Abstract
Peroxisome proliferator-activated receptor (PPAR)-γ is a ligand-activated transcription factor of nuclear hormone receptor superfamily. Thiazolidinedione rosiglitazone is a potent agonist of PPARγ which was shown to induce neuroprotection in animal models of focal ischemia and spinal cord injury. We currently evaluated the therapeutic potential of rosiglitazone (6 mg/kg at 5 min, 6 h and 24 h; i.p.) following controlled cortical impact (CCI)-induced traumatic brain injury (TBI) in adult mice. CCI injury increased the cortical PPARγ mRNA levels which were further elevated by rosiglitazone treatment. In addition, rosiglitazone treatment significantly decreased the cortical lesion volume measured at 7 days compared to vehicle treatment (by 56 ± 7%; p < 0.05; n = 6/group). Following TBI, the spared cortex of the rosiglitazone group showed significantly less numbers of GSI-B4+ activated microglia/macrophages and ICAM1+ capillaries, and curtailed induction of pro-inflammatory genes IL6, MCP1 and ICAM1 compared to vehicle group. Rosiglitazone-treated mice also showed significantly less number of TUNEL+ apoptotic neurons and curtailed induction of caspase-3 and Bax, compared to vehicle control. In addition, rosiglitazone significantly enhanced the post-TBI expression of the neuroprotective chaperones HSP27, HSP70 and HSP32/HO1, and the anti-oxidant enzymes catalase, Cu/Zn-SOD and Mn-SOD, compared to vehicle. Treatment with GW9662 (a specific PPARγ antagonist) prevented all the above PPARγ-mediated actions. Thus, PPARγ activation confers neuroprotection after TBI by anti-inflammatory, anti-apoptotic and anti-oxidative mechanisms.
Keywords: Rosiglitazone, Brain trauma, Inflammation, Oxidative stress, Neuroprotection
1. Introduction
Traumatic brain injury (TBI) is a major disabling condition in young adults all over the world. Several pathological events including inflammation, apoptosis, oxidative stress and excitotoxicity during the acute stage after an injury are known to precipitate the neuronal death and neurological dysfunction (Raghupathi, 2004; Jennings et al., 2008). Hence, therapeutic compounds that can target multiple pathophysiological mechanisms can be extremely useful in preventing post-TBI neuronal death. Peroxisome proliferator-activated receptor-γ (PPARγ) is a ligand-activated transcription factor of nuclear hormone receptor superfamily (Escher and Wahli, 2000). 15-d-Prostaglandin J2 is the natural ligand of PPARγ, while several thiazolidinediones (TZDs) are potent synthetic agonists (Kapadia et al., 2008). As PPARγ plays a significant role in glucose and lipid homeostasis, 2 TZDs rosiglitazone and pioglitazone are currently approved by the United States Food and Drug Administration (FDA) for type-2 diabetes treatment (Sood et al., 2000).
Recent studies showed that both rosiglitazone and pioglitazone are extremely neuroprotective in animal models of acute CNS insults including focal ischemia, spinal cord injury (SCI) and surgical trauma (Sundararajan et al., 2005; Zhao et al., 2005, 2006; Pereira et al., 2006; Collino et al., 2006; Tureyen et al., 2007; Park et al., 2007; McTigue et al., 2007; Hyong et al., 2008). Their efficacy was also shown in animal models of chronic CNS injuries like Parkinson's disease, Amyotrophic lateral sclerosis and Alzheimer's disease (Breidert et al., 2002; Schütz et al., 2005; Pedersen et al., 2006). We currently evaluated the therapeutic potential of rosiglitazone in minimizing secondary lesion expansion, apoptotic markers, infiltration of macrophages/activation of microglia, and the expression of inflammatory, anti-oxidant and heat-shock protein (HSP) genes in adult mice subjected to controlled cortical impact (CCI) injury.
2. Results
2.1. TBI-induced cortical PPARγ expression
At 1 day following CCI injury, the mRNA expression of PPARγ was observed to be upregulated (by 2.1 fold; p < 0.05) in the cortical tissue surrounding the injury epicenter compared to sham control (Fig. 1). Rosiglitazone treatment following CCI injury (6 mg/kg, i.p. at 5 min, 6 h and 12 h) resulted in a significantly higher induction of PPARγ mRNA (by 4.4 fold; p < 0.05) compared to sham (Fig. 1). Whereas, treatment with GW9662 (a PPARγ antagonist; 4 mg/kg, i.p. at 5 min and 12 h) prevented the post-TBI induction of PPARγ mRNA expression (Fig. 1).
Fig. 1.

At 1 day after CCI injury, the PPARγ mRNA expression analyzed with real-time PCR increased significantly in the cortex surrounding the injury compared to sham. Treating with rosiglitazone, but not with PPARγ antagonist GW9662 led to a further significant increase in PPARγ mRNA levels. CCI injury and/or treatment with rosiglitazone or GW9662 had no significant effect on the expression of the house-keeping control 18S rRNA (data not shown). The bars represent mean ± SD of n = 4 in each case. Statistics: ap < 0.05 compared to sham and bp < 0.05 compared to vehicle/TBI group (One way ANOVA followed by Tukey—Kramer multiple comparisons test).
2.2. Rosiglitazone treatment decreased the cortical contusion volume following TBI
A moderate grade CCI injury in adult mice resulted in significant cortical neuronal damage and TUNEL staining by 1 day and a cavitation by 7 days in the ipsilateral cortex. The injury size was observed to be similar at 1 day and 7 days after CCI injury (Fig. 2). At 7 days after CCI injury, the cortical contusion volume in the vehicle treated control group was observed to be 3.09 ± 0.14 mm3 (n = 6) that spanned 0 to 2.4 mm from Bregma (Fig. 3). Treatment with rosiglitazone (i.p.; 6 mg/kg at 5 min, 12 h and 24 h) resulted in a significantly smaller contusion volume compared to vehicle control (by 37 ± 6%; p < 0.05; n = 6 per group). Rosiglitazone treatment led to a similar degree of protection at 1 day after CCI injury as well (data not shown). On the other hand, preventing post-TBI PPARγ activation by treating mice with GW9662 (4 mg/kg, i.p.; at 5 min, 12 h and 24 h) resulted in a significantly larger contusion volume compared to vehicle control (by 31 ± 8%; p < 0.05; n =6) (Fig. 3).
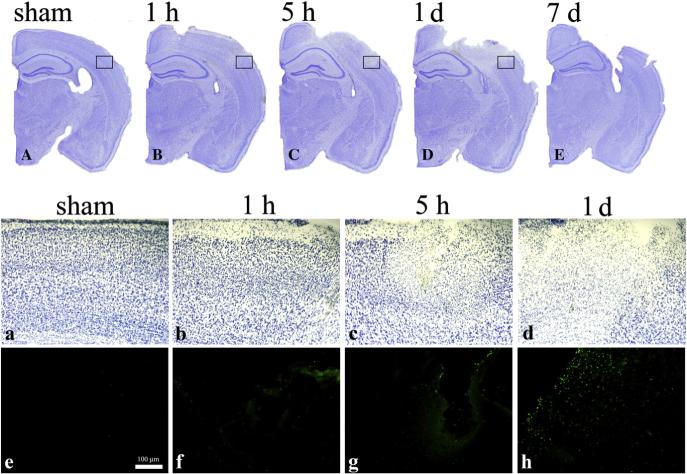
Fig. 2.
Representative Cresyl Violet stained brain sections (A to E) from mice subjected to sham (A), and CCI injury killed at 1 h (B), 5 h (C), 1 day (D) and 7 days (E). Panels a, b, c and d shows the magnified boxed areas of A, B, C and D, respectively. Panels e, f, g and h shows TUNEL staining in the boxed areas of A, B, C and D, respectively.
Fig. 3.

At 7 days after CCI injury, adult mice showed a cortical contusion that spanned from 0 to ∼3.2 mm from Bregma. Rosiglitazone treatment significantly decreased and GW9662 treatment significantly increased the cortical contusion volume. The top panel shows serial sections from representative mice of each group stained with Cresyl Violet. The lesion was outlined in black. Six mice were used per group. Statistics: ap < 0.05 compared to sham and bp < 0.05 compared to vehicle/TBI group (One way ANOVA followed by Tukey—Kramer multiple comparisons test).
2.3. Rosiglitazone curtailed post-TBI apoptosis
The ipsilateral cortex of mice subjected to CCI injury and treated with vehicle showed many TUNEL+ cells at 2 days of survival (Figs. 4A and D). Rosiglitazone-treatment significantly reduced the number of TUNEL+ cells (by 53 ± 11%; p < 0.05; n = 6) compared to the vehicle (Figs. 4B and D). Whereas, the number of TUNEL+ cells in the mice treated with GW9662 showed no significant difference from the vehicle group (Figs. 4C and D). The TUNEL+ cells were counted in the area adjacent to the injury epicenter (Fig. 4E shaded area). Many TUNEL+ cells were observed to be positive for the neuronal marker NeuN (Fig. 4F, arrows), but rarely for the astroglial marker GFAP (Fig. 4G). Rosiglitazone treatment also significantly curtailed the induction of pro-apoptotic genes caspase-3 and Bax compared to vehicle treatment at 1 day after CCI injury (Fig. 6A).
Fig. 4.
PPARγ agonist rosiglitazone treatment decreases TUNEL+ cells in the injured cerebral cortex at 2 days after TBI. Panels A to C show representative images of TUNEL+ cells in vehicle (A), rosiglitazone (B), and GW9662 (C) treated mice. Panel D shows the number of TUNEL+ cells counted in the shaded cortical area shown in panel E. Bars represent mean ± SD of n = 6 per group. Statistics: *p < 0.05 compared to vehicle control by ANOVA followed by Neuman—Keul's multiple comparisons post-test. Many TUNEL+ cells showed co-labeling with the neuronal marker NeuN (F), but not with the astroglial marker GFAP (G).
Fig. 6.

Real-time PCR analysis showed a significant upregulation of pro-inflammatory (IL6, MCP1 and ICAM1) and pro-apoptotic (caspase-3 and Bax) gene expression in the cerebral cortex at 1 day after CCI injury compared to sham (A). Rosiglitazone treatment significantly curtailed the post-TBI induction of all these transcripts (A). Gene expression of neuroprotective genes including HSPs (HSP27, HSP70 and HSP32/HO1) and anti-oxidant enzymes (SOD1, SOD2 and catalase) was also significantly upregulated in the cortex at 1 day after CCI injury (B). All these transcripts showed a further increased expression in the rosiglitazone treated group compared to sham or vehicle/TBI groups (B). The bars represent mean ± SD of n = 4 per group. Statistics: ap < 0.05 compared to sham and bp < 0.05 compared to vehicle/TBI group (One way ANOVA followed by Tukey—Kramer multiple comparisons post-test).
2.4. Rosiglitazone decreased inflammation after CCI injury
Induction of ICAM1 expression on endothelial cells, infiltration of macrophages and activation of microglia are hallmarks of cerebral inflammation. Cerebral cortex of sham-operated mice showed no appreciable staining for either GSI-B4 (macrophage/activated microglia marker) or ICAM1 (Figs. 5A and B). Whereas, mice subjected to CCI injury and treated with vehicle showed several cells and ICAM1+ capillaries at 2 days after TBI (Figs. 5C and D). Rosiglitazone treatment significantly decreased both GSI-B4 and ICAM1 immunostaining (Figs. 5E and F) compared to vehicle group. Treating mice with the PPARγ antagonist GW9662 following TBI had no significant effect on either GSI-B4 or ICAM1 immunostaining (Figs. 5G and H). At 1 day after CCI injury, mRNA levels of the pro-inflammatory genes IL6, MCP1 and ICAM1 were significantly lower in the rosiglitazone-treated group compared to vehicle group (Fig. 6A).
Fig. 5.

Rosiglitazone significantly prevented the macrophage infiltration/microglial activation and ICAM1 induction after CCI injury. The panels shows GSI-B4 stained (A, C, E and G) and ICAM1 immunostained (B, D, F and H) sections from representative mice sacrificed after sham-operation (A and B) and at 2 days after TBI and treated with either vehicle (C and D) or rosiglitazone (E and F) or GW9662 (G and H). Similar pattern of staining was observed with n of 4 mice per group.
2.5. Rosiglitazone induced HSP and anti-oxidant gene expression in the injured brain
HSPs and anti-oxidant enzymes are known to promote neuroprotection. We presently observed that TBI significantly increased the expression of 3 HSPs (HSP70, HSP27 and HSP32/HO1) and 3 anti-oxidant enzymes (SOD1, SOD2 and catalase) at 1 day after injury in the vehicle-treated mice compared to sham control (Fig. 6B). Treating mice subjected to CCI injury with rosiglitazone led to a further significant enhancement of the expression of all these mRNAs in the injured cortex (Fig. 6B). Rosiglitazone treatment also significantly elevated the HSP27 and HO1 immunoreactive protein levels estimated with immunohistochemistry and Western blotting in the cortex at 1 day after CCI injury compared to vehicle treatment (Fig. 7).
Fig. 7.
Rosiglitazone treatment significantly increased the post-TBI induction of HSP27 and HO1 protein expression in the cerebral cortex. The panels show representative sections immunostained for HO1 (A to D) and HSP27 (E to H) from the mice sacrificed after sham-operation (A and E), and 2 days after TBI and treated with either vehicle (B and F) or rosiglitazone (C and G) or GW9662 (D and H). A similar pattern of staining was observed with n of 4 mice per group. Panels I and J shows representative immunoblots of HO1 (I) and HSP27 (J) prepared with the cortical tissue of cohorts of mice subjected to TBI and treated with vehicle, rosiglitazone and GW9662. The house-keeping control β-actin levels were not altered by TBI or any treatment (I and J). The bars in I and J are mean ± SD of n = 4 per group. Statistics: *p < 0.05 compared to the vehicle/TBI group by ANOVA followed by Tukey—Kramer multiple comparisons post-test.
3. Discussion
PPARγ is a transcription factor of nuclear hormone receptor superfamily (Blanquart et al., 2003). The PPAR protein is a complex of several domains including a DNA-binding domain (DBD), a ligand binding domain (LBD) and a D-domain that links DBD and LBD (Escher and Wahli, 2000). In the absence of a ligand, the PPAR will be associated with a corepressor complex to tans-repress the target genes. Whereas, when a specific ligand binds to the LBD, the PPAR complex dimerizes with retinoic acid-X-receptor (RXR) leading to release of the core-pressor (Yi et al., 2007). The PPAR:RXR heterodimer translocates to nucleus and binds to the promoters of several genes that contain a cis-acting element known as peroxisome-proliferator response element (PPRE) to either initiate or suppress the transcription of the target genes (Blanquart et al., 2003).
Of various TZDs which are potent exogenous agonists of PPARγ, rosiglitazone and pioglitazone were shown to be well-tolerated with few side effects in humans (Sood et al., 2000). As they control blood glucose levels by stimulating PPARγ, these 2 TZDs are FDA-approved for type-2 diabetes treatment (Sood et al., 2000). Of these two, the affinity of rosiglitazone to PPARγ was shown to be 10 times higher than pioglitazone (Escher and Wahli, 2000). Recent studies showed that both these TZDs decrease the contusion volume, microglial activation, astrogliosis and myelin loss leading to better preservation of motor neurons with less pain following SCI in adult rodents (Park et al., 2007; McTigue et al., 2007). Mechanistically TZD treatment was observed to curtail post-SCI expression of many chemokines, cytokines, adhesion molecules and pro-inflammatory transcription factors while promoting the expression of neuroprotective genes (Park et al., 2007). Our laboratory and other groups also showed that treatment with rosiglitazone or pioglitazone significantly decreases the infarct volume as well as neurological dysfunction in rodent models of focal ischemia by preventing inflammation and oxidative stress (Sundararajan et al., 2005; Zhao et al., 2005, 2006; Pereira et al., 2006; Collino et al., 2006; Tureyen et al., 2007; Park et al., 2007).
TBI leads to extensive secondary neuronal death (Onyszchuk et al., 2008) which will be mediated by the synergistic action of many pathophysiologic mechanisms including inflammation, glutamate excitotoxicity, apoptosis, ionic imbalance, edema and oxidative stress during the acute period after an injury. Hence, combination therapies with drugs that can target more than one mechanism are beneficial. In addition, compounds that can target multiple pathways simultaneously can be more effective. Recent studies showed that PPARγ agonists are such compounds that induce neuroprotection by targeting several down-stream destructive mechanisms which precipitate brain damage following an acute CNS insult (Park et al., 2007; Tureyen et al., 2007).
Our results showed that rosiglitazone treatment significantly reduced lesion volume compared to vehicle treatment. Previous studies by Luo et al. (2006) following focal ischemia showed that rosiglitazone is effective at 3−12 mg/kg concentration given i.p., with an optimal dose of 6 mg/kg. A recent study from our laboratory showed that multiple doses of rosiglitazone given during the first day after SCI are extremely beneficial (Park et al., 2007). Our studies also showed that this dosing regimen doesn't result in any mortality or overt symptoms such as altered grooming or feeding behavior (Park et al., 2007; Tureyen et al., 2007). Based on these earlier studies, we presently used a dose of 6 mg/kg given i.p. at 5 min, 6 h and 24 h after TBI with an aim to prevent inflammation and oxidative stress which are at peak during the first day after an injury. In concurrence with the earlier studies this dosing regimen induced no observable side effects in adult mice. Interestingly, animals treated with the PPARγ antagonist GW9662 showed significantly greater lesion volume compared to vehicle group suggesting that PPARγ activation by its endogenous ligands is an essential neuroprotective event which can be enhanced by providing exogenous agonists. Indeed, a similar result has been reported in ischemia (Victor et al., 2006).
Reduced inflammation following TBI might have contributed to the neuroprotection induced by rosiglitazone. Following TBI, rosiglitazone-treated mice showed significantly less number of cells in the injured cortex, suggesting a curtailed microglial activation and macrophage infiltration. Activated microglia and macrophages release a host of neurotoxic substances including oxygen free radicals that are extremely toxic to neurons. ICAM1 expression on cerebral capillaries which promotes macrophage infiltration after brain injury (Knoblach and Faden, 2002) was also observed to be less in the rosiglitazone group compared to vehicle group. Post-TBI induction of the pro-inflammatory cytokine IL6 and chemokine MCP1 mRNA expression was also curtailed by rosiglitazone treatment. This is in support of the previous studies that indicated the anti-inflammatory potential of TZDs following cerebral ischemia and SCI (Sundararajan et al., 2005; Zhao et al., 2005, 2006; Collino et al., 2006; Pereira et al., 2006; Tureyen et al., 2007; Park et al., 2007). Recent studies showed that rosiglitazone treatment curtails inflammatory gene expression at 6 h after SCI when the secondary neuronal damage was still evolving indicating prevention of inflammation as a potential cause rather than an effect of rosiglitazone action (Park et al., 2007). We presently observed that rosiglitazone treatment significantly decreased the induction of inflammatory genes after TBI as well, but as the gene expression was estimated at 1 day after TBI, we cannot rule out the possibility that this might be an effect rather than a cause. The reduction in the lesion volume observed in the rosiglitazone-treated animals might be in part due to decreased apoptosis in the injured cerebral cortex. We observed that rosiglitazone treatment significantly decreased the number of TUNEL+ neurons in the cortical area adjacent to the injury core. In addition, post-TBI mRNA induction of the pro-apoptotic genes caspase-3 and Bax was significantly lower in the rosiglitazone group compared to vehicle group.
HSPs act as chaperones to prevent aggregation and denaturation of proteins. In addition, HSPs are known to decrease glutamate release, oxidative stress and apoptosis and hence can confer neuroprotection after acute brain injuries (Kelty et al., 2002; Mehlen et al., 1996). CCI injury led to increased expression of cortical HSP70, HSP27 and HO1 mRNA levels which were further enhanced by rosiglitazone treatment. Rosiglitazone also enhanced the protein expression of HSP27 and HO1 after TBI. In addition, rosiglitazone treatment led to a significant induction of anti-oxidant enzymes SOD1, SOD2 and catalase in the injured brain which indicates an increased capability to handle free radicals and other oxidants leading to better neuronal survival.
In conclusion, our study showed that TBI led to increased expression of PPARγ and treatment with PPARγ agonist rosiglitazone induces significant neuroprotection. Several mechanisms including prevention of inflammation, apoptosis and oxidative stress in addition to induction of HSPs might have synergistically contributed to the beneficial actions of rosiglitazone after TBI. We also showed that PPARγ antagonist GW9662 treatment worsens the outcome after TBI indicating a direct action of rosiglitazone mediated by stimulating PPARγ. While this is the first study showing the efficacy of PPARγ stimulation after TBI, a previous study showed the beneficial effects of PPARα agonist fenofibrate following lateral fluid-percussion-induced TBI in adult rats (Besson et al., 2005). It might be of therapeutic interest to develop potent dual agonists that can induce both the isoforms of PPAR.
4. Experimental procedures
4.1. Controlled cortical impact injury and drug administration
TBI was induced in adult male C57BL/6 mice (22−25 g) with a CCI device as described earlier (Rao et al., 2001). All surgical procedures were conducted and animals were cared for according to the animal welfare guidelines (1985 Principles of the Guide for the Care and Use of Laboratory Animals,U.S.Departmentof Health and Human Services Publ. 85−23) and were approved by the animal care committee of the University of Wisconsin-Madison. In brief, a moderate grade injury was induced through a craniectomy (2 mm in diameter, 1 mm lateral and 1 mm caudal to Bregma) at a velocity of 3 m/s and 1 mm deformation under isoflurane anesthesia. The exposed cortex was covered with Surgicel, the wound was closed with sutures and 0.5% bupivi-caine (100 μl) was injected along the incision to provide local anesthesia. During the surgery and recovery from anesthesia, body temperature was maintained at 37−38 °C with a heating pad and a lamp. After recovering from anesthesia, mice were returned to their cages with free access to food and water. To cohorts of mice, rosiglitazone (6 mg/kg; Cayman Chemicals, Ann Arbor, MI) or GW9662 (4 mg/kg; PPARγ antagonist; Sigma Chemicals, St Louis, MO) or vehicle (4% DMSO) was injected i.p. at 5 min, 6 h and 24 h after TBI.
4.2. Determination of lesion volume
Lesion volume was measured at 7 days after TBI as described earlier (Dempsey and Rao, 2003). In brief, an animal was perfused transcardially with buffered paraformaldehyde, the brain was post-fixed, cryoprotected and sectioned (coronal; 40 μm thick at an interval of 480 μm). 6 serial sections from each brain covering the cortical lesion (from 0 to −3.0 mm from Bregma as per the Mouse Brain Atlas of Paxinos and Watson) were stained with Cresyl violet and scanned using the NIH Image Program (written by Wayne Rasband; http://rsb.info.nih.gov/ij). The volume of the lesion was computed by the numeric integration of data from the serial sections in respect to sectional interval.
4.3. Immunohistochemistry
Parallel sets of 40 μm coronal sections from each mouse were immunostained with antibodies against ICAM1 (1:1000; goat anti-ICAM1; R&D systems inc., MN, USA), HO1 (1:2000; rabbit anti-HO1; Stressgen, BC, Canada) and HSP27 (1:1000; rabbit anti-HSP27; Stressgen, BC, Canada) using the standard procedures. In brief, sections were rinsed in 0.1 M Tris-buffered saline with 0.1% Triton-X100 (TBS-T; 3 × 5 min), incubated in 1% H2O2 for 10 min and washed in TBS-T (3 × 5 min). The sections were incubated in 10% normal donkey serum in TBST for 1 h and at 4 °C overnight in the desired antibodies. The sections were washed in 1% donkey serum in TBS-T (3 × 5 min) and incubated in biotinylated donkey anti-rabbit or anti-goat secondary antibody (1:1000; Santa Cruz, CA, USA) for 1 h. Sections incubated in the absence of primary or secondary antibodies served as negative controls. For GSI-B4 staining, sections were washed in 0.1 M PBS (3 × 10 min), incubated with biotinylated GSI-B4 (1:1000; Sigma Chemical Co.) in PBS for 2 h, incubated for 1 h in streptavidin-HRP (1:300, Dako, Carpinteria, CA, USA) and washed in PBS (3 × 5 min). GSI-B4-reactive cells were visualized using DAB as chromogen as described earlier (Kapadia et al., 2006).
4.4. TUNEL staining and fluorescence double labeling
In situ detection of apoptotic cells was performed using TUNEL staining with the In Situ Cell Death Detection Kit (Roche Molecular Biochemicals, Indianapolis, IN, USA) as described earlier (Kapadia et al., 2006). Briefly, sections were permeabilized with 1% Proteinase K (in 50 mM Tris/5 mM EDTA buffer) for 15 min, rinsed with PBS and incubated in TUNEL reaction mixture for 1 h at 37 °C. Samples were rinsed in PBS (3 × 5 min), coverslipped using Antifade solution (Molecular Probes, Invitrogen) and observed under the fluorescence microscope (Nikon TE2000, Nikon USA). The apoptotic cells (FITC green-stained) were counted in the peri-injury area of 3 ipsilateral cortical sections of each mouse. For double labeling to identify the apoptotic cell type, TUNEL stained sections were washed in TBS (3 × 5 min) and incubated with rabbit anti-GFAP (1:2000: Dako, Carpenteria, CA, USA) or mouse anti-NeuN (1:500, Chemicon, Temecula, CA, USA). The sections were washed in TBS and incubated for 1 h with secondary antibodies (1:200; Alexa Fluor 594 donkey anti-rabbit or anti-mouse, Molecular Probes, Eugene, OR, USA).
4.5. Real-time PCR analysis
Gene expression analysis was conducted as described earlier (Tureyen et al., 2008). The following transcripts were estimated using the primers designed with Primer Express Software (Applied Biosystems USA) based on the mouse GenBank numbers given in parenthesis: PPARγ (NM_011146), IL6 (NM_031168), MCP1 (AF065933), ICAM1 (BC008626), HSP27 (NM_013560), HSP70 (NM_010479), HO1 (NM_010442), SOD1 (NM_011434), SOD2 (NM_013671), catalase (NM_009804), caspase-3 (NM_009810), Bax (NM_007527) and 18S rRNA (M11188). In brief, cohorts of mice subjected to CCI injury or sham operation and treated with vehicle or rosiglitazone were killed at 1 day of survival (n = 4/group). Total RNA was extracted from the ipsilateral cortex of each mouse using the Trizol reagent (Invitrogen, Carlsbad, CA, USA). 1 μg of RNA from each sample was reverse transcribed with oligo(dT)15 and random hexamer primers using M-MuLV reverse transcriptase (Life Technologies, Rockville, MD, USA). 10 ng of cDNA of each sample and gene-specific primers were added to SYBR Green PCR Master Mix (SYBR Green I Dye, AmpliTaq DNA polymerase, dNTPs with dUTP and optimal buffer components; Applied Biosystems) and subjected to PCR amplification in a Perkin-Elmer TaqMan 5700 Sequence Detection System (1 cycle at 50 °C for 2 min, 1 cycle at 95 °C for 10 min, and 40 cycles at 95 °C for 15 s and 1 cycle at 60 °C for 1 min). PCR reactions were conducted in duplicate. The amplified transcripts were quantified with the comparative CT method using 18S rRNA as the internal control as described earlier (Vemuganti et al., 2006).
4.6. Western blotting
Protein levels of Hsp27 and HSP32/HO1 in brain tissue were estimated as described earlier (Satriotomo et al., 2006; Dhodda et al. 2004). Whole ipsilateral cortex from the mice treated with vehicle, rosiglitazone and GW9662 after CCI injury was obtained at 1 day of recovery (n = 4/group). The tissue was homogenized in ice cold-lysis buffer (50 mM Tris, 150 mM NaCl, 1% Triton and 1% SDS) and protease inhibitors (1 mM PMSF, 10 μg/mL leupeptin, 10 μg/mL pepstatin, 10 μg/mL aprotinin and 1 mM EDTA; pH 7.4). The homogenate was centrifuged at 15,000 g for 10 min at 4 °C, and the supernatant (30 μg protein equivalent) from each sample was electro-phoresed on Bio-Rad Criterion precast polyacrylamide gels, transferred to polyvinylidene difluoride membranes and probed with polyclonal anti-HSP27 (1:1000), polyclonal anti-HO1 (1: 1000) and monoclonal anti-β-actin antibodies (1:10,000; Sigma Chemical Co.) followed by HRP-conjugated anti-rabbit or anti-mouse IgG (1:2000). The protein bands recognized by antibodies were detected by enhanced chemiluminescence according to the manufacturer's instructions (Pierce, Rockford, IL, USA).
4.7. Statistical analysis
The data are expressed as mean ± SD. Comparisons among groups were performed by one-way analysis of variance (ANOVA) followed by Tukey—Kramer multiple comparisons post-hoc test.
Acknowledgments
These studies were partially supported by grants from the United States National Institute of Health (RO1 NS044173 and RO1 NS049448), the American Heart Association (Grant-in-Aid 0350164N) and the faculty start-up funds from the University of Wisconsin to R. Vemuganti.
Footnotes
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
REFERENCES
- Besson VC, Chen XR, Plotkine M, Marchand-Verrecchia C. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, exerts neuroprotective effects in traumatic brain injury. Neurosci. Lett. 2005;388:7–12. doi: 10.1016/j.neulet.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Blanquart C, Barbier O, Fruchart JC, Staels B, Glineur C. Peroxisome proliferator-activated receptors: regulation of transcriptional activities and roles in inflammation. J. Steroid Biochem. Mol. Biol. 2003;85:267–273. doi: 10.1016/s0960-0760(03)00214-0. [DOI] [PubMed] [Google Scholar]
- Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson's disease. J. Neurochem. 2002;82:615–624. doi: 10.1046/j.1471-4159.2002.00990.x. [DOI] [PubMed] [Google Scholar]
- Collino M, Aragno M, Mastrocola R, Gallicchio M, Rosa AC, Dianzani C, Danni O, Thiemermann C, Fantozzi R. Modulation of the oxidative stress and inflammatory response by PPAR-gamma agonists in the hippocampus of rats exposed to cerebral ischemia/reperfusion. Eur. J. Pharmacol. 2006;530:70–80. doi: 10.1016/j.ejphar.2005.11.049. [DOI] [PubMed] [Google Scholar]
- Dempsey RJ, Rao VL. Cytidinediphosphocholine treatment to decrease traumatic brain injury-induced hippocampal neuronal death, cortical contusion volume, and neurological dysfunction in rats. J. Neurosurg. 2003;98:867–873. doi: 10.3171/jns.2003.98.4.0867. [DOI] [PubMed] [Google Scholar]
- Dhodda VK, Sailor KA, Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. J. Neurochem. 2004;89:73–79. doi: 10.1111/j.1471-4159.2004.02316.x. [DOI] [PubMed] [Google Scholar]
- Escher P, Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutat. Res. 2000;448:121–138. doi: 10.1016/s0027-5107(99)00231-6. [DOI] [PubMed] [Google Scholar]
- Hyong A, Jadhav V, Lee S, Tong W, Rowe J, Zhang JH, Tang J. Rosiglitazone, a PPAR gamma agonist, attenuates inflammation after surgical brain injury in rodents. Brain Res. 2008;1215:218–224. doi: 10.1016/j.brainres.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JS, Gerber AM, Vallano ML. Pharmacological strategies for neuroprotection in traumatic brain injury. Mini Rev. Med. Chem. 2008;8:689–701. doi: 10.2174/138955708784567377. [DOI] [PubMed] [Google Scholar]
- Kapadia R, Tureyen K, Bowen KK, Kalluri H, Johnson PF, Vemuganti R. Decreased brain damage and curtailed inflammation in transcription factor CCAAT/enhancer binding protein beta knockout mice following transient focal cerebral ischemia. J. Neurochem. 2006;98:1718–1731. doi: 10.1111/j.1471-4159.2006.04056.x. [DOI] [PubMed] [Google Scholar]
- Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelty JD, Noseworthy PA, Feder ME, Robertson RM, Ramirez JM. Thermal preconditioning and heat-shock protein 72 preserve synaptic transmission during thermal stress. J. Neurosci. 2002;22:RC193. doi: 10.1523/JNEUROSCI.22-01-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblach SM, Faden AI. Administration of either anti-intercellular adhesion molecule-1 or a nonspecific control antibody improves recovery after traumatic brain injury in the rat. J. Neurotrauma. 2002;19:1039–1050. doi: 10.1089/089771502760341956. [DOI] [PubMed] [Google Scholar]
- Luo Y, Yin W, Signore AP, Zhang F, Hong Z, Wang S, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. J. Neurochem. 2006;97:435–448. doi: 10.1111/j.1471-4159.2006.03758.x. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi R, Wei P, Lash AT. The PPAR gamma agonist pioglitazone improves anatomical and locomotor recovery after rodent spinal cord injury. Exp. Neurol. 2007;205:396–406. doi: 10.1016/j.expneurol.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Schulze-Osthoff K, Arrigo AP. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J. Biol. Chem. 1996;271:16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- Onyszchuk G, He YY, Berman NE, Brooks WM. Detrimental effects of aging on outcome from traumatic brain injury: a behavioral, magnetic resonance imaging, and histological study in mice. J. Neurotrauma. 2008;25:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- Park SW, Yi JH, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R. Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J. Pharmacol. Exp. Ther. 2007;320:1002–1012. doi: 10.1124/jpet.106.113472. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp. Neurol. 2006;199:265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Pereira MP, Hurtado O, Cardenas A, Bosca L, Castillo J, Davalos A, Vivancos J, Serena J, Lorenzo P, Lizasoain I, Moro MA. Rosiglitazone and 15-deoxy-delta12, 14-prostaglandin J2 cause potent neuroprotection after experimental stroke through noncompletely overlapping mechanisms. J. Cereb. Blood Flow Metab. 2006;26:218–229. doi: 10.1038/sj.jcbfm.9600182. [DOI] [PubMed] [Google Scholar]
- Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VL, Dogan A, Bowen KK, Dempsey RJ. Traumatic brain injury leads to increased expression of peripheral-type benzodiazepine receptors, neuronal death and activation of astrocytes and microglia in rat thalamus. Exp. Neurol. 2001;161:102–114. doi: 10.1006/exnr.1999.7269. [DOI] [PubMed] [Google Scholar]
- Satriotomo I, Bowen KK, Vemuganti R. JAK2 and STAT3 activation contributes to neuronal damage following transient focal cerebral ischemia. J. Neurochem. 2006;98:1353–1368. doi: 10.1111/j.1471-4159.2006.04051.x. [DOI] [PubMed] [Google Scholar]
- Schütz B, Reimann J, Dumitrescu-Ozimek L, Kappes-Horn K, Landreth GE, Schürmann B, Zimmer A, Heneka MT. The oral antidiabetic pioglitazone protects from neurodegeneration and amyotrophic lateral sclerosis-like symptoms in superoxide dismutase-G93A transgenic mice. J. Neurosci. 2005;25:7805–7812. doi: 10.1523/JNEUROSCI.2038-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood V, Colleran K, Burge MR. Thiazolidinediones: a comparative review of approved uses. Diabetes Technol. Ther. 2000;2:429–440. doi: 10.1089/15209150050194297. [DOI] [PubMed] [Google Scholar]
- Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Kapadia R, Bowen K, Satriotomo I, Liang J, Feinstein D, Vemuganti R. Peroxisome proliferators-activated receptor-γ agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J. Neurochem. 2007;101:41–46. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Brooks N, Bowen K, Svaren J, Vemuganti R. Transcription factor early growth response-1 induction mediates inflammatory gene expression and brain damage following transient focal ischemia. J. Neurochem. 2008;105:1313–1324. doi: 10.1111/j.1471-4159.2008.05233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuganti R, Kalluri H, Yi JH, Hazell AS. Microarray analysis reveals gene expression changes associated with inflammation, cellular stress, and structural damage in the thalamus and inferior colliculus following oxidative impairment in experimental Wernicke's encephalopathy. Eur. J. Neurosci. 2006;23:1172–1188. doi: 10.1111/j.1460-9568.2006.04651.x. [DOI] [PubMed] [Google Scholar]
- Victor NA, Wanderi EW, Gamboa J, Zhao X, Aronowski J, Deininger K, Lust WD, Landreth GE, Sundararajan S. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur. J. Neurosci. 2006;24:1653–1663. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- Yi JH, Park SW, Kapadia R, Vemuganti R. Role of transcription factors in mediating post-ischemic cerebral inflammation and brain damage. Neurochem. Int. 2007;50:1014–1027. doi: 10.1016/j.neuint.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Gohlke P, Herdegen T, Culman J. The intracerebral application of the PPARgamma-ligand pioglitazone confers neuroprotection against focal ischaemia in the rat brain. Eur. J. Neurosci. 2005;22:278–282. doi: 10.1111/j.1460-9568.2005.04200.x. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Herdegen T, Gohlke P, Culman J. Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. FASEB J. 2006;20:1162–1175. doi: 10.1096/fj.05-5007com. [DOI] [PubMed] [Google Scholar]