes of European white oaks based on chloroplast DNA and fossil pollen evidence. For. Ecol. Manage. 2002;156:49–74. doi:10.1016/S0378-1127(01)00634-X [Google Scholar]
- Price P.W, Fernandes G.W, Waring G.L. Adaptive nature of insect galls. Environ. Entomol. 1987;16:15–24. [Google Scholar]
- Raman A, Schaeffer C.W, Withers T.M, editors. Biology, ecology and evolution of gall-inducing arthropods. Science Publishers, Inc.; Enfield, NH: 2005. [Google Scholar]
- Rokas A, Atkinson R, Brown G, West S.A, Stone G.N. Understanding patterns of genetic diversity in the oak gallwasp Biorhiza pallida: demographic history or a Wolbachia selective sweep? Heredity. 2001;87:294–305. doi: 10.1046/j.1365-2540.2001.00872.x. doi:10.1046/j.1365-2540.2001.00872.x [DOI] [PubMed] [Google Scholar]
- Rokas A, Atkinson R.J, Webster L, Stone G.N. Out of Anatolia: longitudinal gradients in genetic diversity support a Turkish origin for a circum-Mediterranean gallwasp Andricus quercustozae. Mol. Ecol. 2003a;12:2153–2174. doi: 10.1046/j.1365-294x.2003.01894.x. doi:10.1046/j.1365-294X.2003.01894.x [DOI] [PubMed] [Google Scholar]
- Rokas A, Melika G, Abe Y, Nieves-Aldrey J.-L, Cook J.M, Stone G.N. Lifecycle closure, lineage sorting and hybridisation revealed in a phylogenetic analysis of European oak gallwasps (Hymenoptera: Cynipidae: Cynipini) using mitochondrial sequence data. Mol. Phylogen. Evol. 2003b;26:36–45. doi: 10.1016/s1055-7903(02)00329-9. doi:10.1016/S1055-7903(02)00329-9 [DOI] [PubMed] [Google Scholar]
- Ronquist F. Evolution of parasitism among closely related species: phylogenetic relationships and the origin of inquilinism in gall wasps (Hymenoptera, Cynipidae) Evolution. 1994;48:241–266. doi: 10.1111/j.1558-5646.1994.tb01310.x. doi:10.2307/2410091 [DOI] [PubMed] [Google Scholar]
- Ronquist F. Phylogeny, classification and evolution of the Cynipoidea. Zool. Scr. 1999;28:139–164. doi:10.1046/j.1463-6409.1999.00022.x [Google Scholar]
- Schönrogge K, Stone G.N, Crawley M.J. Spatial and temporal variation in guild structure: parasitoids and inquilines of Andricus quercuscalicis (Hymenoptera: Cynipidae) in its native and alien ranges. Oikos. 1995;72:51–60. doi:10.2307/3546037 [Google Scholar]
- Schönrogge K, Stone G.N, Crawley M.J. Alien herbivores and native parasitoids: rapid development of guild structure in an invading gall wasp, Andricus quercuscalicis (Hymenoptera: Cynipidae) Ecol. Entomol. 1996a;21:71–80. doi:10.1111/j.1365-2311.1996.tb00268.x [Google Scholar]
- Schönrogge K, Stone G.N, Crawley M.J. Abundance patterns and species richness of the parasitoids and inquilines of the alien gall former Andricus quercuscalicis Burgsdorf (Hymenoptera: Cynipidae) Oikos. 1996b;77:507–518. doi:10.2307/3545940 [Google Scholar]
- Schönrogge K, Walker P, Crawley M.J. Complex life cycles in Andricus kollari (Hymenoptera, Cynipidae) and their impact on associated parasitoid and inquiline species. Oikos. 1999;84:293–301. doi:10.2307/3546724 [Google Scholar]
- Schönrogge K, Walker P, Crawley M.J. Parasitoid and inquiline attack in the galls of four alien, cynipid gall wasps: host switches and the effect on parasitoid sex ratios. Ecol. Entomol. 2000;25:208–219. doi:10.1046/j.1365-2311.2000.00244.x [Google Scholar]
- Scott, A. C., Stephenson, J. & Collinson, M. E. 1994 The fossil record of leaves with galls. In Plant galls: organisms, interactions, populations (ed. M. A. J. Williams). Systematics Association Publication, no. 49, pp. 447–470. Oxford, UK: Clarendon Press.
- Shorthouse J.D. Modification of galls of Diplolepis polita by the inquiline Periclistus pirata. Bulletin de la Société Botanique de France. 1980;127:79–84. [Google Scholar]
- Stone G.N, Cook J.M. The structure of cynipid oak galls: patterns in the evolution of an extended phenotype. Proc. R. Soc. B. 1998;265:979–988. doi:10.1098/rspb.1998.0387 [Google Scholar]
- Stone G.N, Schönrogge K. The adaptive significance of insect gall morphology. Trends Ecol. Evol. 2003;18:512–522. doi:10.1016/S0169-5347(03)00247-7 [Google Scholar]
- Stone G.N, Sunnucks P.J. The population genetics of an invasion through a patchy environment: the cynipid gallwasp Andricus quercuscalicis. Mol. Ecol. 1993;2:251–268. doi:10.1111/j.1365-294X.1993.tb00015.x [Google Scholar]
- Stone G.N, Schönrogge K, Crawley M.J, Fraser S. Geographic variation in the parasitoid community associated with an invading gallwasp, Andricus quercuscalicis (Hymenoptera: Cynipidae) Oecologia. 1995;104:207–217. doi: 10.1007/BF00328585. doi:10.1007/BF00328585 [DOI] [PubMed] [Google Scholar]
- Stone G.N, Schönrogge K, Atkinson R.J, Bellido D, Pujade-Villar J. The population biology of oak gallwasps (Hymenoptera: Cynipidae) Annu. Rev. Entomol. 2002;47:633–668. doi: 10.1146/annurev.ento.47.091201.145247. doi:10.1146/annurev.ento.47.091201.145247 [DOI] [PubMed] [Google Scholar]
- Stone G.N, et al. The phylogeographic clade trade: tracing the impact of human-mediated dispersal on the colonisation of northern Europe by the oak gallwasp Andricus kollari. Mol. Ecol. 2007;16:2768–2781. doi: 10.1111/j.1365-294X.2007.03348.x. doi:10.1111/j.1365-294X.2007.03348.x [DOI] [PubMed] [Google Scholar]
- Stone G.N, et al. Evidence for widespread cryptic sexual generations in apparently purely asexual Andricus gallwasps. Mol. Ecol. 2008;17:652–665. doi: 10.1111/j.1365-294X.2007.03573.x. doi:10.1111/j.1365-294X.2007.03573.x [DOI] [PubMed] [Google Scholar]
- Svenning J.-C, Skov F. Limited filling of the potential range in European tree species. Ecol. Lett. 2004;7:565–573. doi:10.1111/j.1461-0248.2004.00614.x [Google Scholar]
- van der Ham R.W.J.M, Kuijper W.J, Kortselius M.J.H, van der Burgh J, Stone G.N, Brewer J.G. Plant remains from the Kreftenheye Formation (Eemian) at Raalte, The Netherlands. Veg. Hist. Archaeobotany. 2008;17:127–144. [Google Scholar]
- Waggoner B.M. Fossil oak leaf galls from the stinking water paleoflora of Oregon (early Miocene) PaleoBios. 1999;19:8–14. [Google Scholar]
- Walker P, Leather S.R, Crawley M.J. Differential rates of invasion in three related alien oak gall wasps (Cynipidae: Hymenoptera) Divers. Distrib. 2002;8:335–349. doi:10.1046/j.1472-4642.2002.00159.x [Google Scholar]
- Washburn J.O, Cornell H.V. Parasitoids, patches and phenology—their possible role in the local extinction of a cynipid gall wasp population. Ecology. 1981;62:1597–1607. doi:10.2307/1941515 [Google Scholar]
- Wiebes-Rijks A.A, Shorthouse J.D. Ecological relationships of insects inhabiting cynipid galls. In: Shorthouse J.D, Rohfritsch O, editors. Biology of insect-induced galls. Oxford University Press; New York, NY: 1992. pp. 238–257. [Google Scholar]
- Wilf P, Labandeira C.C, Johnson K.R, Coley P.D, Cutter A.D. Insect herbivory, plant defense, and early Cenozoic climate change. Proc. Natl Acad. Sci. USA. 2001;98:6221–6226. doi: 10.1073/pnas.111069498. doi:10.1073/pnas.111069498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilf P, Labandeira C.C, Johnson K.R, Ellis B. Decoupled plant and insect diversity after the end-Cretaceous extinction. Science. 2006;313:1112–1115. doi: 10.1126/science.1129569. doi:10.1126/science.1129569 [DOI] [PubMed] [Google Scholar]