Abstract
Under most conditions, resorbed bone is nearly precisely replaced in location and amount by new bone. Thus, it has long been recognized that bone loss through osteoclast-mediated bone resorption and bone replacement through osteoblast-mediated bone formation are tightly coupled processes. Abundant data conclusively demonstrate that osteoblasts direct osteoclast differentiation. Key questions remain, however, as to how osteoblasts are recruited to the resorption site and how the amount of bone produced is so precisely controlled. We hypothesized that osteoclasts play a crucial role in the promotion of bone formation. We found that osteoclast conditioned medium stimulates human mesenchymal stem (hMS) cell migration and differentiation toward the osteoblast lineage as measured by mineralized nodule formation in vitro. We identified candidate osteoclast-derived coupling factors using the Affymetrix microarray. We observed significant induction of sphingosine kinase 1 (SPHK1), which catalyzes the phosphorylation of sphingosine to form sphingosine 1-phosphate (S1P), in mature multinucleated osteoclasts as compared with preosteoclasts. S1P induces osteoblast precursor recruitment and promotes mature cell survival. Wnt10b and BMP6 also were significantly increased in mature osteoclasts, whereas sclerostin levels decreased during differentiation. Stimulation of hMS cell nodule formation by osteoclast conditioned media was attenuated by the Wnt antagonist Dkk1, a BMP6-neutralizing antibody, and by a S1P antagonist. BMP6 antibodies and the S1P antagonist, but not Dkk1, reduced osteoclast conditioned media-induced hMS chemokinesis. In summary, our findings indicate that osteoclasts may recruit osteoprogenitors to the site of bone remodeling through SIP and BMP6 and stimulate bone formation through increased activation of Wnt/BMP pathways.
Keywords: mineralization, osteoblast
Bone is a dynamic tissue that continuously remodels and can undergo regeneration throughout life. This continuous remodeling occurs through a dynamic process of breakdown by osteoclasts and rebuilding by osteoblasts. Bone mass in adults is maintained by a local balance between osteoclastic bone resorption and osteoblastic activities that are mediated via various factors such as hormones, growth factors, cytokines, and matrix proteins. Under most conditions, resorbed bone is nearly precisely replaced in location and amount by new bone. Thus, it has long been recognized that bone loss through osteoclast-mediated bone resorption and bone replacement through osteoblast-mediated bone formation are tightly coupled. We now have clear evidence of how osteoblasts direct osteoclast differentiation through RANK and RANKL as well as other pathways (1). Questions remain as to how osteoblasts are recruited to the site after the resorption phase and how the amount of bone produced is controlled. This has led to consideration of how osteoclasts and/or their activity could promote bone formation.
Mouse models and humans in whom osteoclastogenesis is perturbed have provided important insights into the role of bone resorption in coupling (reviewed in refs. 2 and 3). In mouse models where osteoclasts are present but are not able to resorb bone (c-Src or chloride-7 channel knockout mice), there is no defect in bone formation (4–6). Likewise, in humans with an inactivated chloride-7 channel gene, osteoclasts are present but there is inhibition of bone resorption with no reduction in bone formation (5, 7, 8). These data are in sharp contrast to mouse models that result in defective osteoclastogenesis, such as mice lacking c-Fos or M-CSF, which have no osteoclasts and also exhibit defective bone formation (9–11). Thus, it seems that the presence of osteoclasts, whether or not they are resorbing bone, is needed for normal bone formation and that the release of bone-bound factors may not be required to couple osteoclasts to the bone-formation phase of bone turnover.
Bone formation during skeletal development involves complex coordination between cells resident in the bone environment. Osteoblasts are mesenchymal cells derived from mesodermal and neural crest progenitors. Significant progress has been made over the past decade in our understanding of the molecular framework that controls osteogenic differentiation. A large number of factors have been implicated in regulating osteoblast differentiation, including the Wnt family. Wnts are secreted glycoproteins crucial for the development and homeostatic renewal of many tissues, including bone. Canonical Wnt signaling is central to the regulation of osteoblast development. Wnts stimulate several signaling pathways by binding a receptor complex consisting of LRP5/6 and 1 of 10 Frizzled molecules (12). The canonical Wnt signaling pathway involves stabilization of β-catenin and regulation of Lef/Tcf transcription factors. This pathway is active in all osteoblast lineage cells, including preosteoblasts, lining cells, and osteocytes (13). Wnt signaling is involved in osteoblast commitment from osteo/chondro-progenitors, promotion of osteoblast proliferation and differentiation, and enhancement of osteoblast and osteocyte survival (14). The involvement of the canonical Wnt pathway in bone cells was revealed in seminal studies showing that loss-of-function mutations in LRP5 decrease bone mass whereas gain-of-function mutations increase bone mass in both humans and mice (reviewed in ref. 14). The mouse models also revealed that Lrp5 regulates osteoblast number, through both increasing the proliferation of progenitors and enhancing survival of committed osteoblasts and osteocytes (15–17). Wnt10b stimulates canonical Wnt signaling to promote osteoblast differentiation while inhibiting adipogenesis (18). Moreover, transgenic mice expressing Wnt10b in the bone marrow microenvironment are resistant to bone loss after estrogen depletion (18). Wnt10b expression in osteoblasts using the osteocalcin promoter leads to higher bone mineral density, higher bone volume, and increased trabecular number (19). Wnt10b knockout mice have decreased trabecular bone and serum osteocalcin, confirming the critical role for Wnt10b in osteoblast differentiation (18). Sclerostin is secreted by osteocytes, apparently as a mechanism to reduce bone formation (20, 21). Sclerostin is the product of the SOST gene, which is mutated and down-regulated in patients with sclerosteosis and van Buchem's disease (22, 23). Sclerostin inhibits osteoblast activity and bone formation by sequestering LRP5 and LRP6, thereby repressing the Wnt signaling pathway (24). The LRP5 mutations associated with high bone mass prevent sclerostin from binding LRP5, thus confirming an in vivo role for sclerostin in depressing bone formation (25).
Fifteen BMPs are expressed in humans. They have diverse influences on bone and are divided into functional subgroups. Among the family members that induce bone are BMP2, BMP6, and BMP7. BMP2 synergizes with canonical Wnts to promote bone formation and modulates alkaline phosphatase and mineralization at least in part by induction of Wnt1 and 3a expression in mesenchymal cells (12, 26, 27). Thus, there appears to be considerable cross-talk between the Wnt and BMP pathways. BMP6, but not BMP4, enhances PTH or vitamin D-induced osteocalcin expression in mesenchymal stem cells (28). Using systemic delivery, it has been shown that BMP6 restores bone in aged rats after ovariectomy (29).
There is evidence that osteoclasts may recruit osteoblast lineage cells. Ryu et al. (30) showed that osteoclasts produce the enzyme SPHK1, and consequently its reaction product, S1P, during differentiation. S1P stimulates osteoblast migration and promotes their survival. Thus, osteoclasts may recruit osteoprogenitors to sites of bone resorption and promote mature osteoblast survival by secretion of S1P.
In this study we used an unbiased approach to identify osteoclast-secreted factors that may influence human mesenchymal stem (hMS) cell commitment and differentiation. Our data indicate that osteoclasts secrete Wnt10b, S1P, and BMP6 to promote mineralization.
Results
Mature Osteoclasts Secrete Candidate Coupling Factors That Promote Mesenchymal Stem Cell Mineralization.
We examined the influences of conditioned media from mouse bone marrow-derived osteoclasts, RAW 264.7-derived osteoclasts, and human osteoclasts on hMS cell mineralization. Conditioned media from all 3 osteoclast lineages significantly increased mineralization of the hMS cell cultures (Fig. 1). To determine candidate coupling factors that could be involved in osteoclast-mediated promotion of osteoblast recruitment and maturation, we used an unbiased Affymetrix gene array approach to identify candidate genes expressed by mature osteoclasts and found a number of known osteoblast stimulatory genes that were expressed by mature osteoclasts [supporting information (SI) Table S1]. We determined differential gene expression in bone marrow-derived osteoclasts before and after stimulation with M-CSF and RANKL and validated 14 genes that were expressed at higher levels in mature osteoclasts compared with precursors (Table S2). To narrow this subset further, we compared differentiated RAW 264.7 to undifferentiated cells and found 6 genes that were regulated in this model system as well as the marrow-derived model system (Table S2). Two of the gene products (semaphorin7a and ephrinB2) require cell–cell contact for interactions, so are unlikely to be important in the observed conditioned media response. For this study, we further focused on secreted proteins and identified sclerostin, BMP6, Wnt10b, and the SPHK1 product as potential coupling factors.
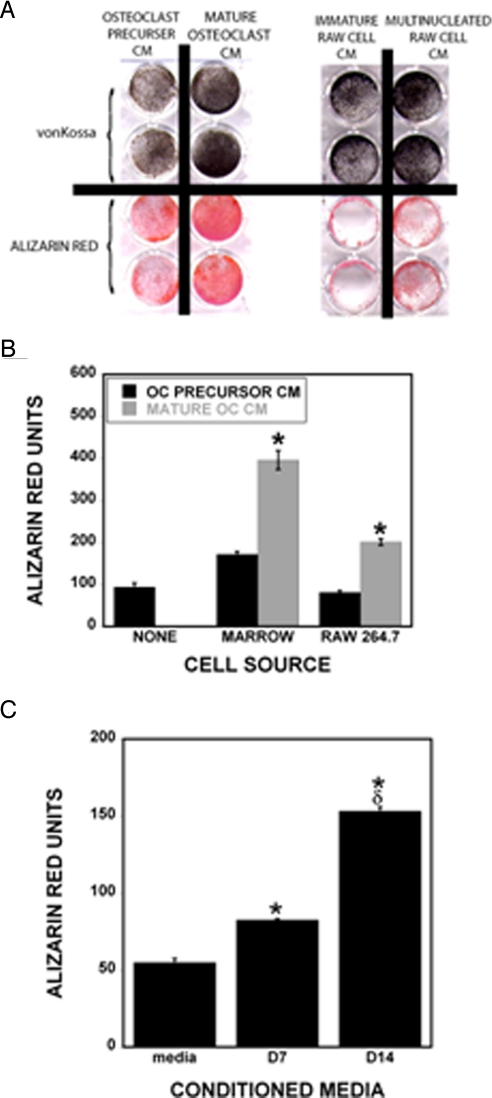
Fig. 1.
Influences of osteoclast conditioned media on hMS cell mineralization. (A) Marrow-derived osteoclast precursor conditioned media or mature osteoclast conditioned media (Left) or RAW 264.7 (RAW) cell conditioned media and mature multinucleated RAW-derived osteoclast conditioned media (Right) were added to hMS cells. Cultures were fixed after 3 weeks (marrow-derived CM) or 1 week (RAW-derived CM) and stained for mineralization by von Kossa or Alizarin red as indicated. (B) Alizarin red was quantified by extraction as described in Experimental Procedures (n = 3 replicates). *, P < 0.05 comparing precursor to mature osteoclast conditioned media. These results are representative of 3 experiments. (C) Human osteoclast precursors were cultured, and CM was collected on day 7 (D7; mature osteoclasts are present after day 5). Cells were fed, and CM was collected on day 14 (D14; mature osteoclasts present throughout). CMs or feeding media were concentrated and added to hMS cells as detailed in Experimental Procedures. Cultures were fixed after 10 days and stained with Alizarin red before quantitation (n = 3 replicates). *, P < 0.05 compared with media; δ, P < 0.05 compared with D7.
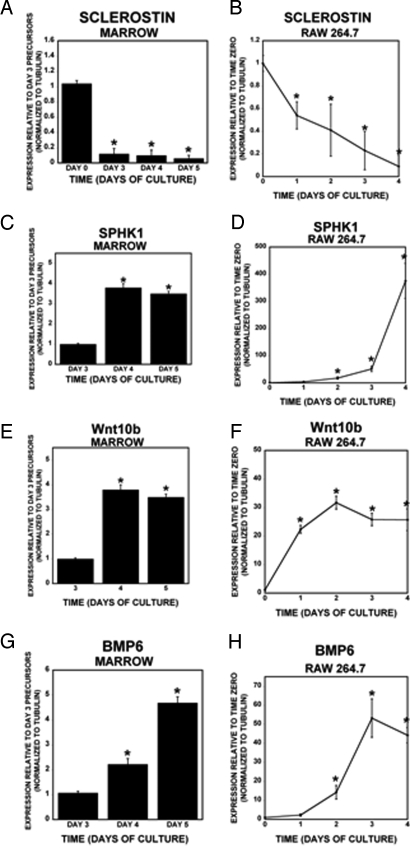
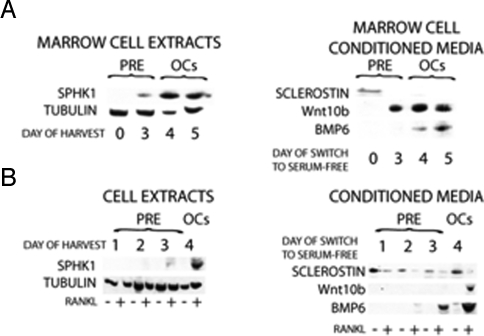
Sclerostin was detected in osteoclast precursors (threshold amplification 35.5 ± 0.2 cycles) but decreased during differentiation and was undetectable in mature osteoclasts (Fig. 2A). Sclerostin expression in osteoclast precursors was unexpected; therefore, we sought to verify that this was not because of contamination of the precursor cultures with osteoblastic cells. To accomplish this, CD11b-positive bone marrow cells were purified by using magnetic activated cell sorting. RNA was immediately harvested from half of the cells, and the remaining cells were cultured overnight with M-CSF. RNA was isolated from the 24-h nonadherent cells from these cultures as a way to examine osteoclast precursors that were further enriched. Real-time PCR analysis of freshly isolated CD11b+ marrow cells and 24-h nonadherent cells revealed that nonadherent cells from the overnight cultures expressed higher levels of sclerostin compared with the freshly isolated CD11b-positive cells (Fig. S1). We examined expression of candidate pro-bone formation coupling factors in marrow-derived and RAW 264.7 cells during differentiation and observed that sclerostin rapidly decreased with RANKL treatment (Fig. 2B). SPHK1 (Fig. 2 C and D), Wnt10b (Fig. 2 E and F), and BMP6 (Fig. 2 G and H) were up-regulated during osteoclast differentiation. To verify that these candidate coupling factors were expressed, we used Western blot analysis (Fig. 3). Mature RAW 264.7 and marrow-derived osteoclasts expressed significantly more SPHK1 protein than immature cells. Also, consistent with our gene expression data, BMP6 and Wnt10b protein expression was higher in concentrated conditioned media from mature RAW 264.7 and marrow-derived osteoclasts compared with conditioned media from precursors. In contrast, sclerostin levels were higher in the conditioned media of undifferentiated precursors than in the mature osteoclast conditioned media.
Fig. 2.
Coupling factor gene expression changes during osteoclast differentiation. (A, C, E, and G) Bone marrow cells were cultured with M-CSF and RANKL for 3, 4, or 5 days as described in Experimental Procedures. Mature osteoclasts appear by day 4 and peak between days 4 and 5. (B, D, F, and H) RAW 264.7 cells were either maintained in the absence of RANKL or cultured with 200 ng/mL RANKL for the indicated number of days. RNA was harvested for real-time PCR analyses (n = 3 replicates). *, P < 0.05 compared with time 0. These results are representative of 3 experiments.
Fig. 3.
Coupling factor protein expression changes during osteoclast differentiation. (A) Bone marrow cells were cultured with M-CSF and RANKL for the indicated time. Cells were either harvested (MARROW CELL EXTRACTS) or switched to serum-free media for 3 days before media collection and 10-fold concentration (MARROW CELL CONDITIONED MEDIA) before Western blotting. (B) RAW cells with or without RANKL treatment for the indicated time were either harvested for Western blotting (RAW CELL EXTRACTS) or rinsed and maintained in serum-free media for 3 days before media collection and 10-fold concentration (RAW CONDITIONED MEDIA) before Western blotting. All samples were normalized to cell extract protein. These results are representative of 2 experiments.
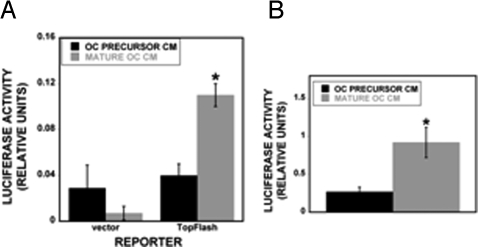
To verify that osteoclast conditioned media stimulated hMS Wnt and BMP signaling, hMS cells were transfected with Wnt and BMP reporters before treatment with conditioned media from immature RAW cells or mature RAW-derived osteoclasts. The hMS cells responded to osteoclast conditioned media with higher activation of both reporters when compared with precursor CM, verifying that the conditioned media contain biologically active Wnt and BMP protein levels (Fig. 4).
Fig. 4.
Wnt and BMP responsive reporter activation by osteoclast conditioned media. hMS cells were transiently transfected with 1 μg of empty reporter vector (vector), the BMP-responsive reporter SMAD6-luc (A), or the Wnt-responsive Tcf/Lef reporter TOP-FLASH-luc (B). Forty-eight hours later, the cells were treated with the indicated conditioned media for 48 h (n = 3 replicates). *, P < 0.05 compared with precursor CM. These results are representative of 2 experiments.
Blocking Wnt, BMP6, or S1P Receptor 1 in Osteoclast Conditioned Media Inhibits Mineralization.
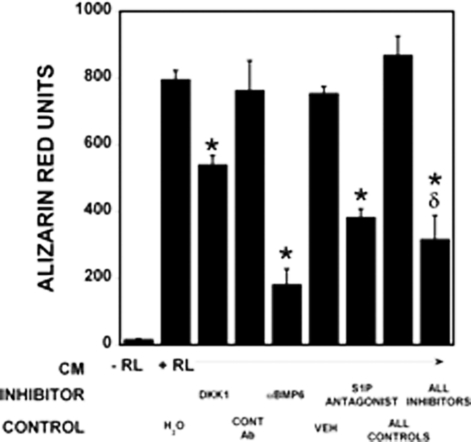
Recombinant DKK1 to block Wnt responses, or anti-BMP6 antibody to neutralize BMP6, or a S1P receptor 1 antagonist to blocked S1P responses were added to RAW-derived osteoclast conditioned media. These were used to treat hMS cells during the mineralization assay. Each of these treatments attenuated osteoclast conditioned media promotion of hMS cell mineralization, and the combination of the 3 inhibitors did not further reduce mineralization repression (Fig. 5). Addition of higher levels of each of these inhibitors did not further enhance blocking of mineralization (data not shown).
Fig. 5.
Blocking coupling factors reduces osteoclast conditioned media-induced hMS cell mineralization. RAW 264.7 precursor (−RL) and multinucleated RAW 264.7-derived osteoclast (+RL) conditioned media were assayed for their effects on osteoblast mineralization. DKK1, control antibody (CONT Ab), αBMP6 antibody, vehicle for the S1P receptor 1 antagonist (VEH), or a S1P receptor 1 antagonist alone or in combination were added to the osteoclast conditioned media as indicated for 7 days before fixing, Alizarin red staining, and quantitation as detailed in Experimental Procedures (n = 3 replicates). *, P < 0.05 compared with vehicle. These results are representative of 2 experiments.
S1P and BMP6, but not Wnt, in Osteoclast Conditioned Media Promote Mesenchymal Stem Cell Chemokinesis.
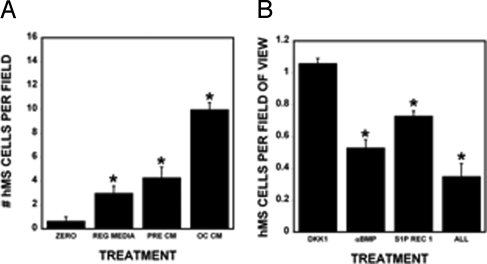
Osteoclast conditioned media significantly increased chemokinesis of hMS cells compared with either osteoclast precursor conditioned media or control conditioned media in a wound-healing assay (Fig. 6A). Receptor antagonist-blocking S1P receptor 1 and BMP6-blocking antibodies in osteoclast conditioned media reduced hMS cell chemokinesis whereas addition of DKK1 had no impact on chemokinesis (Fig. 6B).
Fig. 6.
hMS cell chemokinesis increases with osteoclast conditioned media treatment. hMS cells were grown on coverslips to confluence and assayed for chemokinesis as detailed in Experimental Procedures. (A) Coverslips were fixed (zero) or incubated in the indicated medium (n = 3 per treatment). Sixteen hours later, the remaining cells were fixed and stained. Cells were counted at 200×. *, P < 0.05 compared with other treatments. (B) hMS cells were treated as outlined in A before addition(s) as indicated. Cell counts were normalized to the respective vehicle control (n = 3 replicates). *, P < 0.05 compared with controls. These results are representative of 2 experiments.
Discussion
Here we report that mature osteoclasts secrete products that promote hMS cell migration and mineralization (see ref. 14 for a schematic depiction of this). We did not observe an impact of osteoclast conditioned media on alkaline phosphatase expression, indicating that osteoclast influences may be more focused on later stages of hMS cell differentiation (data not shown). Using Affymetrix gene arrays, real-time PCR, Western blotting, and functional assays, we have identified and validated 4 candidate factors that may couple bone resorption to the recruitment of osteoblast lineage cells and the promotion of osteoblast maturation. We verified that decreased expression of sclerostin and increased expression of SPHK1, Wnt10b, and BMP6 proteins by mature osteoclastic cells correlate with enhanced mineralization. The observations that osteoclast conditioned media activated the BMP and Wnt reporters to a greater extent than osteoclast precursor conditioned media confirms that biologically active Wnt and BMP proteins are enriched in the osteoclast conditioned media. Investigation of hMS cell chemokinesis in response to osteoclast conditioned media showed that a S1P receptor 1 antagonist and blocking BMP6 reduced the migration response to osteoclast conditioned media. We conclude from these data that the SPHK1 product S1P and BMP6 in osteoclast conditioned media stimulates migration of hMS cells. Taken together, these data reveal that Wnt, BMP6, and S1P responses contribute to the ability of osteoclast conditioned media to promote hMS cell mineralization. It has been well-documented that osteocytes express high levels of SOST mRNA and sclerostin protein (21, 31–34). Interestingly, images published by van Bezooijen et al. (32), Bellido et al. (33), and Robling et al. (34) include views of marrow tissue where a small percentage of the marrow cells may be positive for SOST message (32, 34) or protein (33, 34). In preliminary studies, we compared expression between the MLO-Y4 osteocyte cell line and the CD11b+ 24-h nonadherent osteoclast precursors and found that the osteocytic cell line MLO-Y4 (35) expressed ≈50 times more sclerostin message compared with osteoclast precursors (data not shown). These data are not surprising given the reports of high levels of sclerostin mRNA and protein detected in the bone localization studies discussed above. However, the proximity of osteoclast precursors to the stromal cell targets compared with the deeply imbedded osteocytes that express sclerostin may suggest that osteoclast precursor-derived sclerostin has biologically significant influences on stromal cells despite the relatively lower expression levels. In light of the studies reported here, this possibility requires further analysis.
To our knowledge, this is the first report that Wnt10b is expressed by mature osteoclasts and sclerostin is expressed by osteoclast precursors. Recently, Garimella et al. (36) documented that osteoclasts express BMP2, BMP4, BMP6, and BMP7. Thus, decreased expression of a Wnt signaling antagonist coupled to increased production of Wnt10b and BMP6 by osteoclasts elevates bone formation stimuli in the regions in which osteoclasts are localized. There is also evidence that osteoclasts may also recruit osteoblast lineage cells. Our data suggest that osteoclasts likely recruit osteoprogenitors to sites of bone resorption by secretion of S1P and BMP6 and stimulate osteoblast differentiation via secretion of Wnt10b and BMP6. All 3 of these coupling factors likely contribute to cell survival (37–40).
Global knockout of these coupling factors provides insights into respective roles in bone formation, although not necessarily indicative of the specific roles of osteoclast-mediated coupling factor expression. This latter point must await osteoclast-specific deletion of coupling factors. Two studies have examined mice lacking BMP6. In 2005, Kugimiya et al. (41) examined loss of BMP6 in both BMP2 replete and BMP2+/− mice. Mice lacking BMP6 did not exhibit a measurable bone phenotype whereas mice lacking BMP6 in a BMP2+/− background were smaller and had lower bone mineral density, bone formation rates, cortical bone thickness, and trabecular bone volume. Recently, Perry et al. (42) also examined effects of loss of BMP6 in BMP2+/+ mice and observed a decrease in periosteal bone formation in these mice. Thus, BMP6 contributes to bone formation, although redundancy with BMP2 remains unresolved. Global deletion of Wnt10b mice causes decreased trabecular bone and serum osteocalcin, confirming a role for Wnt10b in bone formation (18). Global deletion of SPHK1 and SPHK2 is embryonically lethal, so it will not be possible to resolve the role of S1P in vivo until studies are done in mice with selective deletion of SHPK1/2 in osteoclasts (43).
Other potential osteoblast–osteoclast coupling factors have been described and were confirmed in our studies. Delorme et al. (44) have reported that osteoclasts express Semaphorin 7A and that treatment of MC3T3 cells with recombinant Semaphorin 7A promoted their migration. The bidirectional signaling mediated by the transmembrane proteins ephrinB2 and EphB4, which are expressed on osteoclasts and osteoblasts, respectively, may also contribute to coupling of bone resorption with bone formation in vivo (45). Interactions of these molecules stimulate osteoblast differentiation and repress osteoclast differentiation. Semaphorin–receptor and ephrin–receptor interactions require close physical contact of osteoblastic cells with preosteoclasts and mature osteoclasts. As such, they are not candidates for osteoclast secretion products in the conditioned media and were not pursued in this study. Hepatocyte growth factor is another candidate osteoclast product that is produced by osteoclasts and promotes osteoblast proliferation (46). Hepatocyte growth factor did not appear as highly expressed in osteoclasts in our Affymetrix screen. The interactions among all of these candidate coupling factors and the roles of the release of matrix-bound growth factors in vitro and in vivo require further study to resolve the molecular mechanisms by which osteoclasts recruit osteoblast lineage cells, promote differentiation, and modulate bone formation.
Experimental Procedures
Unless otherwise indicated, all chemicals are from Sigma.
Marrow-Derived Osteoclast Culture.
All protocols were approved by the Mayo Clinic Institutional Animal Care and Use Committee before the start of the studies. Marrow cells were obtained from BALB/c mice as outlined previously (47).
RAW 264.7-Derived Osteoclast Culture.
RAW cells are maintained in an undifferentiated state by culture in Dulbecco's MEM supplemented with 10% FBS and antibiotic/antimycotic at low densities. For differentiation, cells were plated at 3 × 104 cells per well of 6-well plates in maintenance medium supplemented with 200 ng/mL recombinant RANKL, incubated at 37 °C with 5% CO2 in a humidified incubator, and fed daily with RANKL-supplemented medium for 4 days.
Human Osteoclast Culture.
Human osteoclast precursors and differentiation media were obtained from Lonza and cultured according to the provided protocol.
Conditioned Media Preparation.
Marrow-derived cell conditioned media were collected as detailed in the figure legends. RAW cell-derived osteoclasts were incubated at 37 °C with 5% CO2 in a humidified incubator for 3 days, and the conditioned media were harvested. Undifferentiated RAW cell conditioned medium was supplemented with 200 ng/mL RANKL after harvest. Human culture media were collected on days 7 and 14 of culture. Conditioned media were concentrated 10-fold by using Amicon Centricon Plus-70 centrifugation concentrators. After concentration, the units were used to exchange media with fresh Dulbecco's MEM by addition of new media and subsequent centrifugation.
Mesenchymal Stem Cell Culture, Mineralization, and Quantitation.
The hMS cells are transformed by stable expression of a temperature-sensitive large T antigen and are pluripotential cells capable of differentiating into either osteoblasts or adipocytes (48). Cultures were maintained and replated for experiments in αMEM supplemented with 10% FBS. For mineralization, 70% confluent cells were treated with 10-fold concentrated CM supplemented with ascorbic acid (1 μM) and β-glycerol phosphate (4 μM). Cultures were fixed and stained for mineralization with von Kossa or Alizarin red as detailed in the figure legends (49, 50). Alizarin red was eluted with 10% cetylpyridinium chloride, and levels were quantified by comparison to a standard curve.
Reporter Transfection and Analysis.
Seventy-percent-confluent hMS cells were transfected by using Targefect's Osteoblast reagent as outlined by the manufacturer. Forty-eight hours after transfection, cells were treated with precursor or osteoclast CM for an additional 48 h and processed as described (51). Cell extracts were normalized to equal protein concentrations before the luciferase assay.
Quantitative Real-Time PCR.
Cells were rinsed with PBS, and RNA was harvested at times indicated in the figure legends using Qiagene's microRNA purification kit according to the product literature. After quantitation, cDNA was synthesized and real-time PCR analysis was carried out as we have reported (52). Primers are detailed in Table S3.
Western Blotting.
Protocol and antibodies are detailed in SI Methods.
Chemokinesis Assay.
A straight-line region of hMS cells on confluent coverslips was scraped away with a sterile pipette tip. Cultures were treated with control media, osteoclast precursor CM, or mature osteoclast conditioned media for 16 h as detailed in the figure legends. Cell numbers in the gap region were quantitated per unit viewed.
CM treatments.
S1P receptor 1 antagonist VPC 23019 (from Avanti) was resuspended according to the supplier's directions, and 1 μM was added to the media as indicated in the figure legends. The final concentration of the control antibody (normal goat IgG) or BMP6 antibody (catalog no. SC-27409, Santa Cruz Biotechnology) was 2 μg/mL. Mouse DKK1 (R & D Systems) was added to a final concentration of 0.2 μg/mL.
Statistics.
Data were analyzed by using a 1-way ANOVA as compared with controls. Significance was determined at P < 0.05 with KaleidaGraph software.
Supplementary Material
Acknowledgments.
The authors thank Ms. Lindsay Godin for her work in preliminary screening studies. This work was supported by National Institutes of Health Grants AG004875 and AG028936 and the Mayo Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805133106/DCSupplemental.
References
- 1.Khosla S. Minireview: The OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 2.Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11:76–81. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Karsdal MA, Martin TJ, Bollerslev J, Christiansen C, Henriksen K. Are nonresorbing osteoclasts sources of bone anabolic activity? J Bone Miner Res. 2007;22:487–494. doi: 10.1359/jbmr.070109. [DOI] [PubMed] [Google Scholar]
- 4.Marzia M, et al. Decreased c-Src expression enhances osteoblast differentiation and bone formation. J Cell Biol. 2000;151:311–320. doi: 10.1083/jcb.151.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kornak U, et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001;104:205–215. doi: 10.1016/s0092-8674(01)00206-9. [DOI] [PubMed] [Google Scholar]
- 6.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 7.Del Fattore A, et al. Clinical, genetic, and cellular analysis of 49 osteopetrotic patients: Implications for diagnosis and treatment. J Med Genet. 2006;43:315–325. doi: 10.1136/jmg.2005.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleiren E, et al. Albers-Schonberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum Mol Genet. 2001;10:2861–2867. doi: 10.1093/hmg/10.25.2861. [DOI] [PubMed] [Google Scholar]
- 9.Dai XM, Zong XH, Akhter MP, Stanley ER. Osteoclast deficiency results in disorganized matrix, reduced mineralization, and abnormal osteoblast behavior in developing bone. J Bone Miner Res. 2004;19:1441–1451. doi: 10.1359/JBMR.040514. [DOI] [PubMed] [Google Scholar]
- 10.Sakagami N, et al. Reduced osteoblastic population and defective mineralization in osteopetrotic (op/op) mice. Micron. 2005;36:688–695. doi: 10.1016/j.micron.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Tuukkanen J, et al. Mineral density and bone strength are dissociated in long bones of rat osteopetrotic mutations. J Bone Miner Res. 2000;15:1905–1911. doi: 10.1359/jbmr.2000.15.10.1905. [DOI] [PubMed] [Google Scholar]
- 12.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 13.Hens JR, et al. TOPGAL mice show that the canonical Wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro. J Bone Miner Res. 2005;20:1103–1113. doi: 10.1359/JBMR.050210. [DOI] [PubMed] [Google Scholar]
- 14.Khosla S, Westendorf JJ, Oursler MJ. Building bone to reverse osteoporosis and repair fractures. J Clin Invest. 2008;118:421–428. doi: 10.1172/JCI33612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005;280:41342–41351. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- 16.Bodine PV, et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 17.Bodine PV, et al. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J Cell Biochem. 2005;96:1212–1230. doi: 10.1002/jcb.20599. [DOI] [PubMed] [Google Scholar]
- 18.Bennett CN, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett CN, et al. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res. 2007;22:1924–1932. doi: 10.1359/jbmr.070810. [DOI] [PubMed] [Google Scholar]
- 20.van Bezooijen , et al. Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J Bone Miner Res. 2007;22:19–28. doi: 10.1359/jbmr.061002. [DOI] [PubMed] [Google Scholar]
- 21.Winkler DG, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balemans W, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 23.Brunkow ME, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet. 2005;37:945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- 25.Ellies DL, et al. Bone density ligand, Sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res. 2006;21:1738–1749. doi: 10.1359/jbmr.060810. [DOI] [PubMed] [Google Scholar]
- 26.Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18:1842–1853. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 27.Mbalaviele G, et al. Beta-catenin and BMP-2 synergize to promote osteoblast differentiation and new bone formation. J Cell Biochem. 2005;94:403–418. doi: 10.1002/jcb.20253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sammons J, Ahmed N, El-Sheemy M, Hassan HT. The role of BMP-6, IL-6, and BMP-4 in mesenchymal stem cell-dependent bone development: Effects on osteoblastic differentiation induced by parathyroid hormone and vitamin D(3) Stem Cells Dev. 2004;13:273–280. doi: 10.1089/154732804323099208. [DOI] [PubMed] [Google Scholar]
- 29.Simic P, et al. Systemically administered bone morphogenetic protein-6 restores bone in aged ovariectomized rats by increasing bone formation and suppressing bone resorption. J Biol Chem. 2006;281:25509–25521. doi: 10.1074/jbc.M513276200. [DOI] [PubMed] [Google Scholar]
- 30.Ryu J, et al. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006;25:5840–5851. doi: 10.1038/sj.emboj.7601430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poole KE, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 32.van Bezooijen RL, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199:805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellido T, et al. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: A novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 34.Robling AG, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 35.Bonewald LF. Establishment and characterization of an osteocyte-like cell line, MLO-Y4. J Bone Miner Metab. 1999;17:61–65. doi: 10.1007/s007740050066. [DOI] [PubMed] [Google Scholar]
- 36.Garimella R, et al. Expression and synthesis of bone morphogenetic proteins by osteoclasts: A possible path to anabolic bone remodeling. J Histochem Cytochem. 2008;56:569–577. doi: 10.1369/jhc.2008.950394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goetzl E, et al. Mechanisms of lysolipid phosphate effects on cellular survival and proliferation. Ann NY Acad Sci. 2000;905:177–187. doi: 10.1111/j.1749-6632.2000.tb06549.x. [DOI] [PubMed] [Google Scholar]
- 38.Mulroy T, McMahon JA, Burakoff SJ, McMahon AP, Sen J. Wnt-1 and Wnt-4 regulate thymic cellularity. Eur J Immunol. 2002;32:967–971. doi: 10.1002/1521-4141(200204)32:4<967::AID-IMMU967>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Si W, et al. CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol. 2006;26:2955–2964. doi: 10.1128/MCB.26.8.2955-2964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itoh K, et al. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology. 2001;142:3656–3662. doi: 10.1210/endo.142.8.8300. [DOI] [PubMed] [Google Scholar]
- 41.Kugimiya F, et al. Involvement of endogenous bone morphogenetic protein (BMP) 2 and BMP6 in bone formation. J Biol Chem. 2005;280:35704–35712. doi: 10.1074/jbc.M505166200. [DOI] [PubMed] [Google Scholar]
- 42.Perry MJ, McDougall KE, Hou SC, Tobias JH. Impaired growth plate function in bmp-6 null mice. Bone. 2008;42:216–225. doi: 10.1016/j.bone.2007.09.053. [DOI] [PubMed] [Google Scholar]
- 43.Mizugishi K, et al. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delorme G, Saltel F, Bonnelye E, Jurdic P, Machuca-Gayet I. Expression and function of semaphorin 7A in bone cells. Biol Cell. 2005;97:589–597. doi: 10.1042/BC20040103. [DOI] [PubMed] [Google Scholar]
- 45.Zhao C, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 46.Grano M, et al. Hepatocyte growth factor is a coupling factor for osteoclasts and osteoblasts in vitro. Proc Natl Acad Sci USA. 1996;93:7644–7648. doi: 10.1073/pnas.93.15.7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gingery A, Bradley E, Shaw A, Oursler MJ. Phosphatidylinositol 3-kinase coordinately activates the MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival. J Cell Biochem. 2003;89:165–179. doi: 10.1002/jcb.10503. [DOI] [PubMed] [Google Scholar]
- 48.Gori F, Thomas T, Hicok KC, Spelsberg TC, Riggs BL. Differentiation of human marrow stromal precursor cells: Bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. J Bone Miner Res. 1999;14:1522–1535. doi: 10.1359/jbmr.1999.14.9.1522. [DOI] [PubMed] [Google Scholar]
- 49.Bills CE, Eisenberg H, Pallante SL. Complexes of organic acids with calcium phosphate: The von Kossa stain as a clue to the composition of bone mineral. Johns Hopkins Med J. 1971;128:194–207. [PubMed] [Google Scholar]
- 50.Gilmore SK, Whitson SW, Bowers DE., Jr A simple method using alizarin red S for the detection of calcium in epoxy resin embedded tissue. Stain Technol. 1986;61:89–92. doi: 10.3109/10520298609110714. [DOI] [PubMed] [Google Scholar]
- 51.Johnsen SA, Subramaniam M, Janknecht R, Spelsberg TC. TGFbeta inducible early gene enhances TGFbeta/Smad-dependent transcriptional responses. Oncogene. 2002;21:5783–5790. doi: 10.1038/sj.onc.1205681. [DOI] [PubMed] [Google Scholar]
- 52.Karst M, Gorny G, Galvin RJ, Oursler MJ. Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-beta regulation of osteoclast differentiation. J Cell Physiol. 2004;200:99–106. doi: 10.1002/jcp.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.