Abstract
Objective
Osteoarthritis (OA) is characterized by progressive degeneration of articular cartilage and remodeling of the subchondral bone plate (ScBP), comprised of calcified cartilage (CC) and underlying subchondral bone (ScB). CC remodeling due to upward invasion by vascular canals or to CC erosion may contribute to biomechanical alteration of the osteochondral (OC) tissue and its ScBP component. The study hypothesis was that hydraulic conductance of OC tissue and ScBP increases with structural changes indicative of increasing stages of OA.
Methods
OC cores were harvested from knees of cadaveric tissue donors and from discarded fragments of OA knee surgery patients. Tissue donor cores were macroscopically normal, and OA cores had partial- or full-thickness erosion to bone. Cores were perfusion-tested to determine the hydraulic conductance, or ease of fluid flow, in their native state and after enzymatic removal of cartilage. Adjacent portions were analyzed by 3-D histology for CC, ScB, and ScBP thickness and vascular canal density.
Results
The hydraulic conductances of native OC tissue and ScBP were higher (2700- and 3-fold) in fully eroded samples than normal samples. The CC layer was thicker (1.5-fold) in partially eroded samples than normal samples, but thinner and incomplete in fully eroded samples. ScBP vascularity was altered with increasing stages of OA.
Conclusion
During joint loading, increased hydraulic conductance of the OC tissue and ScBP could have deleterious biomechanical consequences for cartilage. Increased fluid exudation from overlying and opposing cartilage, increased fluid depressurization, and increased cartilage tissue strains could lead to chondrocyte death and cartilage damage.
Introduction
Articular cartilage is a low-friction, load-bearing material joined to the subchondral bone plate at the ends of long bones that form a synovial joint. During joint loading, pressurization of interstitial fluid within cartilage protects it from high compressive strains (1, 2). The ability of cartilage to support load through interstitial fluid pressurization is dependent on its low hydraulic permeability. Hydraulic permeability describes the ease of fluid flow through a material, which for articular cartilage is governed by the extracellular matrix. Degenerative changes in cartilage that occur with osteoarthritis (OA) have been correlated with increased hydraulic permeability of cartilage (3–5). However, hydraulic permeability has been determined only for cartilage slices separated from the subchondral bone, not for full-thickness osteochondral tissue. Although fluid transport and resistance to fluid flow through osteochondral tissue is normally governed by cartilage, the contribution of the subchondral bone plate (ScBP) boundary may become increasingly important as the cartilage is eroded with the progression of OA.
In OA, progressive degeneration of the articular cartilage matrix is associated with remodeling of the ScBP. While cartilage decreases in thickness and mechanical integrity with the progression of OA, subchondral bone increases in thickness and stiffness, undergoes accelerated turnover (6), and exhibits altered trabecular architecture (7–9) and cysts (10). Although it is clear that progression of OA involves structural and mechanical changes in both cartilage and bone, it is unknown if such changes are associated with altered fluid transport characteristics of the osteochondral tissue.
While the ease of fluid flow through cartilage is traditionally described by hydraulic permeability, it is useful to describe the ease of fluid flow through osteochondral tissue and ScBP by the related structural property of hydraulic conductance. Since hydraulic conductance describes flow through a structure rather than a material, it can be used to characterize ease of fluid flow through irregular structures, such as osteochondral tissue with cartilage erosion or the undulating CC layer of ScBP. Thus, the hydraulic conductance can include the contribution from ScBP in determining fluid flow through an osteochondral structure, including how that role may change with erosion of cartilage in OA.
Although hydraulic permeability has been measured for cartilage (11), cortical bone (12), and cancellous bone (13), the fluid transport properties of the zone of calcified cartilage (CC) at the osteochondral interface remain unknown. In studies of cartilage nutrition, the CC has been considered an impermeable barrier to material transport from the subchondral bone, particularly after maturation of the joint (14). However, in vivo MRI studies have observed penetration by intravenous Gd(DTPA)2− across the osteochondral interface into the deep regions of human articular cartilage (15). Furthermore, at the osteochondral interface of the intervertebral disc studied ex vivo, fluid flows between the marrow cavity and the cartilaginous disc through vascular channels (16). This suggests that vascular channels in the ScBP may contribute to the fluid flow through, and hydraulic conductance of, the osteochondral interface.
The ScBP is the underlying support structure for articular cartilage in synovial joints. The ScBP begins at the tidemark, which separates uncalcified cartilage from CC, and consists of both the CC layer and the underlying subchondral bone (ScB). Calcified cartilage is composed of hypertrophic chondrocytes enveloped in a calcified matrix and is vascular (17, 18), whereas articular cartilage is normally avascular. The CC layer attaches cartilage to ScB (19, 20) and provides a transitional zone of intermediate stiffness, reducing stress concentrations at the cartilage-bone interface (21). Below the calcified cartilage is the cement line or ossification front which marks the beginning of the porous and vascular ScB.
The CC layer may play a critical role in OA pathogenesis by mediating interactions between cartilage and bone. Calcified cartilage becomes “activated” in OA, increasing in thickness with the formation of new zones of CC, duplications of the tidemark, and vascular invasion into the tidemark (20, 22, 23). Vascular invasion in the initiation or development of OA has been speculated to occur through microcracks that extend between the bone marrow space and the CC after repeated physiological loading (24, 25). The vascular canals in the CC may affect the fluid pressurization load-bearing behavior of the articular cartilage by affecting the hydraulic permeability of the underlying ScBP. Alteration of fluid flow across the cartilage-bone interface could affect the mechanical and chemical environment in ways that promote the progression of OA.
The hypothesis of this study was that the hydraulic conductance of osteochondral tissue and ScBP increases with structural changes indicative of increasing stages of OA. The objectives of this study were to determine, for the human medial femoral condyle with different grades of OA erosion: [1] the hydraulic conductance for osteochondral tissue before and after removal of cartilage, and [2] the thickness and vascularity of the CC and ScBP, as possible structural determinants of hydraulic conductance of ScBP.
Materials and Methods
Sample Harvest
Osteochondral cores (9mm diameter) were harvested from the medial femoral condyles of cadaveric tissue bank donors and discarded knee fragments from total knee replacement surgery patients with Institutional Review Board approval. Each core was obtained from a different donor, and adjacent osteochondral fragments were taken for histology. Tissue bank donor cores were macroscopically normal (n=12, age 24±3 years), and OA cores were graded by visual inspection as having partial erosion of cartilage (n=15, age 71±3 years) or full erosion of cartilage with exposure of bone (n=16, age 71±2 years).
Experimental Design
Cores from normal, partially eroded, and fully eroded ScBP were perfusion-tested first in the intact state at harvest and again after removal of cartilage by papain (Sigma-Aldrich, St Louis, MO). To prepare samples, an Isomet low-speed saw (Buehler, Lake Bluff, IL) was used to trim the bone side of cores to a ScBP thickness of 5mm, leaving the cartilage intact. Core diameter was measured with digital calipers, and all samples were perfusion-tested. Next, uncalcified cartilage was enzymatically removed from all cores by papain digestion (125 μg/mL papain, 0.005 M cysteine HCL, 0.1 M sodium phosphate, pH 6.2) at 60°C for 18–24h (18). Marrow was removed from papain-digested cores by incubation in an ultrasonic bath in saline for 2–5h followed by rinsing via perfusion of 50mL saline through the core in each direction. After rinsing, samples were perfusion-tested again. Additional 9mm-diameter cores (n=3) of normal and partially eroded ScBP were used to assess the effect of papain digestion on hydraulic conductance of bone. Uncalcified cartilage was removed from these samples by mechanical debridement with a curette to isolate ScBP, followed by trimming and marrow removal. Samples were perfusion tested for hydraulic conductance, papain-digested, and retested.
Perfusion Testing
Darcy’s Law was used to estimate the hydraulic conductance constant, c, using a least squares fit of linear fluid velocity, U, vs. pressure drop across the sample, ΔP. Darcy’s Law describes how easily fluid flows through a porous solid and is expressed as:
| (1) |
where U is the linear flow rate, with dimensions of [m/s], Q is the volumetric flow rate, with dimensions of [m3/s] and A is the sample cross-sectional area in the direction of flow, with dimensions of [m2]. Ease of fluid flow through a structure can be described by c, the hydraulic conductance with dimensions of [m/(Pa·s)], or by kp, the hydraulic permeability with dimensions of [m2/(Pa·s)], and h, the sample thickness with dimensions of [m]. For ScBP, c was assessed because the undulating CC layer has an irregular thickness, making it difficult to identify a value for h to allow calculation of kp. Thus, ease of fluid flow through samples was characterized as a hydraulic conductance constant for short-term perfusion, c2h for OC samples and c0.5h for ScBP samples (representing equilibrium values).
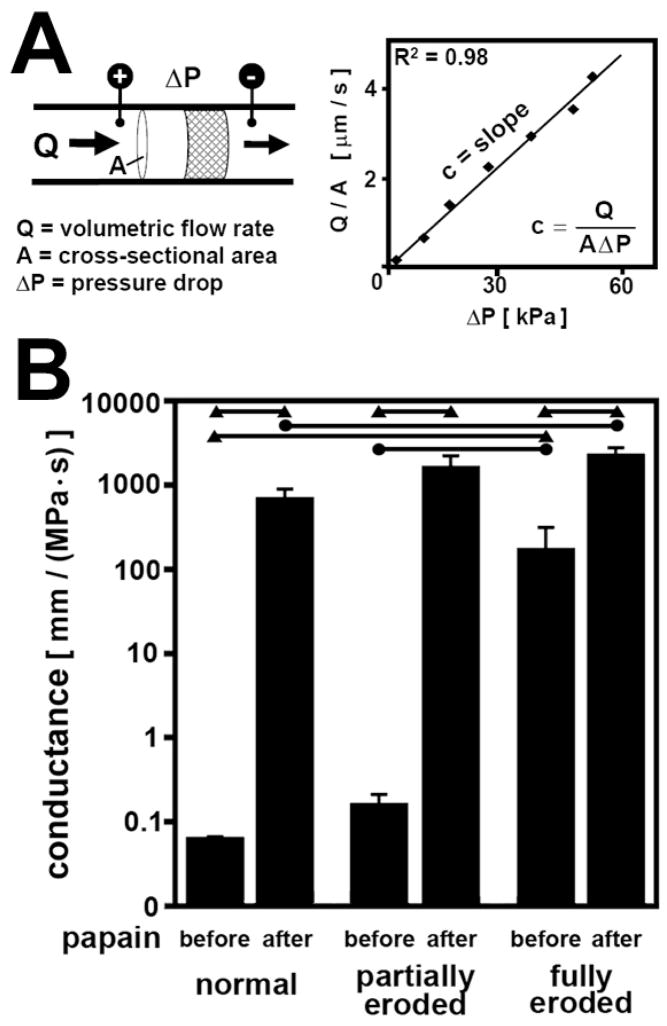
Each sample was inserted into Tygon tubing (Cole-Parmer, Vernon Hills, IL), sealed circumferentially, and tested for hydraulic conductance (Figure 1A). Phosphate-buffered saline (PBS) was perfused through each sample at constant flow rates controlled by a syringe pump (Harvard PHD-2000; Harvard Apparatus, Holliston, MA), and pressure drop across the sample was measured using a low-range pressure transducer (ΔP = 0–55 kPa) (Validyne DP-45, Validyne, Northridge, CA). Different flow rates were used for OC and ScBP cores to keep pressures within the range of the transducer. OC samples were perfused for 2h at flow rates, U, of 0.0013 mm/s and 0.0026 mm/s (or equivalent Q, 0.083 mm3/s and 0.17 mm3/s, normalized to A, 64 mm2). ScBP samples were perfused for 0.5h at flow rates starting from 0.026 mm/s and increased in increments of 0.65 mm/s up to 3.9 mm/s. The pressure drop across the sample at each flow rate was measured, averaging readings over 5s at 2 Hz. Samples were tested both with flow outwards from the joint (from cartilage to bone) and inwards into the joint (from bone to cartilage). Conductance estimates from different flow directions were reproducible (−16% to +20%) and averaged for each sample. Conductance estimates were also reproducible within the same sample in repeated measurements. Thus, three trials per sample per flow direction were used to obtain a best-fit conductance estimate for each sample using a least squares analysis (typical r2 = 0.98).
Figure 1.
(A) Schematic diagram of perfusion test setup and representative Darcy plot to estimate hydraulic conductance constant, c. (B) Effect of OA erosion and papain digestion on hydraulic conductance of osteochondral tissue and ScBP. Conductance values after 2h perfusion of normal, partially eroded, and fully eroded osteochondral tissue before and after removal of cartilage by papain digestion. ● = p<0.013; ▲ = p<0.0033.
Histology
Osteochondral fragments were taken adjacent to each core site; one was left intact and the other was papain-digested along with the core. Paired samples were fixed in 4% paraformaldehyde in PBS, pH 7.4, 4°C for 3 days and then decalcified in 15% EDTA, pH 8.5, 37°C for 3 weeks. For 3D histology, samples (n = 3) were cut to ~3 mm3 blocks and fluorescently stained with 0.1% Eosin-Y (pH 5.0) for 12–16h, embedded in Spurr resin, and imaged at (2.24 μm)3 voxel resolution with a Nikon (Tokyo, Japan) E600 fluorescence microscope (26). Three-dimensional image datasets were visualized using RESView 3.0 software (Resolution Sciences Corporation, Corte Madera, CA), and 2D cross-sections were exported for qualitative viewing and stereological analysis of structural features using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA) and ImageJ (NIH, Bethesda, MD).
Stereological measurements
CC and ScB thickness
The thickness of the CC and ScB was measured by averaging individual thickness measurements from histological sections of a control tissue volume. Ten random 2D cross-sections uniformly spaced by 0.1 mm were exported for a volume of tissue (1 mm)2 × 2 mm deep encompassing the osteochondral interface for each sample. On each vertical (1 mm × 2 mm) cross-section, a grid of 4 lines (each 0.2 mm apart) was overlaid on the image with the lines normal to the tissue surface. The thickness of the CC and ScB was measured along each of the 4 lines for a total of 40 individual measurements across all 10 cross-sections and averaged to produce one CC and one ScB thickness for each sample. The CC thickness was defined as the distance between the tidemark and the cement line, and the ScB thickness was defined as the distance between the cement line and the bottom edge of the cortical bone plate, or the boundary between the solid bone matrix and the large void space associated with trabecular bone porosity. ScBP thickness was the sum of the CC and ScB thicknesses. In OA partially eroded samples where multiple tidemarks were present, the CC thickness was measured between the lowest tidemark (closest to the bone) and the cement line.
Vascular canal density
The number of vessels per cross-sectional area was counted within the same tissue volume used for the thickness measurements. Fifty vertical 2D sections (1 mm × 2 mm) uniformly spaced 0.02 mm apart were exported from the tissue volume. Vessels were defined as void space sheathed by bone that started from the subchondral bone space and ended within the CC or beyond the CC into the cartilage deep zone (in partially eroded samples) or up to the bony ScBP surface (in fully eroded samples). Larger vessels appearing in multiple neighboring sections were counted only once.
Statistics
Data are presented as mean ± SEM. Conductance data were log10 transformed to improve homoscedasticity (27). Log10 transformed data were confirmed to be normally distributed by the Anderson-Darling test (P < 0.01) (28). To assess the effect of erosion and papain digestion on conductance, a repeated-measures ANOVA was used with a fixed factor of the degree of OA erosion (normal, partial, full) and with repeated measures for before and after digestion. Post-hoc comparisons were performed using unpaired t-tests between each group and its three relevant comparison groups (1 to compare before vs. after papain digestion, 2 to compare normal vs. partially eroded vs. fully eroded). For these t-tests, significance was adjusted (P < 0.05/3). To assess structural changes in thickness and vascularity, a repeated measures ANOVA was used with a fixed factor of the degree of OA erosion and with repeated measures for paired samples with and without papain digestion. Tukey post-hoc comparisons were used with P < 0.05 considered significant.
Results
Hydraulic conductance
Hydraulic conductance was found to be dependent on both OA erosion and papain digestion. With increasing severity of OA erosion, the hydraulic conductance of osteochondral samples and ScBP increased significantly (P < 0.01) (Figure 1B). The effect of OA erosion on conductance was evident both in the native state and after cartilage removal in ScBP samples. Osteochondral samples in their native state increased in hydraulic conductance from 0.065 mm/(MPa·s) for normal cartilage and 0.16 mm/(MPa·s) for partially eroded cartilage to 176 mm/(MPa·s) for fully eroded cartilage down to exposed bone. There was a 2700-fold increase in conductance from the normal to the fully eroded samples (P < 0.0033), and a 1000-fold increase from the partially eroded to the fully eroded samples (P < 0.017). Similar trends were evident after papain digestion of all samples to remove cartilage and isolate the ScBP. ScBP conductance followed an increasing trend from 702 mm/(MPa·s) for normal ScBP to 1673 mm/(MPa·s) for partially eroded ScBP to 2316 mm/(MPa·s) for fully eroded ScBP. There was a significant 3-fold increase between hydraulic conductance of normal and fully eroded ScBP (P < 0.017).
After removal of uncalcified cartilage by papain digestion, hydraulic conductance increased significantly for all grades of samples (Figure 1) (P < 0.001). Hydraulic conductance of normal and partially eroded OC samples increased 10800-fold (P < 0.0033) and 10500-fold (P < 0.0033), respectively, while hydraulic conductance of fully eroded samples increased 13-fold (P < 0.0033). For normal and partially eroded ScBP samples isolated by mechanical debridement, ScBP hydraulic conductance increased 2-fold (P < 0.05) after papain digestion.
Histology
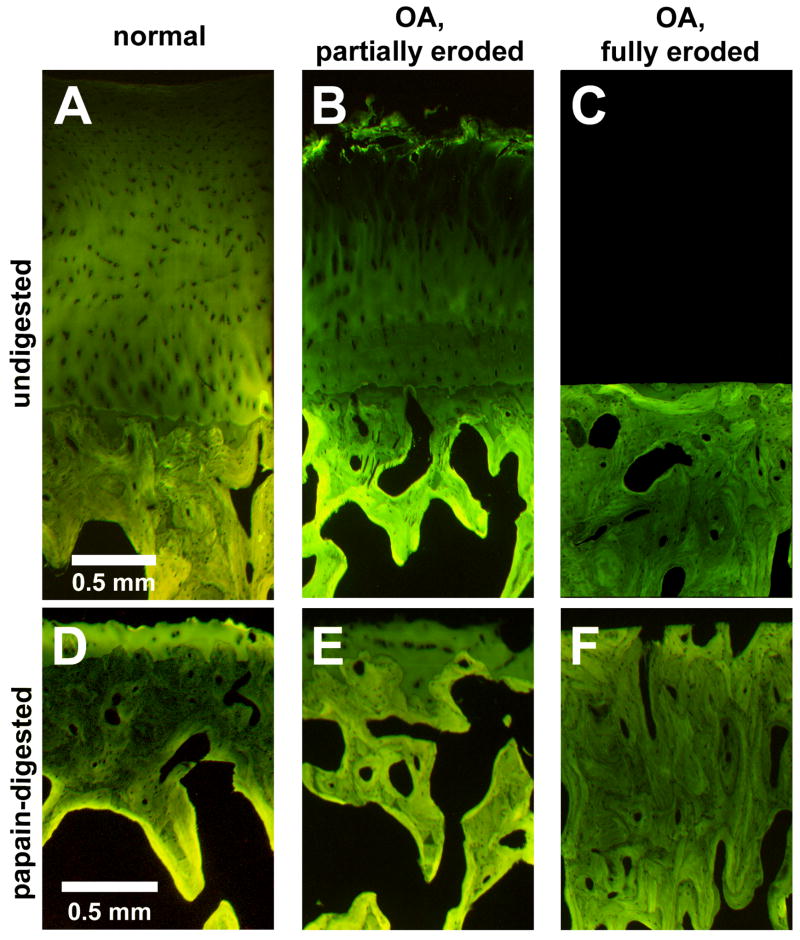
3D histology showed differences in CC and ScBP structure associated with degrees of degeneration. CC was histologically distinct from surrounding tissues in all groups. In normal and partially eroded samples, the CC layer bordered both the overlying uncalcified cartilage with a gently undulating tidemark and the underlying subchondral bone with a highly interdigitated cement line (Figures 2A, B, D, E). In fully eroded samples, the CC was an incomplete layer, appearing in irregular pockets at the smooth bony surface (Figure 2C).
Figure 2.
Normal (A,D), partially eroded (B,E), or fully eroded (C,F) osteochondral samples without (A,B,C) and with (D,E,F) articular cartilage removal by papain digestion
OA samples with partial-thickness erosion of cartilage exhibited fibrillated cartilage, multiple tidemarks, and a thickened CC layer (Figure 2B, E). Papain digestion removed uncalcified cartilage but appeared to preserve the entire CC below the lowest tidemark (Figures 2D, E). OA samples with full-thickness erosion of cartilage with exposure of bone had a smooth bony surface and a thick, dense ScBP (Figures 2C, F), with an incomplete CC layer present in irregular pockets at the surface (Figure 2C). Papain digestion appeared to open up more void space and channels through the ScBP (Figure 2F).
Vascularity was present in normal ScBP as well as OA partial- and full-thickness erosion ScBP, but differed in appearance. In normal ScBP, vascular canals were most commonly seen as long finger-like protrusions sheathed by bone originating from the marrow space and ending within the CC (Figure 3A). In partially eroded ScBP, vascular canals appeared greater in size and number, either ending in the CC or protruding above the CC into deep zone cartilage nearing the duplicate tidemark (Figure 3B). In fully eroded ScBP, vascularity appeared as large, interconnected void spaces or channels starting from within the thickened bone plate and ending at the smooth, bony surface (Figures 2F, 3C).
Figure 3.
Typical vascular canals penetrating (A) into calcified cartilage in normal ScBP, (B) into deep zone cartilage in partially eroded ScBP, and (C) to the surface of fully eroded ScBP.
Stereological measurements
CC and ScB thickness varied with the degree of OA erosion but were not altered by papain digestion. Papain digestion did not have an effect on CC thickness (P = 0.46) or ScB thickness (P = 0.41), which was consistent with qualitative histological observations (Figure 4A). The grade of OA erosion did have a significant effect on both CC thickness (P < 0.01) and ScB thickness (P < 0.01). The CC thickness in partially eroded samples was 157 μm, ~1.5-fold greater than normal (P < 0.01), while the CC thickness in fully eroded samples was 54 μm, ~2-fold less than normal (P < 0.01) and ~3-fold less than in the partially eroded samples (P < 0.001). The ScB thickness of fully eroded samples was 911 μm, ~2-fold greater than that of both normal (P < 0.01) and partially eroded samples (P < 0.01).
Figure 4.
Stereological measurements of subchondral bone plate structure. (A) Thickness of calcified cartilage (black) and underlying subchondral bone (white). Total height of each column is ScBP thickness. (B) Number of vessels penetrating the CC/bone interface per cross-sectional area for normal, partially eroded and fully eroded osteochondral samples before and after cartilage removal by papain digestion. ● = p<0.013; ▲ = p<0.0033.
The density of vessels penetrating into the CC layer or ScBP surface varied with the degree of OA erosion but was not affected by papain digestion. Papain digestion did not have a significant effect on the density of vessels penetrating into or beyond the CC (P = 0.27) (Figure 4B). The grade of OA erosion did have a significant effect on density of vessels (P < 0.01), displaying a similar trend to the one seen for CC thickness. Partially eroded ScBP tended to have the greatest density of vessels penetrating the CC, with 19 vessels per mm2.
Discussion
The results described here indicate that hydraulic conductance of both osteochondral tissue and the isolated ScBP is increased with increasing stage of OA cartilage erosion, in association with structural changes in CC and ScB thickness and vascularity. Increased hydraulic conductance allows for greater ease of interstitial fluid flow through the osteochondral tissue and ScBP. Compared to normal OC tissue and ScBP, partially-eroded OC tissue and ScBP exhibited no significant difference in hydraulic conductance (Figure 1). However, structural changes were evident in partially eroded ScBP, with increased CC thickness (1.5-fold), a trend for increased density of vascular canals penetrating the CC, and larger vascular canals (Figures 2AB, 3AB, 4). Compared to normal tissues, fully-eroded OC tissue and ScBP exhibited a marked increase (2700-fold and 3-fold, respectively) in hydraulic conductance (Figure 1). Structural changes were also evident in fully eroded ScBP, with the appearance of discontinuities in CC, increased ScB thickness (2-fold), decreased density of vascular canals penetrating the ScBP surface, and larger void spaces within the ScBP (Figures 2BC, 3BC, 4). In the progression from normal OC tissue into the partially- and fully-eroded stages of OA, changes in CC thickness and vascularity occur together with increases in hydraulic conductance and ScB thickness. These results suggest that the CC structural and vascular remodeling that occurs with OA contributes to increased ease of fluid flow across the osteochondral structure, a factor which may play a role in advancing OA degeneration (Figure 5).
Figure 5.
Schematic of normal (A), partially eroded (B), and fully eroded (C) osteochondral tissue and potential deleterious effects of increased ScBP permeability leading to increased fluid loss. Wavy arrows indicate fluid flow within cartilage and also fluid loss from from overlying (B) and opposing (C) articular cartilage during loading. Plus signs indicate magnitude of osteochondral and ScBP hydraulic conductance.
The ScBP was isolated from OC tissue with a method that may have affected the hydraulic conductance results. In human ScBP preparations, papain digestion selectively removes uncalcified cartilage down to the tidemark while maintaining CC thickness and contour but not creating perforations through the ScBP (18). Such ScBP preparations are devoid not only of cartilage but also of marrow and soft tissue linings of vascular canals, which could participate in modulating fluid transport across the ScBP. Papain digestion may also remove proteins such as collagen from bone, which could affect the ScBP hydraulic conductance. Papain digestion of normal and partially eroded ScBP (which had been isolated mechanically) increased conductance 2-fold, whereas papain digestion of fully eroded samples increased conductance ~13-fold (Figure 1B). Thus, the estimate of hydraulic conductance of ScBP, as performed in the current study with papain digestion, is likely to be higher than ScBP conductance in vivo. Nevertheless, papain digestion was useful as a repeatable, non-destructive method for removing uncalcified cartilage while preserving CC structure.
The two-hour hydraulic conductance constant (c2h) evaluated in the present study is an upper bound estimate of the overall conductance of full-thickness OC tissue. Hydraulic conductance of OC tissue is determined by the low fluid permeability of the cartilage matrix, which dominates the fluid pressurization behavior and resistance to flow. As fluid enters the cartilage and flows from the superficial towards the deep zone, it exerts drag forces to pull the solid matrix with it. The drag forces result in matrix compaction, which decreases porosity and permeability within the tissue in a depth-varying, non-uniform manner (29–31). A new steady-state is reached when the matrix consolidation and fluid flow through the tissue are balanced, maintaining a constant pressure across the tissue. In the present study, perfusion flow time was standardized to 2 hours, as a physiologically relevant period of loading, and to allow calculation of c2h. At 2h, full-thickness OC tissue would not be fully compacted, and c2h is higher than that at steady-state (cSS). For normal OC tissue, c2h is greater than cSS by ~5-fold (data not shown). For porous, permeable materials with a stiff solid matrix, such as ScBP, the effect of flow-dependent matrix consolidation is negligible, so steady-state is reached on a shorter time scale; for ScBP, c0.5h and cSS were similar (data not shown).
The differences in permeability and structure between normal and OA articular cartilage and ScBP extend the findings of previous studies. The present study extends permeability measurements, traditionally made on isolated cartilage and bone tissues, by directly analyzing the full thickness of articular cartilage still attached to ScBP, providing an overall conductance for the osteochondral unit. Previous measurements of hydraulic permeability on cartilage sections elucidated variations in zonal properties (32) and may be influenced by alterations in matrix organization and water content due to detachment from the underlying CC (11, 33). The c2h and c0.5h of normal osteochondral tissue and ScBP determined in the present study can be converted into corresponding apparent hydraulic permeability (kp) values using average thicknesses for cartilage and ScBP, allowing comparison to previous studies (Table 1). The apparent kp of normal cartilage attached to ScBP in the present study was greater than values from literature for normal cartilage (1, 11, 34). This can be attributed in part to the 2-hour perfusion time, which did not allow the full-thickness cartilage to reach steady-state hydrostatic pressurization and associated matrix compaction, resulting in a higher apparent permeability and precluding direct comparison to literature values of kp, which reflect steady-state. The kp of normal ScBP found in the current study, which reached steady-state on a shorter time scale (<0.5hr), was within the range of values reported for normal cortical and cancellous bone (Table 1) (13, 35, 36).
Table 1. Hydraulic Permeability, kp, of Articular Cartilage and ScBP.
Values are from the current study and others, where noted.
| tissue | type | kp [mm2/(MPa·s)] | average thickness [mm] |
|---|---|---|---|
| cartilage1, 11, 32 | normal | 0.0001 – 0.002 | 0.3 – 0.8 |
| cartilage (+ScBP) | normal | 0.15 | 2.5 |
| OA, partially eroded | 0.27 | 1.8 | |
| cortical bone33, 34 | normal (canine) | 40–80 | 0.5 – 1.0 |
| ScBP | normal | 90 | 0.47 |
| OA, partially eroded | 140 | 0.46 | |
| OA, fully eroded | 190 | 1.1 | |
| ScBP (papain-digested) | normal | 350 | 0.49 |
| OA, partially eroded | 830 | 0.50 | |
| OA, fully eroded | 2,010 | 0.87 | |
| cancellous bone13, 33 | normal | 20,000 – 8,000,000 | 1.0 |
The trend for a small increase in hydraulic conductance of OA OC tissue is consistent with slight increases in hydraulic permeability of OA articular cartilage reported in previous studies (3–5). Increased hydraulic conductance of OC tissue occurring with OA may be due primarily to decreased cartilage thickness, increased hydraulic permeability of the remaining OA cartilage, or a combination of both. However, in the current study, hydraulic conductance of partially eroded OC tissue was not significantly different from normal despite cartilage thickness being 30% lower. Since fluid pressurization is normally maintained by the low hydraulic permeability of deep zone cartilage (31, 32, 37), the overall hydraulic conductance of OC tissue may remain close to normal for cases of partial erosion where the deep zone remains intact. For OC tissue with full-thickness erosion of cartilage, the relatively high hydraulic conductance may be determined by any residual cartilage in the ScBP.
Increased hydraulic conductance of osteochondral tissue and the ScBP could have deleterious biomechanical consequences for cartilage during joint loading (Figure 5). Normally, articular cartilage is able to support joint loads through interstitial fluid pressurization due to its low hydraulic conductance (Figure 5A). Fluid pressurization in cartilage plays a major role in providing load support for a prolonged duration after contact, preventing the solid matrix from deforming to an equilibrium strain state (38). The duration of load support by fluid pressurization can be characterized using a time constant to reach equilibrium that depends on factors affecting the rate of fluid loss from cartilage, including the radial path length, compressive modulus, and hydraulic permeability (39). For normal human cartilage with contact radius, a, of 10 mm, compressive modulus, HA, of 0.5 MPa and kp of 2×10−15 m4/(N·s), the characteristic time constant, a2/(HA·kp), is ~28 hrs, thus protecting cartilage for long durations of load. In OA, increased fluid loss from cartilage during joint loading may occur due to fluid exudation into the underlying ScBP, causing shorter times to equilibrium and potentially leading to large deformations and accelerated cartilage degeneration (Figure 5B).
For full-thickness cartilage erosion with exposed ScBP, the articular cartilage of the opposing joint surface may be damaged through fluid exudation from its superficial zone into and through the eburnated and permeable joint surface (Figure 5C). The depth of the opposing cartilage that undergoes fluid depressurization and concomitant tissue consolidation would depend on the time characteristics of loading. The characteristic time to equilibrium for the opposing cartilage, using confined compression analysis of normal human cartilage with 2 mm thickness, is ~1 hr (40). At the relatively high physiological frequency of gait (0.5 Hz), the characteristic depth of fluid depressurization extends ~40 μm from the articular surface. With a prolonged loading duration typical of standing, e.g. for periods of 15 min (0.001 Hz), the fluid depressurization extends ~1 mm from the surface(34). Even for a short loading duration of 2 min and a conservative contact stress of 0.5 MPa, the opposing cartilage surface would experience a deformation of ~0.2mm (40), which could lead to localized cell death, as when the superficial zone is compressed against a porous platen in vitro (41). Thus, the biomechanical consequences for increased ScBP permeability in OA may contribute to overlying cartilage degeneration as well as the spreading of OA onto the opposing joint surface.
In the normal joint, the CC interface may function as a zone of intermediate hydraulic permeability between articular cartilage and subchondral bone, similar to its role as a zone of intermediate stiffness for the transfer of mechanical loads. Although the CC layer in mature joints has previously been considered impermeable (14, 42), this perception may have been due to low rates of solute diffusion and convection into the adjacent cartilage deep zone rather than low hydraulic permeability of the CC layer itself. In the present study, eburnated ScBP with eroded CC had higher hydraulic conductance than normal ScBP with intact CC, in spite of the greater ScB thickness and density from eburnation which would be expected to decrease conductance. Thus, an intact CC layer appears to be more permeable to fluid than articular cartilage but less permeable than cortical bone, making it a zone of intermediate permeability between deep zone cartilage and ScB in the normal osteochondral junction. With the onset of OA, the relative permeabilities of cartilage, CC and ScB in the osteochondral junction may be altered, which may disrupt the homeostasis of the osteochondral unit and affect progression of the disease.
OA ScBP may undergo additional structural changes due to the intrusion of fluid into eroded or permeable areas, including the development of cartilaginous pockets and bone marrow lesions. Hydraulic conductance of fully eroded ScBP increased 10-fold along with the appearance of void pockets and channels after papain digestion, consistent with residual cartilage within the ScBP. Cartilaginous pockets in OA joints with exposed bone have been documented both within and at the surface of the ScBP and may participate in the repair process (43–45). The void areas may also represent bone cysts or bone marrow lesions found in sclerotic bone which are associated with pain in OA (46, 47). Bone cysts may arise from intrusion of pressurized fluid into bone at the joint surface, as suggested by openings between cysts and the joint cavity and their rounded morphology (10).
The increase in hydraulic conductance of osteochondral tissue and ScBP in OA allows more direct fluid movement between cartilage and bone compared to the normal joint. Fluid movement can, in turn, act as a mechanical signal to cells and enhance the transport of diffusible factors, processes that may mediate the crosstalk between cartilage and bone. In OA, altered boundary conditions at the osteochondral interface may disrupt the mechanical and chemical interactions between the microenvironments of cartilage and bone, which may affect tissue homeostasis and cell physiology. Understanding how altered boundary conditions affect the mechanical and chemical interactions between cartilage and bone will lead to a more complete picture of the multi-tissue pathogenesis of OA.
Acknowledgments
Supported by the NIH, NSF, MTF, and an award to the University of California, San Diego under the Howard Hughes Medical Institute Professors Program (RLS). Additional individual support was received from the NSF Graduate Research Fellowship Program (JH).
References
- 1.McCutchen CW. The frictional properties of animal joints. Wear. 1962;5:1–17. [Google Scholar]
- 2.Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–34. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong CG, Mow VC. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J Bone Joint Surg Am. 1982;64-A:88–94. [PubMed] [Google Scholar]
- 4.Brocklehurst R, Bayliss MT, Maroudas A, Coysh HL, Freeman MA, Revell PA, et al. The composition of normal and osteoarthritic articular cartilage from human knee joints. With special reference to unicompartmental replacement and osteotomy of the knee. J Bone Joint Surg Am. 1984;66(1):95–106. [PubMed] [Google Scholar]
- 5.Setton LA, Mow VC, Muller FJ, Pita JC, Howell DS. Mechanical properties of canine articular cartilage are significantly altered following transection of the anterior cruciate ligament. J Orthop Res. 1994;12:451–463. doi: 10.1002/jor.1100120402. [DOI] [PubMed] [Google Scholar]
- 6.Burr DB. The importance of subchondral bone in osteoarthrosis. Curr Opin Rheumatol. 1998;10(3):256–62. doi: 10.1097/00002281-199805000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Christensen P, Kjaer J, Melsen F, Nielsen HE, Sneppen O, Vang PS. The subchondral bone of the proximal tibial epiphysis in osteoarthritis of the knee. Acta Orthop Scand. 1982;53(6):889–95. doi: 10.3109/17453678208992844. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu M, Tsuji H, Matsui H, Katoh Y, Sano A. Morphometric analysis of subchondral bone of the tibial condyle in osteoarthrosis. Clin Orthop Relat Res. 1993;(293):229–39. [PubMed] [Google Scholar]
- 9.Kamibayashi L, Wyss UP, Cooke TD, Zee B. Trabecular microstructure in the medial condyle of the proximal tibia of patients with knee osteoarthritis. Bone. 1995;17(1):27–35. doi: 10.1016/8756-3282(95)00137-3. [DOI] [PubMed] [Google Scholar]
- 10.Landells JW. The bone cysts of osteoarthritis. J Bone Joint Surg Br. 1953;35-B(4):643–9. doi: 10.1302/0301-620X.35B4.643. [DOI] [PubMed] [Google Scholar]
- 11.Maroudas A, Bullough P. Permeability of articular cartilage. Nature. 1968;219:1260–1. doi: 10.1038/2191260a0. [DOI] [PubMed] [Google Scholar]
- 12.Johnson M, Katz JL. Some new developments in the rheology of bone. Biorheology Suppl. 1984;1:169–74. doi: 10.3233/bir-1984-23s130. [DOI] [PubMed] [Google Scholar]
- 13.Nauman EA, Fong KE, Keaveny TM. Dependence of intertrabecular permeability on flow direction and anatomic site. Ann Biomed Eng. 1999;27(4):517–24. doi: 10.1114/1.195. [DOI] [PubMed] [Google Scholar]
- 14.Maroudas A, Bullough P, Swanson SA, Freeman MA. The permeability of articular cartilage. J Bone Joint Surg Br. 1968;50(1):166–77. [PubMed] [Google Scholar]
- 15.Bashir A, Gray ML, Boutin R, Burstein D. In vivo imaging of GAG in articular cartilage using delayed Gd(DTPA)2- enhanced MRI. Radiology. 1997;205:551–8. doi: 10.1148/radiology.205.2.9356644. [DOI] [PubMed] [Google Scholar]
- 16.Ayotte DC, Ito K, Tepic S. Direction-dependent resistance to flow in the endplate of the intervertebral disc: an ex vivo study. J Orthop Res. 2001;19(6):1073–7. doi: 10.1016/S0736-0266(01)00038-9. [DOI] [PubMed] [Google Scholar]
- 17.Clark JM. The structure of vascular channels in the subchondral plate. J Anat. 1990;171:105–15. [PMC free article] [PubMed] [Google Scholar]
- 18.Clark JM, Huber JD. The structure of the human subchondral plate. J Bone Joint Surg Br. 1990;72(5):866–73. doi: 10.1302/0301-620X.72B5.2211774. [DOI] [PubMed] [Google Scholar]
- 19.Green WT, Jr, Martin GN, Eanes ED, Sokoloff L. Microradiographic study of the calcified layer of articular cartilage. Arch Pathol. 1970;90(2):151–8. [PubMed] [Google Scholar]
- 20.Oegema T, Jr, Carpenter R, Hofmeister F, Thompson RC., Jr The interaction of the zone of calcified cartilage and subchondral bone in osteoarthritis. Microsc Res Tech. 1997;37:324–32. doi: 10.1002/(SICI)1097-0029(19970515)37:4<324::AID-JEMT7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Mente PL, Lewis JL. Elastic modulus of calcified cartilage is an order of magnitude less than that of subchondral bone. J Orthop Res. 1994;12:637–647. doi: 10.1002/jor.1100120506. [DOI] [PubMed] [Google Scholar]
- 22.Oettmeier R, Abendroth K, Oettmeier S. Analyses of the tidemark on human femoral heads. II. Tidemark changes in osteoarthrosis--a histological and histomorphometric study in non-decalcified preparations. Acta Morphol Hung. 1989;37(3–4):169–80. [PubMed] [Google Scholar]
- 23.Dequeker J, Mokassa L, Aerssens J, Boonen S. Bone density and local growth factors in generalized osteoarthritis. Microsc Res Tech. 1997;37(4):358–71. doi: 10.1002/(SICI)1097-0029(19970515)37:4<358::AID-JEMT10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 24.Sokoloff L. Microcracks in the calcified layer of articular cartilage. Arch Pathol Lab Med. 1993;117:191–195. [PubMed] [Google Scholar]
- 25.Mori S, Harruff R, Burr DB. Microcracks in articular calcified cartilage of human femoral heads. Arch Pathol Lab Med. 1993;117:196–198. [PubMed] [Google Scholar]
- 26.Jadin KD, Wong BL, Bae WC, Li KW, Williamson AK, Schumacher BL, et al. Depth-varying density and organization of chondrocyte in immature and mature bovine articular cartilage assessed by 3-D imaging and analysis. J Histochem Cytochem. 2005;53:1109–1119. doi: 10.1369/jhc.4A6511.2005. [DOI] [PubMed] [Google Scholar]
- 27.Glantz SA. Primer of Biostatistics. 3. San Francisco, CA: McGraw-Hill, Inc; 1992. [Google Scholar]
- 28.Anderson TW, Darling DA. Asymptotic theory of certain “goodness of fit” criteria based on stochastic processes. Ann Math Statist. 1952;23(2):193–212. [Google Scholar]
- 29.Mansour JM, Mow VC. The permeability of articular cartilage under compressive strain and at high pressures. J Bone Joint Surg Am. 1976;58-A:509–16. [PubMed] [Google Scholar]
- 30.Mow VC, Holmes MH, Lai WM. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech. 1984;17:377–394. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- 31.Chen AC, Bae WC, Schinagl RM, Sah RL. Depth- and strain-dependent mechanical and electromechanical properties of full-thickness bovine articular cartilage in confined compression. J Biomech. 2001;34:1–12. doi: 10.1016/s0021-9290(00)00170-6. [DOI] [PubMed] [Google Scholar]
- 32.Maroudas A. Physicochemical properties of cartilage in the light of ion exchange theory. Biophys J. 1968;8:575–95. doi: 10.1016/S0006-3495(68)86509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keinan-Adamsky K, Shinar H, Navon G. The effect of detachment of the articular cartilage from its calcified zone on the cartilage microstructure, assessed by 2H-spectroscopic double quantum filtered MRI. J Orthop Res. 2005;23(1):109–17. doi: 10.1016/j.orthres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Frank EH, Grodzinsky AJ. Cartilage electromechanics-II. A continuum model of cartilage electrokinetics and correlation with experiments. J Biomech. 1987;20:629–39. doi: 10.1016/0021-9290(87)90283-1. [DOI] [PubMed] [Google Scholar]
- 35.Sander EA, Nauman EA. Permeability of musculoskeletal tissues and scaffolding materials: experimental results and theoretical predictions. Crit Rev Biomed Eng. 2003;31(1–2):1–26. doi: 10.1615/critrevbiomedeng.v31.i12.10. [DOI] [PubMed] [Google Scholar]
- 36.Li GP, Bronk JT, An KN, Kelly PJ. Permeability of cortical bone of canine tibiae. Microvasc Res. 1987;34(3):302–10. doi: 10.1016/0026-2862(87)90063-x. [DOI] [PubMed] [Google Scholar]
- 37.Setton LA, Zhu W, Mow VC. The biphasic poroviscoelastic behavior of articular cartilage: role of the surface zone in governing the compressive behavior. J Biomech. 1993;26:581–592. doi: 10.1016/0021-9290(93)90019-b. [DOI] [PubMed] [Google Scholar]
- 38.Ateshian GA, Lai WM, Zhu WB, Mow VC. An asymptotic solution for the contact of two biphasic cartilage layers. J Biomech. 1994;27:1347–1360. doi: 10.1016/0021-9290(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong CG, Lai WM, Mow VC. An analysis of the unconfined compression of articular cartilage. J Biomech Eng. 1984;106:165–73. doi: 10.1115/1.3138475. [DOI] [PubMed] [Google Scholar]
- 40.Kwan MK, Lai WM, Mow VC. Fundamentals of fluid transport through cartilage in compression. Ann Biomed Eng. 1984;12:537–558. doi: 10.1007/BF02371448. [DOI] [PubMed] [Google Scholar]
- 41.Milentijevic D, Torzilli PA. Influence of stress rate on water loss, matrix deformation and chondrocyte viability in impacted articular cartilage. J Biomech. 2005;38:493–502. doi: 10.1016/j.jbiomech.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Collins DH. The pathology of articular and spinal disease. London: Arnold; 1949. [Google Scholar]
- 43.Meachim G, Osborne GV. Repair at the femoral articular surface in osteo-arthritis of the hip. J Pathol. 1970;102(1):1–8. doi: 10.1002/path.1711020102. [DOI] [PubMed] [Google Scholar]
- 44.Mankin HJ. The reaction of articular cartilage to injury and osteoarthritis (Part II) N Engl J Med. 1974;291:1335–1340. doi: 10.1056/NEJM197412192912507. [DOI] [PubMed] [Google Scholar]
- 45.Zhang D, Johnson LJ, Hsu HP, Spector M. Cartilaginous deposits in subchondral bone in regions of exposed bone in osteoarthritis of the human knee: Histomorphometric study of PRG4 distribution in osteoarthritic cartilage. J Orthop Res. 2007 doi: 10.1002/jor.20344. [DOI] [PubMed] [Google Scholar]
- 46.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134(7):541–9. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 47.Carrino JA, Blum J, Parellada JA, Schweitzer ME, Morrison WB. MRI of bone marrow edema-like signal in the pathogenesis of subchondral cysts. Osteoarthritis Cartilage. 2006;14(10):1081–5. doi: 10.1016/j.joca.2006.05.011. [DOI] [PubMed] [Google Scholar]