Abstract
In the last few years it has become clear that in cells of the immune system, specialized microdomains present in the plasma membrane, called lipid rafts, have been found to play a central role in regulating signalling by immune receptors. Recent studies have looked at whether lipid rafts may be connected to the abnormalities in signalling seen in T lymphocytes isolated from patients with systemic lupus erythematosus (SLE). These early findings show that in SLE T cells, the expression and protein composition of lipid rafts is different when compared with normal T cells. These results also demonstrate changes in the function and localization of critical signalling molecules such as the LCK tyrosine kinase and the CD45 tyrosine phosphatase.
Keywords: CD45, intracellular signalling, LCK, lipid rafts, T lymphocytes
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune rheumatic disease with a highly variable and unpredictable progression. Patients can present clinically in many ways, with abnormalities observed in musculo-skeletal, cardiopulmonary, gastrointestinal, renal, cerebral, haematological and dermatological systems.1 The principle underlying disorder is an impaired humoral and cellular immune response to self-antigens resulting in the characteristic abnormal production of autoantibodies to nuclear components. However, despite extensive research, the precise actiopathogenesis of SLE remains unclear. Virtually every aspect of the immune response to antigen has been shown to be abnormal in patients with SLE and current evidence suggests a central role is played by underlying defects in T lymphocyte antigen receptor mediated signalling pathways leading to the loss of tolerance to self-antigens.2,3
Abnormalities in immune cell function in SLE have been confirmed by the pattern of gene expression produced from oligonucleotide microarray analysis of peripheral blood mononuclear cells (PBMCs) from SLE patients. In one study, approximately 50% of adult patients with SLE had upregulated interferon (INF)-α associated genes4 and an important study of paediatric patients with severe lupus revealed recently that all patients demonstrated INF-α regulated gene expression. Fourteen out of the 15 genes over-expressed in PBMCs from these patients were identified as targets of INF-α activation including genes for upregulation of auto-antigens Ro and lamin, complement components C2 and C1 inhibitor and molecules associated with dendritic cell (DC) maturation and antigen presentation.5 Genes associated with granulopoiesis were also upregulated and expression of INF-α regulated and neutrophil encoded genes correlated with disease activity.5
Many mechanisms influencing T cell tolerance have been implicated in the actiopathogenesis, of SLE, these include abnormalities in DC maturation, deletion of self-reactive cells, and defects in regulatory T cells and T cell intracellular signalling pathways. Several excellent reviews have discussed these mechanisms in detail.6 11 Nonetheless, work performed recently, investigating lipid raft function in SLE T cells, has provided further insight in to the signalling abnormalities that may contribute to loss of peripheral T cell tolerance in patients with SLE.12 14 This review will focus, therefore, on the role of lipid rafts and associated signalling molecules and how these domains may determine the threshold for T cell activation in this disease.
Proximal T cell signalling events
The immediate consequence of T cell receptor (TCR) ligation by specific MHC/peptide is a rapid increase in protein tyrosine phosphorylation mediated by the sequential action of LCK and ZAP-70 tyrosine kinases, which are responsible for the initiation and amplification of the activation signal. In quiescent T cells, phosphorylation of ITAMs (immunoreceptor tyrosine based activation motif) present on the TCR/CD3 chains, by a pool of active LCK is an ongoing reaction balanced by the presence of protein tyrosine phosphatases, for example CD45.15 TCR activation may shift the balance of phosphorylation by excluding phosphatase molecules from the vicinity of the TCR by formation of the immunological synapse therefore promoting ITAM phosphorylation.16
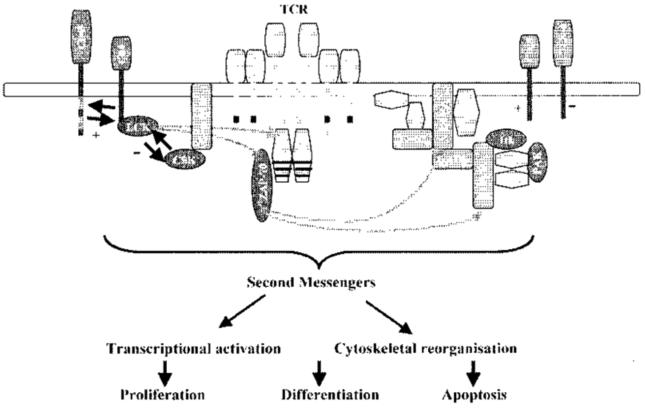
The main substrate for active ZAP-70 is the transmembrane adaptor molecule LAT (linker for activation of T cells), which becomes phosphorylated at multiple positions.15,17 Phosphorylated LAT nucleates protein complexes, the formation of which is critical for propagation of the TCR signal. LCK and LAT are constitutively partitioned within specialized membrane microdomains, termed lipid rafts, and upon TCR ligation, many proteins are targeted to lipid raft domains by protein–protein interactions triggering downstream signalling pathways.18 These include activation of the Ras–MAPK (mitogen activated protein kinases) pathway and hydrolysis of phosphatidylinositol 4,5-biphosphate by phospholipase Cγ 1 (PLCγl) leading to intracellular calcium (Ca2+) release. Activation of these and other pathways results in gene transcription, cytokine synthesis and release, activation or apoptosis19,20 (Figure 1).
Figure 1.
Signalling is initiated by engagement of the TCR and costimulatory molecules, such as CD28. Phosphorylation of proximal signalling molecules TCR-ζ chain, ZAP-70 and adaptor molecules LAT and SLP-76 by protein tyrosine kinases coordinate the activation of second messenger cascades leading to cytoskeletal reorganization and transcriptional activation. The threshold for TCR activation is regulated by the type of costimulatory molecule expressed, CTLA-4 will downregulate TCR activation, and the regulation of the proximal src-family kinase, LCK. LCK homeostasis is controlled by the reciprocal action of CD45 and CSK. Protein tyrosine kinases are shown in blue, adaptor proteins are orange. Modified from Singer and Koretzky.21
Role of lipid rafts in TCR cell signalling
In the last few years, it has become evident that the organization of signalling molecules into discrete membrane associated microdomains, termed lipid rafts, is vital for the regulation of T lymphocyte activation pathways.20,22,23 Lipid raft domains consist of assemblies of tightly packed sphingolipid and cholesterol moieties and were originally defined by their insolubility in cold nonionic detergents (specifically 1% Triton-X-100), which facilitates their isolation by floating in the upper half of a 5–30% sucrose density gradient. Recently, confocal microscopy and other imaging techniques have been used to visualize lipid rafts in intact cells and to provide information about their role during receptor signalling.24 Many of these techniques use cholera toxin B (CTB) subunit, which binds specifically to raft associated glycosphingolipid GM1. This marker has been used widely to detect lipid rafts25,26 and accumulated experimental evidence supports the use of GM1 as a credible indicator for lipid raft domains.
In T cells, signalling molecules that partition to lipid raft domains include the src-family kinases, LCK and FYN, GTP-binding proteins, GPI-linked receptors, CD4 and CD824 and adaptor molecules LAT and Cbp/PAG (CSK binding protein/protein associated with GEMs).18,27,28 Transient incorporation of other receptors and signalling proteins has also been observed during TCR activation, including the TCR itself.24,29,30 Some reports also suggest that CD45 may copurify with lipid rafts although other reports found CD45 to be excluded from these domains.31,32
In naive and resting T cells, the proportion of the plasma membrane adopting a raft conformation is low, measured by expression of the raft associated sphingolipid, GM1. TCR activation induces GM1 synthesis, and targets cytosolic LCK to the plasma membrane, thereby increasing the availability of lipid rafts and associated signalling molecules.26 Although their role in the formation of the immunological synapse is not fully understood, current evidence places lipid raft domains and associated signalling molecules at the centre of the supramolecular activation complex (cSMAC).33 Costimulation by CD28 bolsters signalling via the TCR by upregulating lipid raft expression and initiating changes in the actin cytoskeleton that stabilizes the T cell/antigen presenting cell (APC) contact area.34 It is interesting to note that lipid rafts are enriched with the cytoskeletal component, F-actin,35 thus changes in the cytoskeleton, stimulated by CD28 engagement, are likely to control the polarization of lipid rafts and their associated signalling molecules to the immunological synapse.36 Conversely, signalling via the inhibitory coreceptor CTLA-4 blocks surface upregulation of rafts.37 Thus the mechanism via which CD28 and CTLA-4 control T cell activation may, at least in part, be due to their ability to regulate lipid raft expression and their association with the immunological synapse.24,37
T-lymphocyte activation needs to be strictly regulated to maintain the specificity and fidelity of the immune response. The expression of lipid rafts is central to the control of T cell signalling pathways suggesting that alteration in the properties or composition of lipid rafts may lead to inappropriate T lymphocyte signalling and result in the development of pathological conditions including autoimmunity.
Abnormal intracellular signalling in T cells from patients with SLE
The threshold for T cell activation is lowered in patients with SLE.38 Moreover, defects in the expression and function of a number of signalling molecules have been reported, which may be responsible for the heightened sensitivity and the prolonged response to TCR activation seen in T lymphocytes from lupus patients.3 Principally, these defects involve molecules associated with early TCR/CD3 complex and CD28 costimulation pathways, although abnormalities have also been described in downstream signalling molecules and nuclear transcription factors.3,11 A summary of the T cell intracellular signalling abnormalities described in patients with SLE is shown in Table 1.
Table 1.
Summary of T cell signalling abnormalities in patients with SLE
| Defects of signal transduction | Reference |
|---|---|
| • Reduced expression of lipid raft associated LCK and increased LCK ubiquitination | 12 |
| • Altered association of signalling molecules with lipid raft domains and increased expression of lipid raft associated sphingolipid GM1 | 13 |
| • Decreased CD3/T cell receptor ζ chain | 10 |
| • Increased intracellular free calcium levels | 72 |
| • Enhanced tyrosine phosphorylation of proximal signalling molecules | 44,46 |
| • Enhanced activity of PTK LCK | 45,47 |
| • Decreased CD45 phosphatase activity | 74 |
| • Decreased protein kinase A isoenzyme activity | 80 |
| • Decreased expression of protein kinase C | 11 |
| • Altered mitochondrial hyperpolarization, induction of reactive oxygen intermediates and T cell apoptosis | 11 |
| • Increased binding of transcriptional inhibitor pCREM to the IL-2 promoter | 10 |
| • Decreased levels of p65-RelA subunit of the NF-κB nuclear transcription factor | 10 |
Altered expression of the lipid raft associated glycosphingolipid GM1
T cells freshly isolated from patients with SLE were found to express higher levels of the glycosphingolipid GM1, a constituent of lipid rafts.13 Increased levels of GM1 most likely indicate that a higher proportion of the plasma membrane assumes a lipid raft structure and could be linked to the phenotype manifested by SLE T cells. An increase in lipid raft formation could increase the strength of the T cell signal by reducing the threshold for T cell stimulation. This in turn may influence immune cell function in SLE patients, and, as such, lipid rafts may provide targets for future therapy.24 Lipid raft heterogeneity, in terms of both protein and lipid content, has been described in relation to T cell activation and function.26,39-42 Thus, decreased lateral mobility of T cell surface receptors and reduced capping in T cells from patients with SLE may reflect abnormalities in lipid raft composition.43 Increased lipid raft expression has also been reported previously in activated and memory T cells compared with naive T cells.25,26 Thus the increased raft expression seen in SLE T cells could be indicative of an ‘activated’ phenotype which may result from exposure of these cells to differentiated dendritic cells expressing relevant coreceptors, cytokines, circulating immune complexes or a combination of these factors.
Expression and regulation of LCK in SLE T cells
Aberrant expression and tyrosine phosphorylation of LCK have been described previously in PBMCs from patients with SLE and it has been suggested that LCK and FYN have increased activity in these cells.44-47 These results were confirmed recently by another report showing that expression of LCK was significantly reduced in both raft and non raft fractions in T lymphocytes from patients with active disease compared with patients with rheumatoid arthritis (RA) and healthy controls.12
LCK is essential for T cell differentiation and signalling48 and its partition to lipid rafts via a unique domain at the N terminus, is essential for its function.49 Experiments using LCK deficient cell lines indicate that LCK is responsible for most, if not all, of the ITAM phosphorylation that follows TCR engagement.50 Tsokos and colleagues have described reduced expression and mutations of the TCR-ζ chain in lupus T cells which may affect their activation,10,11 although the chains of the CD3 complex, which also contain ITAMs, may compensate for the reduced TCR-ζ expression.51 Interestingly, SLE T cells express the Fc receptor gamma chain (FcR-γ) which may make up for the absence of TCR-ζ.52 In addition to its kinase activity, the SH3 and SH2 domains of LCK interact with a wide range of adaptor and signalling molecules including the ubiquitin ligase c-Cbl and costimulatory molecules CD28 and CD2 that mediate remodelling of the cytoskeleton and assembly of raft domains within the immunological synapse.53,54 Thus, reduced expression of LCK in SLE T cells is likely to affect immune function. Little is known about the effect of reduced LCK on the function of primary T-lymphocytes. In LCK deficient mice, LCK is critical for the generation and maturation of thymocytes since these animals develop very few T cells, which are phenotypically abnormal.48 Absence of LCK in peripheral blood T cells leads to defects in their proliferative potential55 and diminished numbers of CD8+ cells, poor responses to CD28 costimulation and reduced calcium mobilization after stimulation.56 The effect of reduced LCK in patients with SLE is not clear, however, a similar reduction has been observed in patients with type I diabetes57 and conformational changes affecting LCK function have been reported in synovial T cells from patients with RA.58 In both cases, abnormal LCK expression was associated with T cell hyporesponsiveness. Correspondingly, lupus patients also show considerable evidence of reduced T cell proliferative responses to recall antigens.59
Reduced LCK expression in lupus T cells was found to be independent of the treatment regime or the activation status of the cells as determined by expression of the activation markers CD69, CD25 and CD95.12 However, there was increased ubiquitination of LCK in SLE T cells compared with normal controls, which could result in accelerated proteasomal degradation of the molecule. This observation supports recent studies showing an increased ubiquitination of CD3-ζ chain in T cell lysates from lupus patients.14 Protein ubiquitination targets proteins for degradation by the 26S proteasome and is a critical process regulating signal transduction in eukaryotic cells,60 thus the increased level of ubiquitinated proteins and ubiquitinated LCK may be the result of continuous T cell activation by autoantigens in SLE. However, this seems unlikely as LCK was reduced in both activated and nonactivated lupus T cells. In addition, SLE T cells activated in vitro with antibodies to CD3 and CD28 did not show differences in LCK expression over a 60 minute time course compared to controls, although differences in protein ubiquitination and phosphorylation were observed. These findings suggest that T cell activation is not solely responsible for the low levels of LCK observed in T cells from patients with SLE.12
Abnormal regulation of LCK activity in T cells from patients with SLE
Abnormal LCK ubiquitination and phosphotyrosine profiles observed in lipid rafts might indicate that the pool of LCK is dysregulated in patients with SLE.12 The precise regulation of LCK is critical for maintaining T cell homeostasis and for initiating activation of signalling cascades. There is good evidence that LCK function is controlled by the reciprocal action of protein tyrosine phosphatase (PTPase) CD45 and the protein tyrosine kinase c-terminal src kinase (CSK)61,62 (Figure 2). Cbl, an E3 ubiquitin ligase, also plays an important regulatory role in the immune response and is associated with negative regulation of active LCK.63,64 Precise LCK regulation ensures that the immune system can respond rapidly to foreign antigen. However, an imbalance in its regulation may predispose T cells to autoimmunity.65
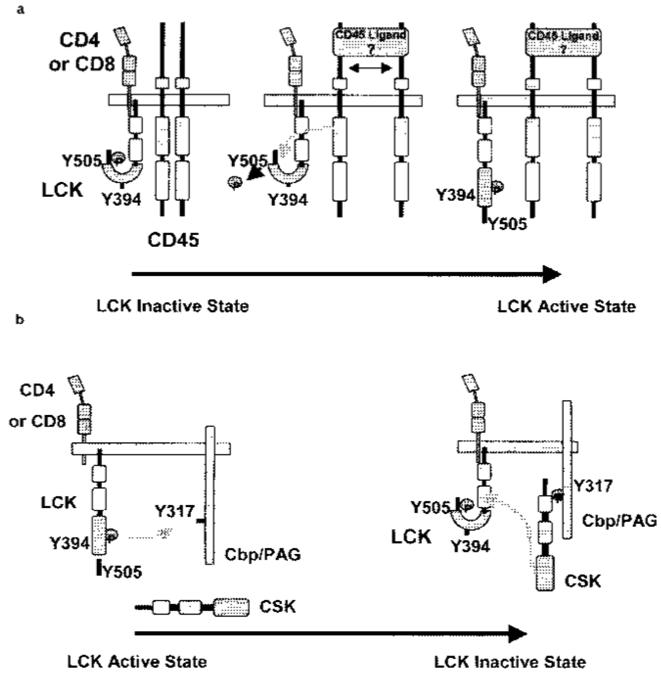
Figure 2.
Regulation of LCK by CD45 and CSK. LCK is controlled by the reciprocal action of the protein tyrosine phosphatase CD45 and the protein tyrosine kinase CSK. a) CD45 dephosphorylates the regulatory Tyr-505 residue, promoting LCK to adopt its open conformation. Subsequently, LCK becomes fully activated by autophosphorylation of Tyr-394. b) Src-family kinases, LCK and FYN, entering lipid rafts phosphorylate the adapter molecule Cbp/PAG resulting in the recruitment of CSK from the cytosol. Recruited CSK phosphorylates raft localized LCK at Tyr-505 inducing its inactive. ‘tail-bite’ conformation.
LCK conformation and kinase activity are regulated by the phosphorylation of two important tyrosines, an autophosphorylation site (Tyr-394) and an opposing negative regulatory site (Tyr-505). Phosphorylation of the negative regulatory site in the carboxy tail induces an intramolecular interaction with the SH2 domain, whereby the molecule folds to adopt an inactive or closed LCK conformation. This so-called ‘tail-bite’ structure is further stabilized by interaction of the SH3 domain with the linker region. Dephosphorylation of Tyr-505 generates an open conformation, where the SH2, SH3 and kinase domains are exposed and available for intermolecular interactions. Autophosphorylation of Tyr-394 in the kinase activation loop, displaces the loop from the kinase active site, resulting in greater accessibility to the site and full kinase activation66 (Figure 2).
The role of CD45
The CD45 phosphatase plays critical and diverse roles during T cell signalling. It positively regulates src-family PTKs, such as LCK, by dephosphorylating the negative regulatory residue Tyr-505, resulting in the kinase adopting an active conformation. In the resting T cell. CD45 maintains LCK in a primed state ready to respond to TCR activation. But CD45 can negatively regulate TCR signalling by dephosphorylating the TCR-ζ chain.67 It is also possible for CD45 to dephosphorylate Tyr-394, thus reducing LCK activity, although the latter is not believed to be the dominant interaction.68
The possibility that reduced LCK expression results from dysregulated LCK homeostasis in T cells from SLE patients has been reported recently.13 An increase in the localization of CD45 to lipid raft domains is observed along with a parallel increase of activated LCK in these domains. Also, an increase in the physical association of CD45 with LCK has been detected in SLE T cells compared with controls.13 The location of CD45 is important for its function as a regulator of LCK activity. A body of evidence suggests that CD45 is excluded from lipid rafts and that it regulates LCK activity by its proximity to raft domains.32,61,68,69 However, recent reports have shown that CD45 is associated with T cell lipid raft domains in T cell lines and that it is excluded only after T cell activation,70,71 thus suggesting that the lipid raft association of CD45 is dependent upon the activation status of the cell. T cells from patients with SLE demonstrate a more pronounced inclusion of CD45 to raft domains, and CD45 is maintained in these domains even after stimulation of the TCR, pointing to the possibility that alterations in the distribution of CD45 in lupus T cells may contribute to the abnormalities seen in these cells.
The consequences of increased LCK activation in lipid rafts, if LCK levels remain above a critical threshold for signalling, may result in hyper-responsive T cells, primed to respond to TCR/CD28 stimulation more readily. This assumption is confirmed by the correlation between increased levels of active LCK, and increased protein tyrosine phosphorylation following T cell activation13 and increased calcium mobilization in SLE.72 T cells from patients with SLE are therefore ‘primed’ for activation and respond more rapidly to antigenic triggers than T cells from normal controls. This may explain the exaggerated response to environmental antigens observed in SLE patents.73 However, other reports show reduced CD45 activity in SLE,46,75 which may be associated with an altered CD45 phenotype.59 The activity of CD45 is regulated by dimerization.75 The CD45RO isoform (associated with memory or activated T cells), dimerizes more readily than CD45RA (associated with naive/resting T cells). The phosphatase domain of CD45RO forms a symmetrical dimer in which the catalytic site of one molecule is blocked by specific contacts with a wedge from the other (reviewed in Hermiston et al.68). Furthermore, in vivo experiments using ‘knock-in’ mice with an inactivating point mutation in CD45 prevent the formation of the inhibitory wedge and develop lymphoproliferation and lupus-like nephritis.76 It will be interesting to investigate whether CD45 dimerization is different in lupus T cells compared with controls.
Evidence from fluorescent resonance energy transfer (FRET) analysis has shown that CD45RO localizes preferentially with raft partitioned CD4 and CD8 and is associated with an increase in protein tyrosine phosphorylation.77 Furthermore, CD26, a raft associated molecule with increased expression in activated and memory T cells, binds the cytoplasmic domain of CD45RO, bringing it to close proximity with LCK and increasing LCK activity.78 The distribution of CD26 and CD45RO can be affected by cytokine signalling. For example, the ThI type cytokine IL-12 affects the distribution of CD45RO by excluding it from lipid rafts.79 These observations support the idea that low molecular weight CD45 isoforms associate with rafts, but larger molecular weight isoforms are excluded thereby influencing CD45 PTPase activity. Thus, the activation of LCK by CD45 depends upon the membrane location and isoform of CD45, which, in turn, is influenced by T cell maturation and the cytokine environment.
The role of CSK
The action of CD45 on LCK phosphorylation is balanced by CSK, a 50 kDa soluble PTK that interacts via its SH2 domain with the raft bound adapter protein, Cbp/PAG, and downregulates LCK activity in lipid rafts.28,61 The most prominent tyrosine phosphoproteins in lipid raft domains from unstimulated T cells are LCK and Cbp/PAG. Whether CD45 can dephosphorylate Cbp/PAG, in addition to LCK, leading to dissociation of CSK in lipid raft domains, is currently unknown. Although no alterations in the association of CSK with lipid raft domains have been observed,13 the possibility that association of CD45 with lipid rafts in SLE T cells increases LCK activity not only by dephosphorylating Tyr-505 on LCK, but also by dephosphorylating PAG/Cbp and thus disrupting the CSK-PAG/Cbp inhibitory complex, cannot be excluded.
In 1994, Kammer and colleagues reported deficiency of the PKA isoenzyme in SLE T cells.80 PKA, a cAMP dependent protein kinase, is an important membrane associated immune cell modulator that increases the activity of CSK, when the latter is bound to Cbp/PAG in lipid raft domains.81 Downmodulation of PKA activity diminishes the negative regulatory feedback mechanism of T cell activation.82 CSK activity is regulated by raft associated PKA and by its interaction via its SH3 domain to cytoplasmic PTPase molecules.61 PKA induces a 2–4-fold increase in CSK activity through phosphorylation of serine(Ser)-364 in CSK.83 Thus, abnormalities in PKA function could contribute to abnormal LCK homeostasis by reducing CSK activity in T cells from patients with SLE.
Cbl family proteins and T cell activation
The c-Cbl and Cbl-b members of the Cbl-family (Casitas B cell lymphoma-b) of proteins have been identified as E3 ubiquitin ligases and shown to be important in setting signalling thresholds in peripheral T cell responses. Both c-Cbl and Cbl-b contain highly conserved N-terminal phosphotyrosine binding domains (SH2) and a zinc binding domain (RING finger motif) which recruits E2 ubiquitin conjugating enzymes. In addition, a C terminal proline rich motif can associate with SH3 domains on target signalling proteins. Thus, a dual mode of action has been proposed for the Cbl proteins, one that involves ubiquitination mediated degradation of target proteins, and a second where the function of signalling proteins is directly inhibited following their association to Cbl.84
Specifically it has been demonstrated that c-Cbl promotes TCR-ζ chain and Fyn ubiquitination and degradation.85 T cells from mice lacking c-Cbl consistently demonstrated increased levels of TCR, CD3, CD4 and CD69 expression and hyperphosphorylation of proximal signalling molecules, ZAP-70, LAT and SLP-76.86,87 c-Cbl is also important in the regulation of LCK activity. Hawash and colleagues64 demonstrated that the SH3 domain of LCK binds to non raft associated c-Cbl and its over expression resulted in depletion of LCK from lipid raft domains. c-Cbl has also been shown to directly regulate activated LCK by enhancing its ubiquitin mediated proteasomal degradation via binding to the SH3 domain of activated LCK.63
Cbl-b is also involved in the regulation of T cell activation by promoting the ubiquitination of the p85 subunit of PI3 kinase through binding to its SH3 domain. This modulates its recruitment to CD28 and the TCR complex which, in turn, regulates activation of guanine nucleotide exchange factor protein, Vav-1.85 Studies comparing Cbl-b−/− and with c-Cbl−/− knockout mice (KOs) revealed differences in their T cell phenotype. Cbl-b−/− KOs demonstrate T and B cell hyperproliferation, develop spontaneous autoimmunity and hyperphosphorylation of Vav following TCR stimulation, suggesting that TCR signalling has been cut off from the requirement for CD28 costimulation.84,88 In support of this model, Cbl-b has been shown to act as a negative regulator of lipid raft aggregation and TCR activation in the absence of CD28 engagement.89
In SLE T cells, the increase in LCK ubiquitination and subsequent degradation12 could result from increased consumption of the activated form of LCK, possibly due to the altered localization of CD45 and its increased association with LCK. Interestingly, the expression of c-Cbl is reduced in the lupus T cells.13 This observation is supported by recently described abnormalities in the c-Cbl-mediated regulation of signalling pathways in T cell lines derived from patients with SLE.90 Thus, the reduction observed in c-Cbl molecules may have profound consequences on T cell activation threshold in lupus T cells.
The effect of TCR engagement on SLE T cells
When purified SLE T cells were ‘rested’ in vitro the changes seen in LCK, CD45 and lipid raft expression in freshly purified cells were reversed. Addition of SLE serum during the in vitro incubation did not prevent this reversion of cell phenotype, suggesting that factors present in SLE serum are not sufficient for reproducing the disease phenotype in vitro.13 It is possible that while in the body of the patient, T cells come into contact with other cell types and this interaction is responsible, at least in part, for the observed alterations in the proximal signalling pathways. Relevant to this hypothesis is a recent report by Blanco and colleagues,91 which demonstrated that, CD14+ monocytes differentiate into plasmacytoid dendritic cells, under the influence of SLE serum. These results revealed that INF-α, whose levels are increased in patients,92 is a factor driving dendritic cell differentiation and activation in SLE. An increase in the differentiation of plasmacytoid dendritic cells resulting in increased presentation of autoantigens to T and B lymphocytes could lower the threshold for T and B cell activation. Hence, when lupus T cells were rested in vitro, the proportion of plasma membrane adopting a lipid raft structure, measured by GM1 expression, was reduced. However, lipid raft formation on the cell surface was rapidly expanded following subsequent activation with anti-CD3 and anti-CD28.13
These results corroborate studies by Tsokos and colleagues showing that T cells from patients with SLE are ‘rewired’ and signal using alternative pathways,10 including signalling via the Fc receptor (FcR)γ chain which, although not present in normal T cells, it is expressed in lupus T cells.52 Therefore, anomalous expression and changes in membrane raft location of signalling molecules could contribute to changes in the threshold for T cell activation, characteristic of individuals with SLE.
Summary
Numerous abnormalities in T cell function have been described in SLE patients, although no collective abnormality is present in all patients. A common feature appears to be a reduced threshold for T cell activation. Thus, defects in intracellular biochemical pathways or the provision of costimulatory signals may be responsible for the heightened sensitivity and the prolonged response to TCR activation seen in T lymphocytes from patients with SLE. Recent work has indicated that abnormalities in the localization of signalling or costimulatory molecules to lipid raft domains could contribute to T cell dysfunction in these patients. It is likely that in vivo factors, either soluble mediators or cell–cell interactions or both, influence the abnormal distribution of signalling molecules to lipid raft domains, although the possibility that inherent abnormalities also exist in lupus T cells cannot been excluded. Recent work highlights the importance of lipid rafts in maintaining T cell homeostasis and suggests that altering their integrity could promote untimely protein–protein interactions, which can influence T cell function and possibly break T cell tolerance. Future work exploring the precise reasons underlying the increased expression and altered occupancy of lipid raft domains in T cells from patients with SLE may reveal further insights into the pathogenesis of SLE and uncover possibilities for new therapies.
Acknowledgements
PS Kabouridis is supported by a Wellcome Trust Career Development Award (058408). The Arthritis Research Campaign supports EC Jury. The authors thank David Isenberg for his support.
References
- 1.Morrow J, Nelson L, Watts R, Isenberg D. Systemic lupus erythematosus. In: Morrow J, Nelson L, Watts R, Isenberg D, editors. Autoimmune rheumatic disease. second edition Oxford, UK: Oxford University Press; 1999. pp. 56–103. [Google Scholar]
- 2.Dayal AK, Kammer GM. The T cell enigma in lupus. Arthritis Rheum. 1996;39:23–33. doi: 10.1002/art.1780390104. [DOI] [PubMed] [Google Scholar]
- 3.Tsokos GC, Liossis S-NC. Immune cell signalling defects in lupus: activation, anergy and death. Immunol Today. 1999;20:199–124. doi: 10.1016/s0167-5699(98)01395-4. [DOI] [PubMed] [Google Scholar]
- 4.Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohashi PS. T cell signalling and autoimmunity: molecular mechanisms of disease. Nat Rev Immunol. 2002;2:427–438. doi: 10.1038/nri822. [DOI] [PubMed] [Google Scholar]
- 7.Pascual V, Banchereau J, Palucka AK. The central role of dendritic cells and interferon-alpha in SLE. Curr Opin Rheum. 2003;15:548–556. doi: 10.1097/00002281-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 8.White S, Rosen A. Apoptosis in systemic lupus erythematosus. Curr Opin Rheum. 2003;15:557–562. doi: 10.1097/00002281-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 10.Tsokos GC, Nambiar MP, Tenbrock K, Juang Y-T. Rewiring the T-cell: signalling defects and novel prospects for the treatment of SLE. Trends Immunol. 2003;24:259–263. doi: 10.1016/s1471-4906(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 11.Kammer GM, Perl A, Richardson BC, Tsokos GC. Abnormal T cell signal transduction in systemic lupus erythematosus. Arthritis Rheum. 2002;46:1139–1154. doi: 10.1002/art.10192. [DOI] [PubMed] [Google Scholar]
- 12.Jury EC, Kabouridis PS, Abba A, Mageed RA, Isenberg DA. Increased ubiquitination and reduced expression of LCK in T lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1343–1354. doi: 10.1002/art.10978. [DOI] [PubMed] [Google Scholar]
- 13.Jury EC, Kabouridis PS, Flores-Borja F, Mageed RA, Isenberg DA. Altered lipid raft-associated signalling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 2004;113:1176–1187. doi: 10.1172/JCI20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nambiar MP, Enyedy EJ, Fisher CU, et al. Abnormal expression of various molecular forms and distruption of T cell receptor ζ chain in patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:163–174. doi: 10.1002/1529-0131(200201)46:1<163::AID-ART10065>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 15.Mustelin T, Taskén K. Positive and negative regulation of T-cell activation through kinases and phosphatases. Biochem J. 2003;371:15–27. doi: 10.1042/BJ20021637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis SJ, Van der Merwe PA. The structure and ligand interactions of CD2: implications for T cell function. Immunol Today. 1996;17:177–187. doi: 10.1016/0167-5699(96)80617-7. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Samelson LE. The role of membrane-associated adaptors in T cell receptor signalling. Cell. 2000;12:35–41. doi: 10.1006/smim.2000.0205. [DOI] [PubMed] [Google Scholar]
- 19.Simons K, Toomre D. Lipid rafts and signal transduction. Nature Rev. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 20.Janes PW, Ley SC, Magee AI, Kabouridis PS. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin Immunol. 2000;12:23–34. doi: 10.1006/smim.2000.0204. [DOI] [PubMed] [Google Scholar]
- 21.Singer AL, Koretzky GA. Control of T cell function by positive and negative regulators. Science. 2002;296:1639–1640. doi: 10.1126/science.1071551. [DOI] [PubMed] [Google Scholar]
- 22.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 23.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 24.Dykstra M, Cherukuri A, Sohn HW, Tzeng S-J, Pierce SK. Location is everything: Lipid rafts and immune cell signalling. Annu Rev Immunol. 2003;21:457–481. doi: 10.1146/annurev.immunol.21.120601.141021. [DOI] [PubMed] [Google Scholar]
- 25.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganisation of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 26.Tuosto L, Parolini I, Schroder S, Sariacomo M, Lanzavecchia A, Viola A. Organisation of plasma membrane functional rafts upon T cell activation. Eur J Immunol. 2001;31:345–349. doi: 10.1002/1521-4141(200102)31:2<345::aid-immu345>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 27.Tomlinson MG, Lin J, Weiss A. Lymphocytes with a complex: adapter proteins in antigen receptor signalling. Immunol Today. 2000;21:584–591. doi: 10.1016/s0167-5699(00)01716-3. [DOI] [PubMed] [Google Scholar]
- 28.Brdicka T, Pavlistova D, Leo A, et al. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 30.Giurisato E, McIntosh DP, Tassi M, Gamberucci A, Benedetti A. T cell receptor can be recruited to a subset of plasma membrane rafts, independently of cell signaling and attendantly to raft clustering. J Biol Chem. 2003;278:6771–6778. doi: 10.1074/jbc.M210758200. [DOI] [PubMed] [Google Scholar]
- 31.Freiberg BA, Kupfer H, Maslanik JD, Kappler J, Zaller DM, Kupfer A. Staging and resetting T cell activation in SMACs. Nature Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 32.Rodgers W, Rose JK. Exclusion of CD45 inhibits activity of p56lck associated with glycolipid-enriched membrane domains. J Cell Biol. 1996;135:1515–1523. doi: 10.1083/jcb.135.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burack WR, Lee KH, Holdorf AD, Dustin ML, Shaw AS. Quantitative imaging of raft accumulation in the immunological synapse. J Immunol. 2002;169:2837–2841. doi: 10.4049/jimmunol.169.6.2837. [DOI] [PubMed] [Google Scholar]
- 34.Acuto O, Cantrell D. T cell activation and the cytoskeleton. Annu Rev Immunol. 2000;18:165–184. doi: 10.1146/annurev.immunol.18.1.165. [DOI] [PubMed] [Google Scholar]
- 35.Rodgers W, Zavzavadjian J. Glycolipid-enriched membrane domains are assembled into membrane patches by associating with the actin cytoskeleton. Exp Cell Res. 2001;267:173–183. doi: 10.1006/excr.2001.5253. [DOI] [PubMed] [Google Scholar]
- 36.Jordan S, Rodgers W. T cell glycolipid-enriched membrane domains are constitutively assembled as membrane patches that translocate to immune synapses. J Immunol. 2003;171:78–87. doi: 10.4049/jimmunol.171.1.78. [DOI] [PubMed] [Google Scholar]
- 37.Martin M, Schneider H, Azouz A, Rudd CE. Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T cell function. J Exp Med. 2001;194:1675–1681. doi: 10.1084/jem.194.11.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vratsanos GS, Jung S, Park YM, Craft J. CD4(+) T cells from lupus-prone mice are hyperresponsive to T cell receptor engagement with low and high affinity peptide antigens: a model to explain spontaneous T cell activation in lupus. J Exp Med. 2001;193:329–337. doi: 10.1084/jem.193.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schade AE, Levine AD. Lipid raft heterogeneity in human peripheral blood T lymphoblasts: A mechanism for regulating the initiation of TCR signal transduction. J Immunol. 2002;168:2233–2239. doi: 10.4049/jimmunol.168.5.2233. [DOI] [PubMed] [Google Scholar]
- 40.Drevot P, Langlet C, Guo XJ, et al. TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. EMBO J. 2002;21:1899–1908. doi: 10.1093/emboj/21.8.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pike LJ. Lipid rafts: heterogeneity on the high seas. Biochem J. 2004;378:281–292. doi: 10.1042/BJ20031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez-Mouton C, Abad JL, Mira E, et al. Segregation of leading edge and uropod components into specific lipid rafts during T cell polarization. Proc Natl Acad Sci USA. 2001;98:9642–9647. doi: 10.1073/pnas.171160298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kammer GM. Impaired T cell capping and receptor regeneration in active systemic lupus erythematosus: evidence for a disorder intrinsic to the T lymphocyte. J Clin Invest. 1983;72:1686–1697. doi: 10.1172/JCI111128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matache C, Stefanescu M, Onu A, et al. Tyrosine phosphorylation in peripheral lymphocytes from patients with systemic lupus erythematosus. Autoimmunity. 1996;24:217–228. doi: 10.3109/08916939608994714. [DOI] [PubMed] [Google Scholar]
- 45.Matache C, Stefanescu M, Onu A, et al. P56lck activity and expression in peripheral blood lymphocytes from patients with systemic lupus erythematosus. Autoimmunity. 1999;29:111–120. doi: 10.3109/08916939908995380. [DOI] [PubMed] [Google Scholar]
- 46.Blasini AM, Alonzo E, Chacon R, Riera R, Stekman IL, Rodriguez MA. Abnormal pattern of tyrosine phosphorylation in unstimulated peripheral blood T lymphocytes from patients with systemic lupus erythematosus. Lupus. 1998;7:515–523. doi: 10.1191/096120398678920604. [DOI] [PubMed] [Google Scholar]
- 47.Blasini AM, Brundula V, Paris M, et al. Protein tyrosine kinase activity in T lymphocytes from patients with systemic lupus erythematosus. J Autoimmunity. 1998;11:387–393. doi: 10.1006/jaut.1998.0230. [DOI] [PubMed] [Google Scholar]
- 48.Molina TJ, Kishihara K, Siderovski DP, et al. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 49.Kabouridis PS, Magee AI, Ley SC. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Straus D, Weiss A. Genetic evidence for the involvement of the lck tryrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 51.Ardouin L, Boyer C, Gillet A, et al. Crippling of CD3-ζ ITAMs does not impair T cell receptor signalling. Immunity. 1999;10:409–420. doi: 10.1016/s1074-7613(00)80041-2. [DOI] [PubMed] [Google Scholar]
- 52.Enyedy EJ, Nambiar MP, Liossis S-NC, Dennis G, Kammer GM, Tsokos GC. Fcε receptor type I γ chain in T cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44:1114–1121. doi: 10.1002/1529-0131(200105)44:5<1114::AID-ANR192>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 53.Holdorf AD, Green JM, Levin SD, et al. Proline residues in CD28 and Src homology (SH)3 domain of Lck are required for T cell costimulation. J Exp Med. 1999;190:375–384. doi: 10.1084/jem.190.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nature Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 55.Seddon B, Legname G, Tomlinson P, Zamoyska R. Long-term survival but impaired homeoststic proliferation of naive T cells in the absence of p56lck. Science. 2000;290:127–131. doi: 10.1126/science.290.5489.127. [DOI] [PubMed] [Google Scholar]
- 56.Trowbridge PA, Levin SD. Lck plays a critical role in Ca2+ moblization and CD28 costimulation in mature primary T cells. Eur J Immunol. 2001;31:3567–3579. doi: 10.1002/1521-4141(200112)31:12<3567::aid-immu3567>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 57.Nervi S, Atlan-Gepner C, Kahn-Perles B, et al. Specific deficiency of p56lck expression in T lymphocytes from type I diabetic patients. J Immunol. 2000;165:5874–5883. doi: 10.4049/jimmunol.165.10.5874. [DOI] [PubMed] [Google Scholar]
- 58.Romagnoli P, Strahan D, Pelosi M, Cantagrel A, van Meerwijk JPM. A potential role for protein tyrosine kinase p56lck in rheumatoid arthritis synovial fluid T lymphocyte hyporesponsiveness. Int Immunol. 2001;13:305–312. doi: 10.1093/intimm/13.3.305. [DOI] [PubMed] [Google Scholar]
- 59.Horwitz DA, Stohl W, Gray JD. T lymphocytes, natural killer cells and immune regulation. In: Wallace DJ, Hann BH, editors. Dubois lupus erythematosus. sixth edition Philadelphia, USA: Lippincott, Williams and Wilkins; 2002. pp. 157–185. [Google Scholar]
- 60.Ben-Neriah Y. Regulatory functions of ubiquitination in the immune system. Nature Immunol. 2002;3:20–26. doi: 10.1038/ni0102-20. [DOI] [PubMed] [Google Scholar]
- 61.Hermiston ML, Xu Z, Majeti R, Weiss A. Reciprocal regulation of lymphocyte activation by tyrosine kinases and phosphatases. J Clin Invest. 2002;109:9–14. doi: 10.1172/JCI14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Penninger JM, Irie-Sasaki J, Sasaki T, Oliveira-dos-Santos AJ. CD45: new jobs for an old acquaintance. Nature Immunol. 2001;2:389–396. doi: 10.1038/87687. [DOI] [PubMed] [Google Scholar]
- 63.Rao N, Miyake S, Reddi AL, et al. Negative regulation of Lck by Cbl ubiquitin ligase. Proc Natl Acad Sci USA. 2002;99:3794–3799. doi: 10.1073/pnas.062055999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hawash IY, Kesavan KP, Magee AI, Geahlen RL, Harrison ML. The Lck SH3 domain negatively regulates localisation to lipid rafts through an interation with c-Cbl. J Biol Chem. 2002;277:5683–5691. doi: 10.1074/jbc.M110002200. [DOI] [PubMed] [Google Scholar]
- 65.Leitenberg D, Balamuth F, Bottomly K. Changes in the T cell receptor macromolecular signalling complex and membrane microdomains during T cell development and activation. Semin Immunol. 2001;13:129–138. doi: 10.1006/smim.2000.0304. [DOI] [PubMed] [Google Scholar]
- 66.Williams JC, Wierenga RK, Saraste M. Insights into Src kinase functions – structural comparisons. Trends Biochem Sci. 1998;23:179–184. doi: 10.1016/s0968-0004(98)01202-x. [DOI] [PubMed] [Google Scholar]
- 67.Furukawa T, Itoh M, Krueger NX, Streuli M, Saito H. Specific interaction of the CD45-protein tyrosine phosphorylated CD3 ζ chain. Proc Natl Acad Sci USA. 1994;25:942–946. doi: 10.1073/pnas.91.23.10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signalling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 69.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;174:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edmonds SD, Ostergaard HL. Dynamic association of CD45 with detergent-insoluble microdomains in T lymphocytes. J Immunol. 2002;169:5036–5042. doi: 10.4049/jimmunol.169.9.5036. [DOI] [PubMed] [Google Scholar]
- 71.Irles C, Symons A, Michel F, Bakker TR, van der Merwe PA, Acuto O. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nat Immunol. 2003;4:189–197. doi: 10.1038/ni877. [DOI] [PubMed] [Google Scholar]
- 72.Vassilopoulos D, Kovacs B, Tsokos GC. TCR/CD3 complex-mediated signal transduction pathway in T cell lines from patients with systemic lupus erythematosus. J Immunol. 1995;155:2269–2281. [PubMed] [Google Scholar]
- 73.Dar O, Salamam MR, Seifert MH, Isenberg DA. Spontaneous antibody secreting cells against DNA and common environmental antigens in systemic lupus erythematosus. J Autoimmunity. 1990;3:523–530. doi: 10.1016/s0896-8411(05)80018-6. [DOI] [PubMed] [Google Scholar]
- 74.Takeuchi T, Pang M, Amano J, Koide J, Abe T. Reduced protein tyrosine phosphatase (PTPase) activity of CD45 on peripheral blood lymphocytes in patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1997;109:20–26. doi: 10.1046/j.1365-2249.1997.4371334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu Z, Weiss A. Negative regulation of CD45 by differential homodimerisation of the alternatively spliced isoforms. Nature Immunol. 2002;3:764–771. doi: 10.1038/ni822. [DOI] [PubMed] [Google Scholar]
- 76.Majeti R, Xu Z, Parslow TG, et al. An inactivating point mutation in the inhibitory wedge of CD45 causes lymphoproliferation and autoimmunity. Cell. 2000;103:1059–1070. doi: 10.1016/s0092-8674(00)00209-9. [DOI] [PubMed] [Google Scholar]
- 77.Dornan S, Sebestyen Z, Gamble J, et al. Differential association of CD45 isoforms with CD4 and CD8 regulates the actions of specific pools of p56lck tyrosine kinase in T cell antigen receptor signal transduction. J Biol Chem. 2002;277:1912–1918. doi: 10.1074/jbc.M108386200. [DOI] [PubMed] [Google Scholar]
- 78.Ishii T, Ohnuma K, Murakami A, et al. CD26-mediated signalling for T cell activation occurs in lipid rafts through its association with CD45RO. PNAS. 2001;98:12138–12143. doi: 10.1073/pnas.211439098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salgado FJ, Lojo J, Alonso-Lebrero JL, et al. A role for IL-12 in the regulation of T cell plasma membrane compartmentation. J Biol Chem. 2003;278:24849–24857. doi: 10.1074/jbc.M212978200. [DOI] [PubMed] [Google Scholar]
- 80.Kammer GM, Khan IU, Malemud CJ. Deficient type I protein kinase A isoenzyme activity in systemic lupus erythematosus T lymphocytes. J Clin Invest. 1994;94:422–430. doi: 10.1172/JCI117340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Torgersen KM, Vang T, Abrahamsen H, et al. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (CSK) from lipid rafts. J Biol Chem. 2001;276:29313–29318. doi: 10.1074/jbc.C100014200. [DOI] [PubMed] [Google Scholar]
- 82.Aandahl EM, Moretto WJ, Haslett PA, et al. Inhibition of antigen-specific T cell proliferation and cytokine production by protein kinase A type I. J Immunol. 2002;169:802–808. doi: 10.4049/jimmunol.169.2.802. [DOI] [PubMed] [Google Scholar]
- 83.Vang T, Sundvold V, Levy FO, et al. Activation of the COOH-terminal Src kinase (CSK) by cAMP-dependent protein kinase inhibits signalling through the T cell receptor. J Exp Med. 2001;193:497–507. doi: 10.1084/jem.193.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krawczyk C, Penninger JM. Molecular controls of antigen receptor clustering and autoimmunity. Trends Cell Biol. 2001;11:212–220. doi: 10.1016/s0962-8924(01)01981-x. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y-C, Gu H. Cbl and Cbl-b in T-cell regulation. Trends Immunol. 2002;23:140–143. doi: 10.1016/s1471-4906(01)02157-3. [DOI] [PubMed] [Google Scholar]
- 86.Murphy MA, Schnall RG, Venter DJ, et al. Tissue hyperplasia and enhanced T cell signalling via ZAP-70 in c-Cbl deficient mice. Mol Cell Biol. 1998;18:4872–4882. doi: 10.1128/mcb.18.8.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Naramura M, Kole HK, Hu RJ, Gu H. Altered thymic positive selection and intracellular signals in cbl-deficient mice. PNAS. 1998;95:15547–15552. doi: 10.1073/pnas.95.26.15547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nature Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 89.Krawczyk C, Bachmaier K, Sasaki T, et al. Cbl-b is a negative regulator of receptor clustering and raft aggregation in T cells. Immunity. 2000;13:463–473. doi: 10.1016/s1074-7613(00)00046-7. [DOI] [PubMed] [Google Scholar]
- 90.Yi Y, McNerney M, Datta SK. Regulatory defects in Cbl and mitogen-activated protein kinase (extracellular signal-related kinase) pathways cause persistent hyperexpression of CD40 ligand in human lupus T cells. J Immunol. 2000;165:6627–6634. doi: 10.4049/jimmunol.165.11.6627. [DOI] [PubMed] [Google Scholar]
- 91.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 92.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301:5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]