Abstract
Increased levels of unfolded proteins in the endoplasmic reticulum (ER) of all eukaryotes trigger the unfolded protein response (UPR). Lower eukaryotes solely use an ancient UPR mechanism, whereby they up-regulate ER-resident chaperones and other enzymatic activities to augment protein folding and enhance degradation of misfolded proteins. Metazoans have evolved an additional mechanism through which they attenuate translation of secretory pathway proteins by activating the ER protein kinase PERK. In mammalian professional secretory cells such as insulin-producing pancreatic β-cells, PERK is highly abundant and crucial for proper functioning of the secretory pathway. Through a modeling approach, we propose explanations for why a translation attenuation (TA) mechanism may be critical for β-cells, but is less important in nonsecretory cells and unnecessary in lower eukaryotes such as yeast. We compared the performance of a model UPR, both with and without a TA mechanism, by monitoring 2 variables: (i) the maximal increase in ER unfolded proteins during a response, and (ii) the accumulation of chaperones between 2 consecutive pulses of stress. We found that a TA mechanism is important for minimizing these 2 variables when the ER is repeatedly subjected to transient unfolded protein stresses and when it sustains a large flux of secretory pathway proteins which are both conditions encountered physiologically by pancreatic β-cells. Low expression of PERK in nonsecretory cells, and its absence in yeast, can be rationalized by lower trafficking of secretory proteins through their ERs.
Keywords: pancreatic beta cells, modeling, negative feedback loops, stress response
The endoplasmic reticulum (ER) is an organelle where secretory and transmembrane proteins are folded, modified, and assembled into multiprotein complexes. The ER is of crucial importance to the functioning of “professional” secretory cells—for example, ≈200–1,000 immunoglobulins are synthesized per second in the ER of plasma cells, a secretory load approximately equal to their own weight in proteins per day (1). Other cell types that make extensive use of the ER are insulin-producing pancreatic β-cells, pancreatic exocrine cells that secrete digestive enzymes at a rate of 2 million per minute (2), and secretory cells of the skeletal system: chondrocytes and osteoblasts. Proteins in the ER are folded with the help of chaperones. A sudden increase in unfolded proteins, a condition called ER stress, activates the unfolded protein response (UPR), a molecular network that evolved to keep the concentration of ER unfolded proteins low. All eukaryotes share a conserved UPR strategy through which they mount a response to increasing levels of unfolded proteins: they up-regulate chaperones and other ER-resident enzymatic activities to augment ER protein folding, as well as to increase the functional capacity of proteolytic systems to identify, remove, and degrade irreversibly misfolded ER proteins (3). These activities are up-regulated through a conserved pathway in both yeast and mammals.
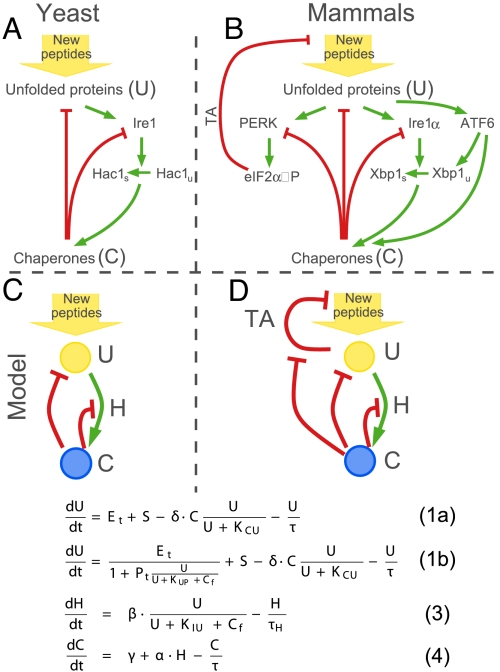
In yeast, an increase in unfolded proteins (U), activates an ER stress sensor, Ire1 (3), which in turn splices Hac1 mRNA (see Fig. 1A). Spliced Hac1 mRNA, Hac1s, is translated to produce the potent transcription factor Hac1, which activates transcription of chaperones, oxidoreductases, glycosylating enzymes, and ER degradation components—these activities, in their aggregate, remodel the entire secretory pathway, thereby increasing protein-folding capacity. For brevity, these activities will henceforth be referred to simply as chaperones (C). Chaperones (C), reduce levels of unfolded proteins (U), and inhibit the activity of Ire1, thereby restoring homeostasis (red arrows to unfolded protein (U) and Ire1 in Fig. 1A). In mammalian cells, up-regulation of chaperones is mainly controlled by 2 pathways: Ire1α, homologous to yeast Ire1, fulfills this function through splicing Xbp1 mRNA; and another ER stress transducer, the ATF6 transcription factor, is activated by ER-unfolded proteins, producing downstream targets whose functions overlap extensively with those of Ire1α (3, 4) (see Fig. 1B). ATF6 activation also increases the pool of Xbp1 mRNA for subsequent splicing by Ire1α.
Fig. 1.
Minimal model of the UPR. The UPR networks in yeast (A) and mammalian cells (B). Green arrows show positive regulation, red arrows show negative regulation. Simplified version of the UPR without TA mechanism (C) and with TA mechanism (D) and corresponding mathematical models. The only difference in each of the models is in the equations describing the change of the unfolded proteins (U); the up-regulation of Hac1 (H) and chaperones (C) are identical in C and D and therefore are placed in the middle.
Metazoans have additionally evolved another unique mechanism to counter ER stress: they transiently attenuate translation and thus reduce the entry of nascent polypeptides into the ER (3) (Fig. 1B). This translation attenuation pathway, henceforth abbreviated TA, is mediated through the ER kinase PERK: increased levels of unfolded proteins activate PERK, which in turn phosphorylates the translation initiation factor eIF2α. The increase in levels of phosphorylated eIF2α, eIF2α-P, leads to partial translation attenuation, because eIF2α-P cannot form the ribosomal preinitiation complex necessary for translation initiation. PERK, a highly expressed protein in secretory cells, plays an important role in the physiology and survival of secretory cells: In mice lacking both copies of the PERK gene, the major secretory cells of the pancreas and the skeletal system display a rough ER distended with protein aggregates, and undergo accelerated cell death. This results in skeletal defects at birth and progressive diabetes mellitus neonatally (5, 6). A growing body of evidence indicates that optimal function and survival of diverse secretory cells depends on translational regulation via this PERK/eIF2α branch of the UPR (7).
These studies raise several interesting questions: What unique challenges do specialized secretory cells of higher eukaryotes face that necessitate the additional TA component in the conserved UPR core? Can differences in cell functions and their environments explain why the TA mechanism is important for one cell type and not another? In particular, what makes the TA mechanism so critical for proper functioning of β-cells? Conversely, why do nonsecretory cells rely less on a TA mechanism, and why did lower eukaryotes not need to evolve such a mechanism? We address these and other conceptually important questions with the help of a mathematical model of the UPR.
Minimal Model of the UPR
To capture the key points relevant for addressing our questions, we reduced the UPR networks in Fig. 1 A and B to their essential components, which are represented conceptually in Fig. 1 C and D. In our model U is the free amount of unfolded proteins, C is the total amount of chaperones, and Cf is the amount of free chaperones. The UPR's outputs initially restore homeostasis if challenges to homeostasis are manageable. These remediable stresses to ER function are the situations we are considering in our minimal model. We are specifically excluding situations of unmitigated stress that overwhelm homeostatic outputs, and switch cells into the alternate fate of apoptosis, i.e., this is a minimal model of how homeostasis might be achieved in the UPR and its main assumptions are:
Up-regulation of chaperones, green arrow from U to C in Fig. 1 C and D, occurs on a slower timescale than the timescale of the TA mechanism, red line from U to New peptides in Fig. 1D. Indeed, it was shown experimentally that there is a time separation in the activities of each of the 3 pathways in mammalian cells: TA is activated first, and takes effect soon after induction of stress as eIF2α becomes phosphorylated within 5 min (unpublished observations). In contrast, transcriptional effects proceeding from ATF6 and Ire1α become maximal many hours after the stress induction (4). Although PERK signaling also has downstream transcriptional targets, we only consider the immediate translational attenuation effects of PERK. In our model we assume that chaperone production continues even when the TA mechanism is active. This effect has been reported experimentally and may be due to a combination of continued translation of some messages even during global translation cessation (i.e., translational privilege) (8) and/or increased transcription of mRNAs encoding chaperones. The main outcome of the model does not change when translation of C is attenuated by the TA mechanism (see supporting information (SI) Appendix, Fig. S2). We approximate Ire1/Ire1α and ATF6 activation, consequent production of the active downstream transcription factors, and target (chaperone) gene up-regulation by a 2-step process: (i) Up-regulation of the transcription factor Hac1/Xbp1 (H) and (ii) consecutive up-regulation of chaperones (C). For simplicity, these 2 steps are shown by a single green link from U to C in Fig. 1 C and D. We further reduce the Hac1 mRNA splicing steps and translation of the spliced mRNA, Hac1s into 1 ordinary differential equation (ODE), assuming that the concentration of Hac1 protein at any time point will be proportional to the amount of its spliced mRNA, Hac1s. This assumption is possible because of the short half-life of the Hac1 proteins [1.5 min (9)], that is much shorter than the half-life of the Hac1 mRNA, the estimate of which varies from τH ≈ 13 min (10) to τH ≈ 30 min (11).
We assume that chaperones inhibit the activity of PERK and Ire1 in a similar way. It was shown that chaperones bind PERK and Ire1/Ire1α to prevent formation of dimers, a step necessary for activation of both Ire1/Ire1α and PERK (3).
This simplified system can be described by 3 ODEs (see Fig. 1 C and D Bottoms). Eqs. 2 and 3 are identical for both systems, but Eq. 1 has additional terms when the TA mechanism is present.
The rate of change in free unfolded proteins, dU/dt, depends on 4 terms: (i) the translation rate of new polypeptides into the ER, Et; (ii) stress, S, which can be provoked by physiological challenges, or induced artificially in the lab by using chemical toxins; (iii) the effect of chaperones, C, on sequestering, folding/refolding or degrading unfolded proteins; and (iv) dilution of free unfolded proteins through cell division. Terms ii through iv are identical, and the only difference between the 2 systems is in how the translation rate is regulated in response to the increase in free unfolded proteins. The translation rate is proportional to the amount of unphosphorylated eIF2α, which, in the presence of the TA mechanism [e(+TA)], is inversely proportional to the amount of activated PERK, Pact, e(+TA) = . In our model PERK is activated through binding of unfolded proteins and is deactivated through binding of free chaperones, Cf (i.e., chaperones that are not in complex with unfolded proteins), Pact = Pt . See Methods and SI Appendix for a detailed derivation of all of the equations in the model.
Results
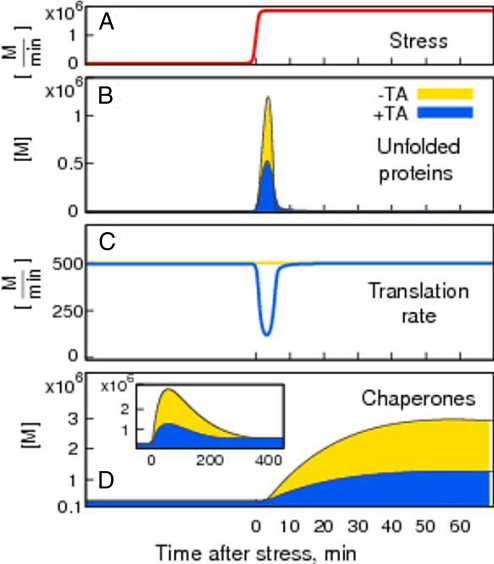
In Fig. 2 we show the outcome of the ODE integration >400 min after stress, S, is induced at time t = 0. With and without the TA mechanism, an acute stress proceeding from a millionfold increase in the production rate of unfolded proteins (measured in molecules per minute, M/min), leads to a sudden increase in the number of molecules of unfolded proteins (U) (Fig. 2B). In our model, this would be a remediable stress that could result from exposure of cells to DTT (which causes chemical reduction of ER proteins) at amounts to which cells could adapt through the UPR. After stress initiation (Fig. 2A), U reaches its maximal level, then decreases back to its initial, low, steady-state level (Fig. 2B), as chaperones become up-regulated (Fig. 2D). As unfolded proteins return to their low steady-state level, Ire1 signaling is shut off (see SI Appendix, Eq. S6); however, the concentration of chaperones continues increasing because spliced Hac1(Xbp1) mRNA and Hac1(Xbp1) protein is still present in the cell. This delay between the shutoff in Ire1 signaling and the decrease in chaperone transcription depends on the slowest step in Hac1(Xbp1) processing. Because Hac1 protein is very unstable, with a half-life of ≈1.5 min, the timescale of the slowest step in yeast will depend on the stability of spliced mRNA, which we have assumed to be the same as that for unspliced mRNA, with the half-life τH = 30 min.
Fig. 2.
TA decreases unfolded proteins and chaperones. The response to stress (A) without TA, and thus with constant translation rate, is shown in yellow, and the response with TA is shown in blue. The translation rate (C) drops down in response to the increase in unfolded proteins (B), and starts recovering only after the amount of chaperones (D) has increased enough to accommodate newly translated proteins. In both cases the initial increase in unfolded proteins, U, is taken care of by increased level of chaperones, C.
In the absence of a TA, the translation rate remains constant throughout the response (Fig. 2C, yellow line); in the presence of a TA (Fig. 2C, blue line) the translation rate is inversely proportional to U: as U increases, the translation rate drops during the first 4 min to its minimal level, and then gradually recovers to the prestressed rate of translation, as the effect of chaperone up-regulation on containing unfolded proteins becomes manifest.
An increasing number of experimental studies show that the accumulation of misfolded proteins results in protein aggregates that cause cell toxicity and eventual cell death (12, 13). Chaperones and proteases maintain the concentration of unfolded proteins at low levels. However, excessive accumulation of chaperones can be toxic when out of proportion to the amount of unfolded proteins (14, 15). To capture these 2 physiological aspects in our model we compare the quality of the response in the presence and absence of the TA mechanism by monitoring (i) how well it can minimize the levels of U and (ii) how effective the response is in preventing excessive accumulation of chaperones. We first quantify the performance of the response by measuring the maximum amount of unfolded proteins, Umax, during the adaptation to ER stress (see Fig. 2B). The higher the quality of the response, the smaller the maximal amount of unfolded proteins, Umax. Translation attenuation in response to increased levels of U (Fig. 2C) provides a higher quality of the response by reducing the total amount of U during adaptation to stress, Umax(+TA) < Umax(−TA); compare yellow (no TA) with blue (with TA) curves in Fig. 2B. Observe that the TA not only reduces the amount of U, but it also decreases the amount of chaperones needed to handle the stress (Fig. 2D).
The TA mechanism thereby allows for a tight regulation of the response. It minimizes the amount of unfolded proteins by allowing for the production of only as much proteins as can be handled by existing chaperones, until de novo synthesis of chaperones further increases folding capacity. This is demonstrated in the changes of translation rate and chaperone production in Fig. 2 C and D. A response where translation is constant (yellow) results in higher accumulation of U, and requires more chaperones than when translation is attenuated through the TA mechanism (blue).
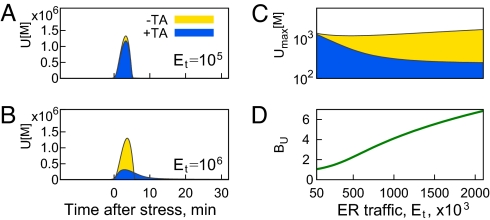
TA Is Needed When the Traffic Through ER Is Large.
High traffic of newly synthesized proteins into the ER is characteristic of mammalian professional secretory cells, which therefore have a greater ER protein-folding burden than other cell types not specialized for secretion. For example, the synthesis rates for hepatocytes is estimated to be 13 million proteins per minute, and ≈2.6 million per minute in pancreatic exocrine cells (2). We have investigated how the quality of the UPR varies with ER traffic, Et, defined as the amount of new peptides translated into the ER per unit time. We find that the presence of TA makes little difference in UPR quality when the traffic is low (Fig. 3A). However, the difference between TA-aided UPR, Umax(+TA), and UPR without TA, Umax(−TA), becomes dramatic as traffic increases (Fig. 3B). The reason for this increase is that with addition of the TA mechanism Umax is reduced as the traffic increases: Umax(+TA, Et = 1,000·103) < Umax(+TA, Et = 100·103).
Fig. 3.
Benefit of TA increases with increased traffic into the ER. The amount of unfolded proteins is comparable in both +TA (blue) and −TA (yellow) cases in A, when the traffic is low (Et = 100·103), but is significantly different in B, where traffic is high (Et = 1,000·103). (C) Maximal amount of unfolded proteins during the response, Umax, increases slightly with ER traffic in the absence of TA and is decreasing in the presence of translation attenuation. The ratio BU = quantifies the increasing benefit of translation attenuation.
In Fig. 3C we show how the maximum concentration of unfolded proteins depends on traffic through the ER. As ER traffic increases, the quality of UPR without a TA mechanism does not change much, whereas the quality of a TA-aided UPR improves [Umax(+TA) goes down] because of its increased ability to buffer the stress by stopping translation. We can further quantify the benefit of the TA mechanism through the ratio BU = , which shows how the basic UPR of lower eukaryotes performs compared with a TA-aided UPR. As seen in Fig. 3D, translation attenuation is more beneficial when the ER traffic is high.
Higher ER traffic necessitates large concentration of ER chaperones to deal with the flux of incoming polypeptides. In the presence of the TA mechanism this large pool of available chaperones can be quickly redirected to counter the unfolded proteins generated at the time of stress. This implies that a cell accustomed to high ER traffic will be buffered against acute unfolded proteins increase, but only when a TA mechanism is available.
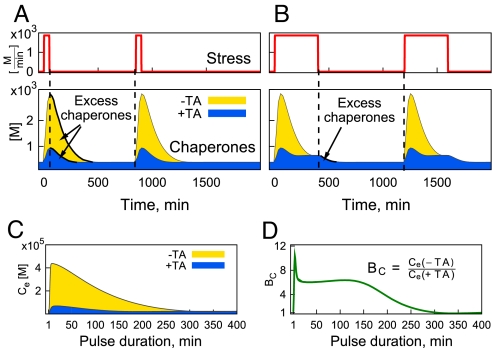
TA Is Beneficial Under Conditions of Pulsatile Stress.
Physiological functioning of insulin-secreting pancreatic β-cells occurs in response to highly variable patterns of blood glucose level. Rapidly changing glucose levels engender correspondingly rapid changes in insulin production and secretion: Glucose directly up-regulates both transcription and translation of preproinsulin, which has to be processed and folded in the ER (16). This creates a highly fluctuating ER load over time. We investigated the role of the translation attenuation mechanism under conditions of transient, recurrent stress. Fig. 4A shows the dynamics of the response to short pulses of stress with and without translation attenuation. TA minimizes the amount of chaperones in the interval between the stresses, and is particularly beneficial when the pulses are short, as shown in Fig. 4A Bottom. However, as the duration of each pulse increases, the contribution of the TA mechanism in minimizing the excess accumulation of chaperones becomes reduced (Fig. 4B). This can be easily understood from the temporal separation of the 2 mechanisms: TA is a fast-acting response; it only requires 2 phosphorylation steps (first PERK autophosphorylation, then eIF2α phosphorylation by activated PERK) to stop protein translation. In contrast, the up-regulation of chaperones is a slower synthetic process, requiring both transcription and translation steps. Thus, if the stress is transient and very brief (Fig. 4A), the TA pathway alone is enough to handle the stress. This leads to fewer excess chaperones after the stress goes back to zero. However, if the duration of the stress approaches the timescale it takes for chaperones to reach their new steady-state level (≈400min in Fig. 4B), the TA mechanism does not reduce excess chaperones.
Fig. 4.
The role of TA in reducing the amount of chaperones diminishes as the duration of the stress pulse increases. A and B are the chaperone profiles in response to a pulsatile stress of various durations: (A) short pulse, t = 50min, and (B) long pulse, t = 400min. C shows the excess chaperones defined as the total amount of chaperones between the 2 consecutive stress pulses and D shows the benefit of TA, BC, calculated as the ratio of the 2 curves in C), BC = .
To quantify this property of the TA to reduce excessive amounts of chaperones between stresses, we define the benefit, BC = , where Ce(+TA) and Ce(−TA) are the amounts of chaperones between 2 consequent stress pulses, with and without a TA. Fig. 4 C and D summarizes these findings; Fig. 4C shows the amount of chaperones between stresses versus the duration of the stress pulse, and Fig. 4D demonstrates the beneficial role of the TA for short-pulse durations. The peak in BC at ≈4 min in Fig. 4D corresponds to the time at which the amount of free unfolded proteins reaches its maximal level (see Fig. 2B). The initial increase in BC during the first 4 min is due to the different rates of chaperone up-regulation: the amount of U increases, causing rapid increase in C(−TA), and a much slower increase in C(+TA), where the stress is compensated by an instantly acting translation attenuation pathway. A decrease in BC after the first 4 min is because C(+TA) continues increasing at about the same rate, whereas C(−TA) slows down. A further plateau in BC for pulses that last for ≈40–120 min corresponds to the same rate of chaperone decay, that is, chaperones have already reached their maximal levels and are decaying back to the new steady state at the time when stress goes back to zero after 40–120 min. The final decay in BC captures the difference in levels of chaperones when the stress is removed after ≈120 min. Although C(−TA) is still decaying to its steady-state level, C(+TA) has already reached steady state at the time when stress is removed.
In our study we only considered the stress pulses separated by a long period, long enough for the chaperones to reset to the initial (no-stress) steady-state level. If this separation between pulses is much shorter than chaperone half-life, the excess of chaperones will be beneficial, because it will reduce the Umax during the response to the following pulse (data not shown). Thus, the excess of chaperones can be both protective or toxic, depending on the context of the environment: this observation is indirectly supported by chaperone overexpression studies (14, 15, 17, 18).
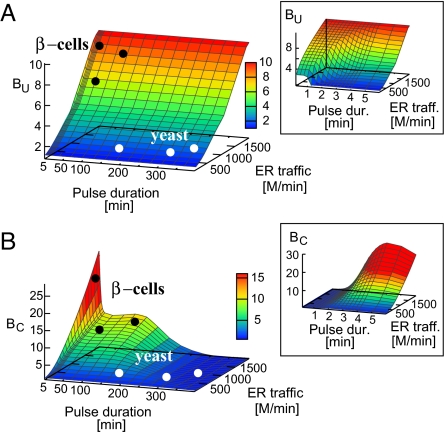
Finally we summarize our results on the beneficial role of translation attenuation in the unfolded protein response in Fig. 5 where we show the importance of the TA mechanism in minimizing the amount of unfolded proteins (Fig. 5A), and in reducing the excess of chaperones, between stress pulses (Fig. 5B), for a range of different values of ER traffic and pulse durations. Let us first examine the role of TA mechanisms in reducing the amount of unfolded proteins. From Fig. 5A it is evident that TA mechanism is highly effective in reducing the free unfolded proteins under the conditions of high ER traffic (Et = 2,000·103, BU = 10), independent of the duration of the stress pulse. However, the presence of a TA does make a difference under the conditions of lower ER traffic, e.g., Et = 1,000·103 when the stress comes in short pulses (compare a pulse duration of 1 min with that of 10 min in Fig. 5A). The duration of these short pulses is determined by the time at which U reaches its maximum, Umax.
Fig. 5.
The benefit of translation attenuation pathway quantified in terms of reduction in unfolded proteins during stress response, BU = (A) and in terms of TA's ability to minimize the amount of chaperones between consecutive pulses of stress, BC = , as a function of ER traffic and pulse duration (B). The black and white circles illustrate the believed typical conditions for β-cells and nonsecretory cells per yeast correspondingly. (Insets) represent the first 5 minutes.
Fig. 5B shows that the TA is effective in reducing the excess of chaperones under the conditions of high ER traffic and short (the peak in Fig. 5B) and intermediate (the plateau in Fig. 5B) pulses of stress. The timescale of the short pulses is determined by the delay between the stress induction and the time when chaperones take an initial effect (i.e., ≈5 min in our case). The timescale of the intermediate pulses is set by the chaperone half-life. Both criteria show that TA is of high importance under conditions of transient recurrent stress and high ER traffic—conditions imposed on mammalian β-cells where indeed PERK and the whole TA pathway has been shown to be central for proper functioning (5, 6).
Discussion
Failure of the ER-folding machinery to fold and assemble client proteins of the secretory pathway as they transit through the ER results in protein aggregation and eventual cell death. The TA branch of the UPR is particularly important for insulin-producing pancreatic β-cells, as evidenced by diabetes syndromes in transgenic mice models lacking the UPR components PERK, eIF2α (5), and PERK repressor, P58IPK (19). A high expression level of PERK in secretory cells (20), as opposed to low levels in virtually all other cell types, suggests that PERK may have an important regulatory role in secretory cells under normal physiological conditions.
We have built a mathematical model to assess the role of PERK and the translation attenuation component of the UPR. We have focused on the early homeostatic events in the UPR, i.e., the part of the network that allows cells to respond to containable stresses, without undergoing programmed cell death. To this end, we monitored and compared the quality of the UPR with, and without, the TA mechanism under a range of stressful conditions physiologically encountered by pancreatic β-cells—high rates of basal ER polypeptide production (ER traffic) punctuated further by transient and recurrent increases in protein production (e.g., insulin)—to those more typical for nonsecretory cells and yeast where ER traffic is low. Additional factors may explain the absence of a TA in yeast: (i) Activation of chaperones is much faster in yeast, possibly because of the “ready-to-go” pool of mHac1u, unspliced Hac1 messenger RNA with preassembled ribosomes, which can be rapidly translated on Ire1 splicing (3, 10). (ii) A TA may result in a slower growth rate, which could impose negative selective pressure on these fast-growing unicellular organisms. In populations of unicellular organisms, some fraction of the cells may be killed to achieve a faster growth rate of the population as a whole. This particular consideration is irrelevant for pancreatic β-cells, because their doubling in the adult is very slow, and survival of every functional cell is highly advantageous. (iii) Fast doubling rate of yeast and overall shorter chaperone half-life will result in lower amounts of excess chaperones, thus eliminating the necessity for the tight control afforded by PERK.
The primary goal of our model is not to attempt a direct comparison of the yeast and mammalian UPR, but rather to contrast 2 different stress response strategies—one with a single negative feedback loop, and the other with the combination of fast and slow negative feedback loops. In this scheme, yeast—because of the lack of PERK—can be thought of as an extreme example of mammalian nonsecretory cells, where levels of PERK are low.
Overall, we found that addition of the TA mechanism to the UPR results in a tighter response, with fewer unfolded proteins during stress adaptation and fewer excess chaperones between consecutive stresses. Essentially, the TA mechanism allows a tighter adjustment of the translation of new peptides to the amount of chaperones present in the ER at any moment of time. This comes as a result of the TA response being fast compared with chaperone up-regulation.
We found that the TA mechanism reduces the amount of free unfolded proteins through its ability to rapidly redirect chaperones from newly synthesized peptides (a typical substrate in unstressed ER) to unfolded proteins that appear as the result of any particular ER stress. The TA mechanism reaches its maximal effect when ER traffic is high, i.e., under the conditions specific to professional secretory cells, and the effect of TA is minimal when ER traffic is low, a condition more characteristic of nonsecretory cells. This result is particularly interesting in view of recent findings that PERK/eIF2α targeting strategies, such as preemptive eIF2α phosphorylation or small-molecule inhibition of eIF2α dephosphorylation, seem to protect against only a few chemically induced ER stresses, and the effects are cell-type specific (21, 22). Our model predicts that activation of the TA mechanism, by targeting the PERK/eIF2α pathway, for example, will be particularly effective in minimizing the concentration of unfolded protein under the following conditions: (i) in professional secretory cells where ER traffic, and thus the buffering capacity of TA, is high (see Figs. 3 and 5 A and B), and (ii) during acute stresses, where the fast timescale of the TA is of benefit. If the stress is induced gradually, slower than the time it takes for chaperones to take an effect, the addition of a TA component becomes redundant (data not shown).
Another aspect of why professional secretory cells may benefit from the presence of a TA mechanism is its ability to minimize the amount of chaperones needed to deal with transient stresses. Chaperones are generally long-lived proteins, so once they become up-regulated in response to transient stress, they will be present in the ER long after the stress is gone. There are numerous reports suggesting that an excess of chaperones in the absence of folding stress imposes a burden that may even cause cell toxicity (15, 17). Our model predicts that the presence of a TA mechanism is especially important for minimizing the excess chaperones when the duration of the transient stress is approximately the same or smaller than the time it takes for chaperones to be induced.
Our results and conclusions should be general to other signaling networks—not necessarily limited to the UPR—wherein 2 negative feedback loops act on different timescales. Indeed the basic wiring of translational attenuation already exists in yeast in the GCN pathway, which becomes activated under amino acid and glucose starvation leading to transient Gcn2 kinase activation (3, 23). Similar to the UPR, in the GCN pathway, a fast TA mechanism is combined with a slower transcriptional response upregulating amino acid synthesis.
In conclusion, in this present work we have taken a step toward building a framework for the quantitative description of the mammalian unfolded protein response to more rigorously understand the role of ER stress and the UPR in protein-folding diseases.
Methods
Let us first consider common terms in Eq. 1. The stress, S, is a sudden perturbation to ER homeostasis, to which cells can adapt through the unfolded protein response. A physiologically relevant example might be a sudden increase in insulin translation caused by increased level of glucose in pancreatic β-cells, or ER stress induced experimentally by applying chemical reductant DTT in Saccharomyces cerevisiae. Chaperones and proteases (C) fold newly synthesized proteins, sequester free unfolded proteins, refold or degrade misfolded proteins (shown by red link from C to U in Fig. 1 C and D). We approximate this concerted contribution of chaperone function in decreasing free unfolded proteins by a Michaelis–Menten process where chaperones (C) act as enzymes, δ·C (here KU is the Michaelis–Menten constant and δ is the parameter defining the efficiency of the process). Another way to decrease free unfolded proteins is by dilution due to cell division, U/τ, where τ is the cell doubling time. We do not include ER expansion in response to ER stress, which is an additional mechanism to dilute the unfolded proteins in both yeast and mammalian cells.
Now we consider the key difference between the 2 systems in our model: the increase in free unfolded proteins determined by the translation rate of the new polypeptides into the ER (represented by the yellow arrow to U in Fig. 1 C and D). The translation rate is determined by the amount of unphosphorylated eIF2α, e. We assume that without TA (−TA), the unphosphorylated eIF2α, e(−TA), is maintained at a constant level at all times, i.e., e(−TA) = Et.
With TA (+TA) we set the level of unphosphorylated eIF2α to be the same as in the (−TA) case in the absence of free unfolded proteins, i.e., e(+TA) → Et as U → 0. However, with TA, the level of unphosphorylated eIF2α decreases as the amount of U increases. Assuming that activation of PERK by unfolded proteins is a fast process, much faster than the changes in the concentration of chaperones, we can derive the expression for the activated PERK to be
where Pt is proportional to the total amount of PERK and KUP is the dissociation constant for PERK to U binding(for detailed derivation, see SI Appendix). We assume U binds with the same strength to PERK as to Ire1. The term corresponds to the inhibition of PERK by chaperones, where Cf is the amount of free chaperones. Assuming that binding/unbinding events reach steady state much faster than all other processes, we can derive the expression for the amount of free chaperones to be
When there is no ER stress, U is at its minimal level (U/KUP ≪ 1) and, thus, the sequestering of chaperones by unfolded proteins is minimal, resulting in maximal levels of Cf that bind and inhibit PERK, the amount of activated PERK,
Under ER stress, large amounts of U (U/KUP ≫ 1) sequester chaperones, so that Cf → 1. Thus, under ER stress PERK is activated to its maximal level Pact ∝ Pt. From the law of mass conservation for eIF2α, the amount of unphosphorylated eIF2α is inversely proportional to the concentration of activated PERK:
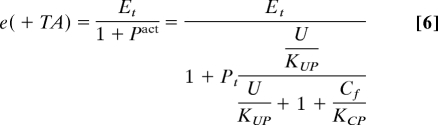 |
We assume a nonzero basal level of chaperone transcription, γ. The increase in chaperone production in response to ER stress is proportional to the amount of Hac1/Xbp1 transcription factor, H with coefficient of proportionality α (see Eq. 2 in Fig. 1). Chaperones are generally long lived; for example, Bip half-life in yeast was estimated to be 2 h (24), which is similar to the yeast doubling time. This is represented by the dilution term, C/τ, with τ = 120 min.
The amount of Hac1/Xbp1 transcription factor H is proportional to the amount of the spliced mRNA Hac1s. Splicing is controlled by the amount of active Ire1, which, similarly to PERK, is derived to be
where It is the total amount of Ire1. With the half-life of Hac1 mRNA, τH ≈ 30 min (10, 11), the change in Hac1 protein is described by
where β is the parameter that depends on the amount of total Ire1, splicing efficiency, and Hac1 translation rate. The details about the choice of the rest of the parameters as well as step-by-step derivations of all equations are presented in SI Appendix.
Supplementary Material
Acknowledgments.
We thank Morten Kloster, Mariana Babor, and Philip Merksamer for useful discussions and critical reading of the manuscript. This work was supported in part by Alexander M. and June 1. Maison Foundation Award within the Sandler Program, National Science Foundation Grant DMR-0804183, and Chinese Ministry of Science and Technology Award 2009CB918504.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803476105/DCSupplemental.
References
- 1.Federovitch CM, Ron D, Hampton RY. The dynamic ER: Experimental approaches and current questions. Curr Opin Cell Biol. 2005;17(4):409–414. doi: 10.1016/j.ceb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Mullins C, editor. The Biogenesis of Cellular Organelles. New York: Kluwer Academic/Plenum Publishers; 2005. [Google Scholar]
- 3.Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- 4.Rutkowski DT, Kaufman RJ. A trip to the ER: Coping with stress. Trends Cell Biol. 2004;14(1):20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Harding HP, et al. Diabetes mellitus and exocrine pancreatic dysfunction in Perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7(6):1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhang PC, et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22(11):3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gass JN, et al. Stressed-out B cells? Plasma-cell differentiation and the unfolded protein response. Trends Immunol. 2004;25(1):17–24. doi: 10.1016/j.it.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Gulow K, Bienert D, Haas IG. BiP is feed-back regulated by control of protein translation efficiency. J Cell Sci. 2002;115(11):2443–2452. doi: 10.1242/jcs.115.11.2443. [DOI] [PubMed] [Google Scholar]
- 9.Pal B, et al. SCFCdc4-mediated degradation of the Hac1p transcription factor regulates the unfolded protein response in Saccharomyces cerevisiae. Mol Biol Cell. 2007;18(2):426–440. doi: 10.1091/mbc.E06-04-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axelsen JB, Sneppen K. Quantifying the benefits of translation regulation in the unfolded protein response. Phys Biol. 2004;1(3):159–165. doi: 10.1088/1478-3967/1/3/003. [DOI] [PubMed] [Google Scholar]
- 11.Ruegsegger U, Leber JH, Walter P. Block of HAC1 mRNA translation by long-range base pairing is released by cytoplasmic splicing upon induction of the unfolded protein response. Cell. 2001;107(1):103–114. doi: 10.1016/s0092-8674(01)00505-0. [DOI] [PubMed] [Google Scholar]
- 12.Xu SH. Aggregation drives “misfolding” in protein amyloid fiber formation. Amyloid. 2007;14(2):119–131. doi: 10.1080/13506120701260059. [DOI] [PubMed] [Google Scholar]
- 13.Muchowskil PJ. Protein misfolding, amyloid formation, and neurodegeneration: A critical role for molecular chaperones? Neuron. 2002;35(1):9–12. doi: 10.1016/s0896-6273(02)00761-4. [DOI] [PubMed] [Google Scholar]
- 14.Landsverk ML, et al. The UNC-45 chaperone mediates sarcomere assembly through myosin degradation in Caenorhabditis elegans. J Cell Biol. 2007;177(2):205–210. doi: 10.1083/jcb.200607084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feder JH, et al. The consequences of expressing hsp70 in drosophila cells at normal temperatures. Genes Dev. 1992;6(8):1402–1413. doi: 10.1101/gad.6.8.1402. [DOI] [PubMed] [Google Scholar]
- 16.Docherty K, Clark AR. Nutrient regulation of insulin gene-expression. FASEB J. 1994;8(1):20–27. doi: 10.1096/fasebj.8.1.8299887. [DOI] [PubMed] [Google Scholar]
- 17.Krebs RA, Feder ME. Deleterious consequences of Hsp7O overexpression in Drosophila melanogaster larvae. Cell Stress Chaperones. 1997;2(1):60–71. doi: 10.1379/1466-1268(1997)002<0060:dcohoi>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman JS, Heckathorn SA, Hallberg RL. Heat-shock proteins and thermotolerance: Linking molecular and ecological perspectives. Trends Ecol Evol. 1995;10(8):305–306. doi: 10.1016/s0169-5347(00)89112-0. [DOI] [PubMed] [Google Scholar]
- 19.Ladiges WC, et al. Pancreatic beta-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes. 2005;54(4):1074–1081. doi: 10.2337/diabetes.54.4.1074. [DOI] [PubMed] [Google Scholar]
- 20.Shi Y, et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18(12):7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyce M, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307(5711):935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 22.Cnop M, et al. Selective inhibition of eukaryotic translation initiation factor 2 alpha dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic beta-cell dysfunction and apoptosis. J Biol Chem. 2007;282(6):3989–3997. doi: 10.1074/jbc.M607627200. [DOI] [PubMed] [Google Scholar]
- 23.Yang RJ, Wek SA, Wek RC. Glucose limitation induces GCN4 translation by activation of gcn2 protein kinase. Mol Cell Biol. 2000;20(8):2706–2717. doi: 10.1128/mcb.20.8.2706-2717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belle A, et al. Quantification of protein half-lives in the budding yeast proteome. Proc Natl Acad Sci USA. 2006;103(35):13004–13009. doi: 10.1073/pnas.0605420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.