Abstract
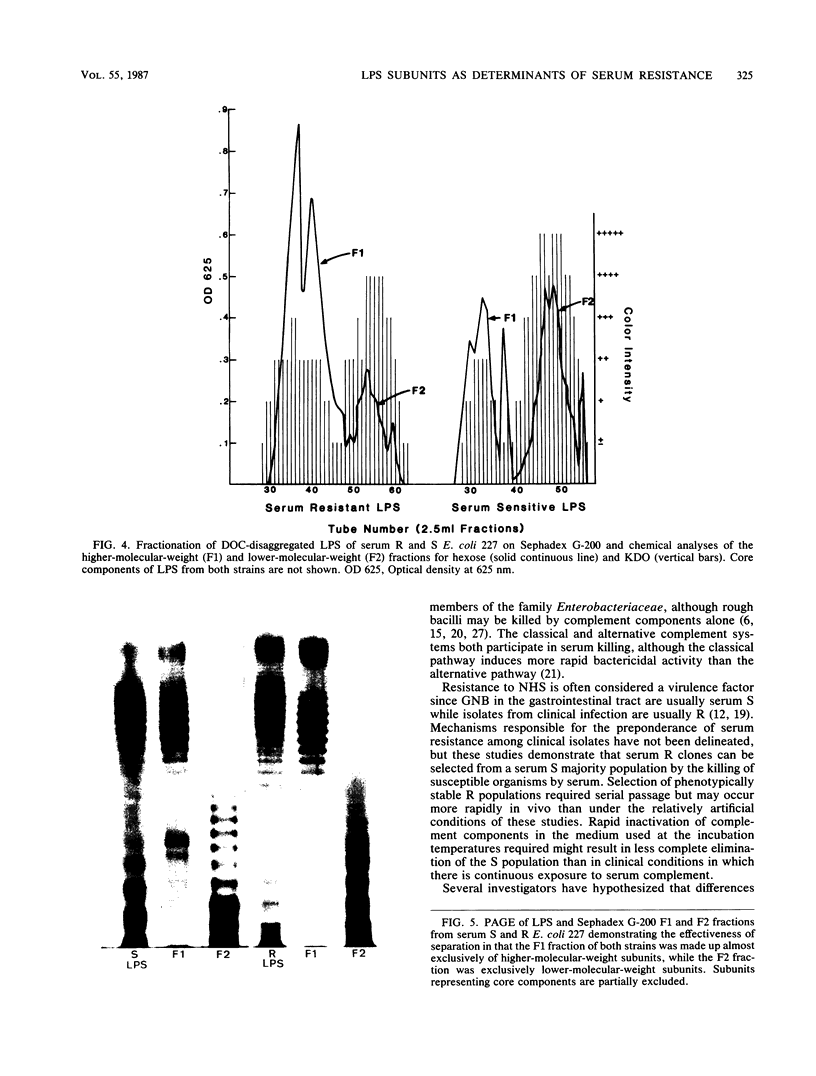
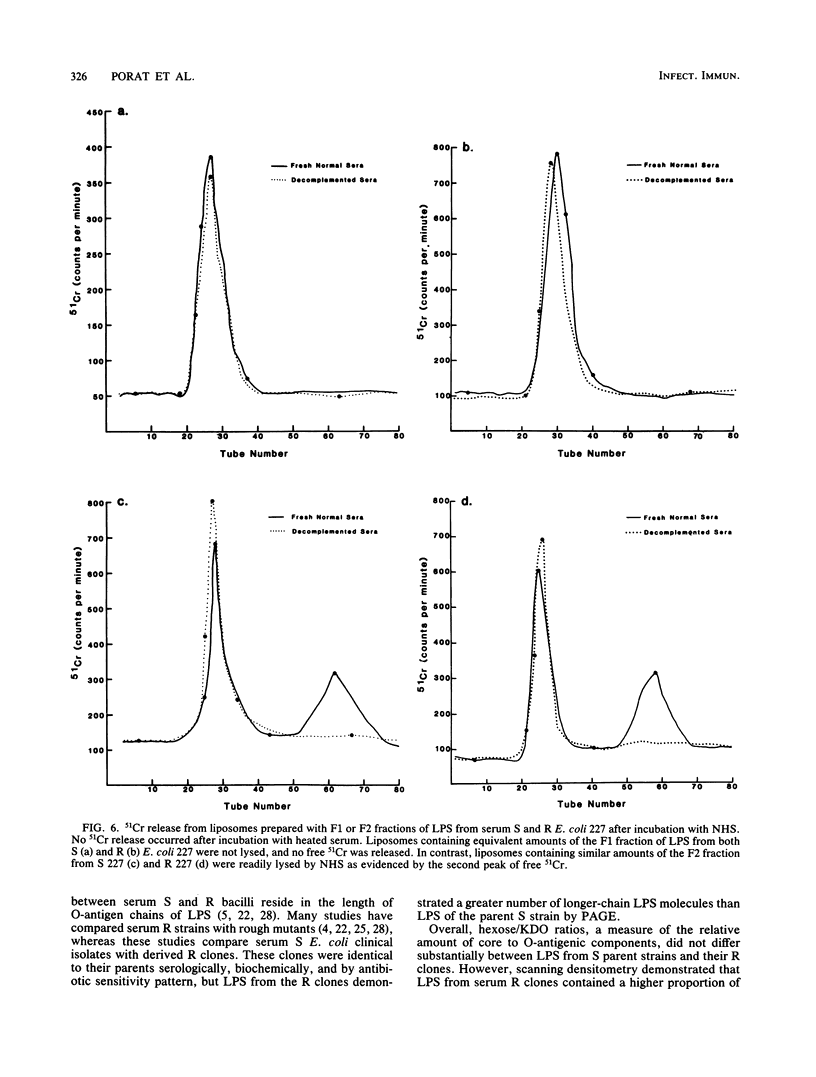
Differences in molecular composition of lipopolysaccharides (LPS) between serum-sensitive (S) clinical isolates of Escherichia coli and serum-resistant (R) clones derived by serial passage in serum were demonstrated to determine sensitivity or resistance to killing by normal human serum (NHS). LPS from R clones had a greater proportion of higher-molecular-weight, more highly O-antigen-substituted subunits than LPS from their serum S parents. Utilization of a liposomal model with inserted LPS simulating bacterial cell walls established LPS as the site of serum bactericidal action. Liposomes containing S LPS were lysed, while liposomes containing R LPS were unaffected by NHS. R and S LPS were fractionated into higher (F1)- and lower (F2)-molecular-weight fractions. Liposomes containing R LPS or the F1 fraction of S and R LPS were not lysed by serum. Liposomes containing the F2 fraction of S or R LPS were lysed by serum analogous to that observed with liposomes containing intact S LPS. These findings establish LPS to be one site of serum bactericidal activity and demonstrate that the higher-molecular-weight, highly O-antigen-substituted LPS subunits mediate resistance to killing by NHS.
Full text
PDF

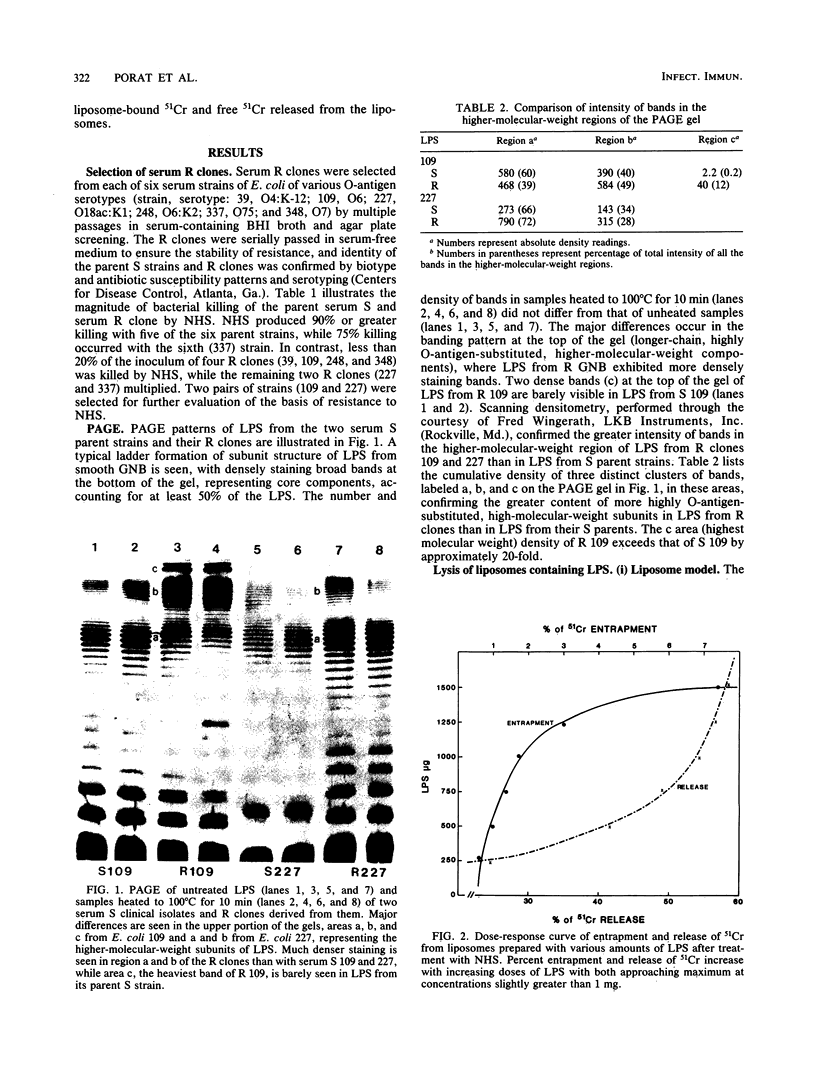
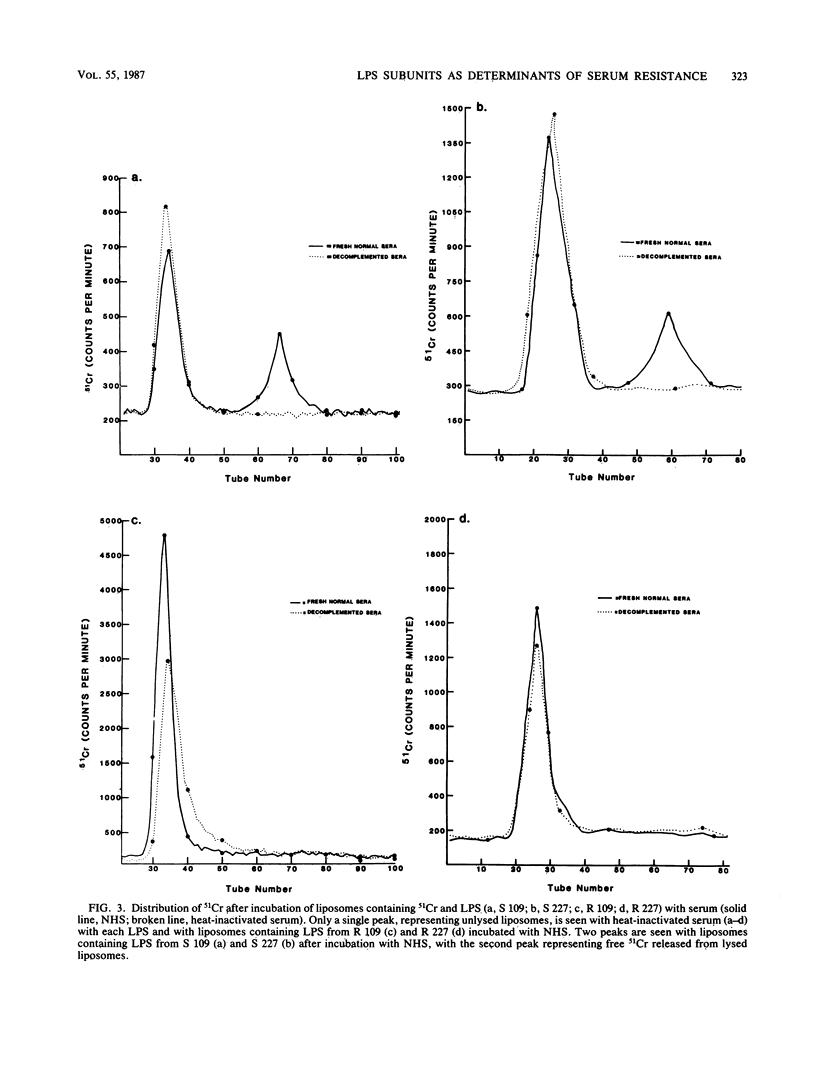



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DuPont H. L., Formal S. B., Hornick R. B., Snyder M. J., Libonati J. P., Sheahan D. G., LaBrec E. H., Kalas J. P. Pathogenesis of Escherichia coli diarrhea. N Engl J Med. 1971 Jul 1;285(1):1–9. doi: 10.1056/NEJM197107012850101. [DOI] [PubMed] [Google Scholar]
- Fierer J., Finley F., Braude A. I. A plaque assay on agar for detection of gram-negative bacilli sensitive to complement. J Immunol. 1972 Nov;109(5):1156–1158. [PubMed] [Google Scholar]
- Goldman J. N., Ruddy S., Austen K. F., Feingold D. S. The serum bactericidal reaction. 3. Antibody and complement requirements for killing a rough Escherichia coli. J Immunol. 1969 Jun;102(6):1379–1387. [PubMed] [Google Scholar]
- Goldman R. C., Joiner K., Leive L. Serum-resistant mutants of Escherichia coli O111 contain increased lipopolysaccharide, lack an O antigen-containing capsule, and cover more of their lipid A core with O antigen. J Bacteriol. 1984 Sep;159(3):877–882. doi: 10.1128/jb.159.3.877-882.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Yonemasu K., Takamizawa A., Amano T. [Studies on the immune bacteriolysis. XIV. Requirement of all nine components of complement for immune bacteriolysis]. Biken J. 1968 Sep;11(3):203–206. [PubMed] [Google Scholar]
- Johanson W. G., Jr, Woods D. E., Chaudhuri T. Association of respiratory tract colonization with adherence of gram-negative bacilli to epithelial cells. J Infect Dis. 1979 Jun;139(6):667–673. doi: 10.1093/infdis/139.6.667. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Inoue K., Lüderitz O., Kinsky S. C. Antibody- and complement-dependent damage to liposomes prepared with bacterial lipopolysaccharides. Eur J Biochem. 1971 Jul 15;21(1):80–85. doi: 10.1111/j.1432-1033.1971.tb01442.x. [DOI] [PubMed] [Google Scholar]
- Kinsky S. C., Haxby J. A., Zopf D. A., Alving C. R., Kinsky C. B. Complement-dependent damage to liposomes prepared from pure lipids and Forssman hapten. Biochemistry. 1969 Oct;8(10):4149–4158. doi: 10.1021/bi00838a036. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCabe W. R., Kaijser B., Olling S., Uwaydah M., Hanson L. A. Escherichia coli in bacteremia: K and O antigens and serum sensitivity of strains from adults and neonates. J Infect Dis. 1978 Jul;138(1):33–41. doi: 10.1093/infdis/138.1.33. [DOI] [PubMed] [Google Scholar]
- Mintz C. S., Apicella M. A., Morse S. A. Electrophoretic and serological characterization of the lipopolysaccharide produced by Neisseria gonorrhoeae. J Infect Dis. 1984 Apr;149(4):544–552. doi: 10.1093/infdis/149.4.544. [DOI] [PubMed] [Google Scholar]
- Moll A., Manning P. A., Timmis K. N. Plasmid-determined resistance to serum bactericidal activity: a major outer membrane protein, the traT gene product, is responsible for plasmid-specified serum resistance in Escherichia coli. Infect Immun. 1980 May;28(2):359–367. doi: 10.1128/iai.28.2.359-367.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata R. T., Levine R. P. Characterization of complement resistance in Escherichia coli conferred by the antibiotic resistance plasmid R100. J Immunol. 1980 Oct;125(4):1494–1498. [PubMed] [Google Scholar]
- Opal S., Cross A., Gemski P. K antigen and serum sensitivity of rough Escherichia coli. Infect Immun. 1982 Sep;37(3):956–960. doi: 10.1128/iai.37.3.956-960.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS J. B., KENNY K., SUTER E. THE ISOLATION AND BIOLOGICAL ACTIVITIES OF RABBIT GAMMA M- AND GAMMA G-ANTI-SALMONELLA TYPHIMURIUM ANTIBODIES. J Exp Med. 1965 Aug 1;122:385–402. doi: 10.1084/jem.122.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roantree R. J., Rantz L. A. A STUDY OF THE RELATIONSHIP OF THE NORMAL BACTERICIDAL ACTIVITY OF HUMAN SERUM TO BACTERIAL INFECTION. J Clin Invest. 1960 Jan;39(1):72–81. doi: 10.1172/JCI104029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Ellman L., Frank M. M. Bactericidal and opsonic properties of C4-deficient guinea pig serum. J Immunol. 1972 Sep;109(3):477–486. [PubMed] [Google Scholar]
- Rowley D. Sensitivity of rough gram-negative bacteria to the bactericidal action of serum. J Bacteriol. 1968 May;95(5):1647–1650. doi: 10.1128/jb.95.5.1647-1650.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg Edén C., Gotschlich E. C., Korhonen T. K., Leffler H., Schoolnik G. Aspects on structure and function of pili on uropathogenic Escherichia coli. Prog Allergy. 1983;33:189–202. doi: 10.1159/000318330. [DOI] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Genetical studies of serum resistance in Escherichia coli. J Gen Microbiol. 1975 Jul;89(1):57–66. doi: 10.1099/00221287-89-1-57. [DOI] [PubMed] [Google Scholar]
- Vukajlovich S. W., Morrison D. C. Conversion of lipopolysaccharides to molecular aggregates with reduced subunit heterogeneity: demonstration of LPS-responsiveness in "endotoxin-unresponsive" C3H/HeJ splenocytes. J Immunol. 1983 Jun;130(6):2804–2808. [PubMed] [Google Scholar]
- Wright B. G., Rebers P. A. Procedure for determining heptose and hexose in lipopolysaccharides. Modification of the cysteine-sulfuric acid method. Anal Biochem. 1972 Oct;49(2):307–319. doi: 10.1016/0003-2697(72)90433-2. [DOI] [PubMed] [Google Scholar]