Abstract
Photopigment optical density (OD) of middle-(M) and long-(L) wavelength-sensitive cones was determined to evaluate the hypothesis that reductions in the amount of photopigment are responsible for age-dependent sensitivity losses of the human cone pathways. Flicker thresholds were measured at the peak and tail of the photoreceptor’s absorption spectrum as a function of the intensity of a bleaching background. Photopigment OD was measured at 0 (fovea), 2, 4, and 8 deg in the temporal retina by use of a 0.3-deg-diameter test spot. Seventy-two genetically characterized dichromats were studied so that the L- and M-cones could be analyzed separately. Subjects included 28 protanopes with M- but no L-cones and 44 deuteranopes with L- but no M-cones (all male, age range 12–29 and 55–83 years). Previous methods have not provided estimates of photopigment OD for separate cone classes in the foveola. In this study, it was found that foveolar cones are remarkably efficient, absorbing 78% of the available photons (OD = 0.65). Photopigment OD decreased exponentially with retinal eccentricity independently of age and cone type. Paradoxically, the OD of perifoveal cones increased significantly with age. Over the 70-year age range of our participants, the perifoveal M- and L-cones showed a 14% increase in capacity to absorb photons despite a 30% decrease in visual sensitivity over the same period.
Keywords: Cones, Photopigment optical density, Aging, Dichromacy, Color blindness
Introduction
Human trichromatic vision is established by three different cone photoreceptor types, absorbing maximally at short (S), middle (M), or long (L) wavelengths (i.e., λmax ≈ 40, 540, and 565 nm, respectively). The capacity of the cone photoreceptor to absorb light can be described in terms of its photopigment optical density (OD). Following the Beer-Lambert relation, photopigment OD is a function of the pigment’s extinction spectrum, concentration of molecules, and pathlength. Because cone outer segment length decreases with retinal eccentricity (Polyak, 1941), photopigment OD is maximal in the foveola and decreases with eccentricity (Pokorny et al., 1976; Marcos et al., 1997). It is not known whether outer segment length varies with age, but a central question in this study was whether there are senescent changes in photopigment OD that may account for age-related losses in the sensitivity of M- and L-cone pathways (Werner et al., 2000; Knoblauch et al., 2001). Quantitative modeling indicates that much of the senescent loss in cone sensitivity is due to degradations that are mathematically equivalent to a loss in quantal efficiency and/or an elevation in neural noise (Schefrin et al., 1992, 1995).
Previous studies have reported age-related decreases in photo-pigment OD based on reflection densitometry (Kilbride et al., 1986; Keunen et al., 1987; Coile & Baker, 1992) and color matching (Eisner et al., 1987; Elsner et al., 1988; Swanson & Fish, 1996). Because these aging studies have been conducted with trichromats, the photopigment OD of M- and L-cones may have been confounded. It is presently unclear whether the M- and L-cones differ in photopigment OD because studies carried out with dichromats have typically included only a few observers of each type, and all studies report a great deal of variability in photopigment OD among observers of the same age. With retinal reflection densitometry and a 2-deg measuring field, Rushton (1963a) obtained a value of 0.35 for a single protanope, and King-Smith (1973a,b) obtained individual photopigment OD values ranging from 0.38–0.93 for deuteranopes. More recently, Berendschot et al. (1996) used densitometry and found no significant difference between M- and L-cone photopigment OD. Two psychophysical studies (Miller, 1972; Smith & Pokorny, 1973) report higher L-cone than M-cone photopigment OD while axial values estimated from transverse microspectrophotometry measurements of single human cones are not different for M- and L-cones (Bowmaker & Dartnall, 1980). All these studies yield values that are too low compared to photopigment ODs of 0.7–0.9 required to explain self-screening effects measured by the change in color when monochromatic lights are obliquely incident on the central fovea (Walraven & Bouman, 1960; Enoch & Stiles, 1961; but see Burns & Elsner, 1993).
In the present study, M- and L-cone photopigment OD was measured for small localized areas (0.3-deg diameter) at the fovea and at 2, 4, and 8 deg in the temporal retina using a large number of younger and older X-linked dichromats who were phenotypically identified and genetically characterized. Our methods permitted us to (1) quantify differences in photopigment OD at multiple retinal locations for younger and older groups of observers, (2) compare the photopigment OD in M- and L-cones, and (3) determine whether differences exist in photopigment OD between single-gene and multi-gene dichromats. Measurements of separate M- and L-cone photopigment OD in the foveola have not been reported, and it was found that these photoreceptors are remarkably efficient at absorbing photons, with an average OD = 0.65. Photopigment OD decreases exponentially with retinal eccentricity, but paradoxically increases with age in the perifovea from 0.35 to 0.44 over a span of seven decades.
Materials and methods
Subject phenotyping
Phenotypic classification of subjects was based on a series of standardized tests including the American Optical HRR pseudo-isochromatic plates, the F-2 tritan plate, and Rayleigh matches on a Neitz anomaloscope. Only those subjects who obtained meta-meric matches between the yellow and each of the green and red primaries alone were accepted as dichromatic. All 72 subjects were male between 12 and 83 years old; there were 28 protanopes and 44 deuteranopes, of which 27 were younger (12–29 years) and 45 were older (55–83 years).
At the time of testing, all subjects were examined by an optometrist and/or ophthalmologist and found to be free of systemic, ocular, or neurological diseases. The presence of retinal disease and abnormal ocular media in the tested eye were ruled out by ocular examination including visual acuity, slit lamp examination, intraocular pressure, and both direct and indirect ophthalmoscopy. Stereofundus photographs were assessed as normal by a retinal specialist. The retinae of all subjects were found to have no more than five small (≤63 µm) drusen and no vascular, retinal, choroidal, or optic nerve findings known to disrupt visual function. Intraocular pressure was < 22 mm Hg. All but seven subjects demonstrated a corrected Snellen acuity of ≥ 20/20 in the tested eye; six subjects had 20/25 visual acuity, and one subject had 20/30 visual acuity. Exclusion of subjects whose visual acuity was worse than 20/20 would not affect the conclusions.
Subject genotyping
Genomic DNA was isolated from whole blood, and used in genetic analyses to confirm the phenotype and to distinguish between dichromats who had one versus more than one visual pigment gene on the X-chromosome. The relative number of L and M genes per X-chromosome array was determined using real-time quantitative polymerase chain reaction. These analyses are presented elsewhere in this journal (Neitz et al., 2004).
Apparatus and calibration
Stimuli were presented using four channels of a conventional Maxwellian-view optical system. The sources were 1-kW and 300-W xenon arc lamps powered by regulated dc supplies. Water baths were used to filter out infrared radiation. The stimuli were rendered monochromatic by use of a holographic grating mono-chromator (Instruments SA, V-20) or an interference filter (Ealing, Rocklin, CA), both having ≤ 8-nm half-band passes. Calibrated neutral density filters and wedges were placed in collimated and focused portions of the beams, respectively. Wedge positions were computer controlled and monitored with potentiometers and a linear read-out system. Mirrors were front surfaced, and lenses were achromatic doublets. A rotating sectored mirror placed at a focal point was used to produce square-wave flicker. A 0.04-mm-diameter aperture (0.2 deg) was used to define the (600 nm) fixation point. The background was bichromatic, composed of a 430-nm light of fixed intensity superposed on a 580-nm light of varying intensity. The final Maxwellian-view lens focused a 1.5-mm-diameter image onto the plane of the observer’s pupil. Presentations of test spots and background were controlled by a calibrated mechanical shutter (Vincent Associates, Rochester, NY) and custom timing circuitry. Observers were aligned with respect to the optic axis of the Maxwellian-view optical system by use of a dental-impression bite bar mounted to a milling-machine table that permitted adjustments along three orthogonal directions. A pupil viewer was used to monitor subject fixation and to align the subject’s eye in relation to the Maxwellian-view image. Correction of individual refractive error, if necessary, was provided by appropriately selected trial lenses mounted in the spectacle plane.
Radiometric measurements of the spectral lights and calibrations of the neutral density filters and wedges were made using a silicon photodiode and linear read-out system (United Detector Technology, Hawthorne, CA; 81 Optometer) that was calibrated relative to standards of the National Institute of Standards and Technology. Photometric measurements were made with a Minolta Chromameter (CS-100) and barium sulfate plate and then converted to retinal illuminance using the method outlined by West-heimer (1966). The monochromators were calibrated with a scanning spectroradiometer (Photo Research PR-703 A) at a series of spectral lines emitted by Hg and Ne low-pressure calibration lamps (Oriel, Hg 6034, Ne 6032).
Procedure
A flickering (18 Hz) target of 0.3-deg diameter was superimposed in the center of a bichromatic 16-deg-diameter background, consisting of (1) a 430-nm adapting field fixed at 11 log quanta s−1 deg−2, chosen to saturate the rod photoreceptors and desensitize the S-cones (which bleached about 33.2% and 27.8% of the M- and L-cone pigment, respectively, and less than 50% of the S-cone pigment, the amount depending on individual variation in lenticular and macular pigmentation) and (2) a 580-nm bleaching field increased in 0.5 log unit steps from 8.0 log quanta s−1 deg−2 to 11.5 log quanta s−1 deg−2 for peripheral measurements and to 11.75 log quanta s−1 deg−2 for foveal measurements, chosen to progressively bleach the M- or L-cone photopigment. These light intensities are below the safe exposure limits recommended by the CIE international standard S-009E-2002 and ANSI RP27.1-1996 which follow the American Conference of Governmental Industrial Hygienists (2003).
Subjects used the method of adjustment to find the threshold at which flicker was just visible. Sensitivity at 545 and 615 nm for protanopes or at 565 and 650 nm for deuteranopes and color normals were compared under bleached and unbleached conditions. Although individual and age-related variation in the optical density of the crystalline lens might be expected to contribute to some variation in the retinal illuminance at short wavelengths, our method depends on changes from the bleached to the unbleached state so inert pigments have a negligible effect on our photopigment OD measurements.
Stimuli were imaged in Maxwellian-view at 0 (fovea), 2, 4, and 8-deg eccentricity in the temporal retina. A fixation point was used for stimulus presentations outside the fovea. Psychophysical testing was preceded by 8 min of dark adaptation and 2.5 min adaptation to each new bleaching level before measuring thresholds. Subjects were told to blink frequently in order to prevent stimulus fading due to Troxler’s effect.
Each subject was tested 4–6 times at each retinal location. Only one retinal location was tested per session and usually there was only one session per day. For subjects traveling a long distance to the laboratory, two sessions were conducted per day, but separated by at least 45 min to allow recovery from previous bleaches. All 72 subjects completed the tasks with foveal viewing first. Some subjects were not able to devote enough time to complete all of the perifoveal measurements. This appears unrelated to age.
Written informed consent was obtained following the Tenets of Helsinki, and with approval of the Office of Human Research Protection of the University of California, Davis and the Medical College of Wisconsin.
Data analysis
Photopigment optical densities were calculated following Miller (1972):
| (1) |
where
Δ log W = difference in log relative spectral sensitivity,
(KλP − KλT) = the difference in log sensitivity (unbleached state) between wavelengths falling on the peak (λp) and on the long-wave tail (λt) of the cone spectral sensitivity function,
ODλmax = αλpc02.3, or the photopigment optical density at the peak,
αλ = molecular extinction coefficient,
c = no. of molecules per unit area of receptor,
p′ = I0/(I + I0), or the fraction of unbleached pigment,
I = intensity of bleaching background, and
I0 = half-bleaching constant.
For the analyses presented here, we used a fixed half-bleaching constant (I0) of 3.89 log Tds for deuteranopes and protanopes (Rushton, 1963b, 1965). The bleaching effect of both background lights has been taken into account and calculated (1) for the peak of the L-cone pigment of deuteranopes; and (2) for the peak of the M-cone pigment of protanopes, as tabled by Stockman and Sharpe (2000).
Our analyses thus estimate photopigment optical density based upon the assumption that (1) there is no significant bleaching product that absorbs in the spectral region of our test stimuli, and (2) there is no change in I0 with age. We are not aware of any evidence that is inconsistent with these assumptions.
Results
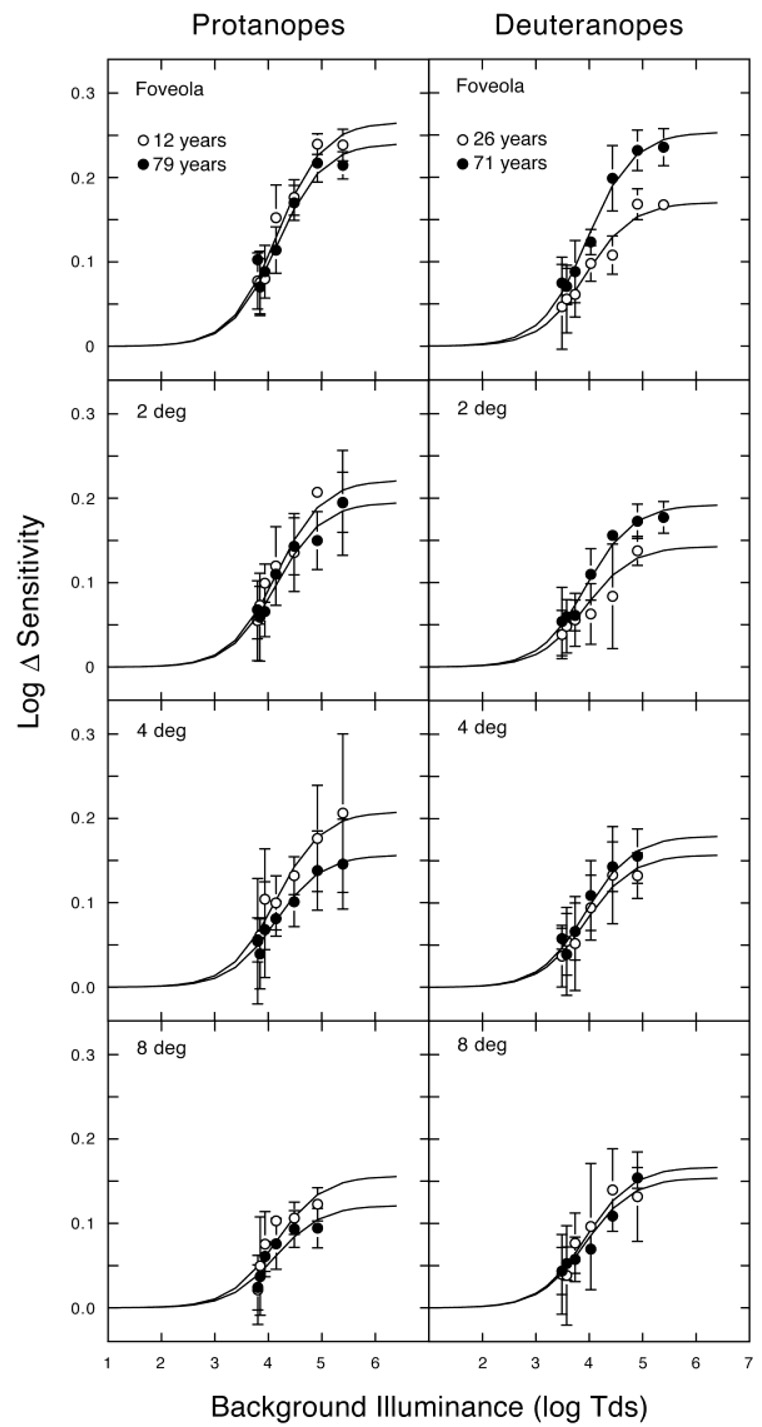
Fig. 1 shows log Δ sensitivity versus log background illuminance for individual younger and older protanopes and deuteranopes for each retinal location tested. Log Δ sensitivity rises with bleaching level for both types of dichromat. With increasing light intensity, we progressively bleached the photopigment up to 98.2% for L-cones (deuteranopes) and 97.2% for M-cones (protanopes) in the fovea. In the parafovea, the maximum bleaching levels were 96.9% and 95.2% for L- and M-cones, respectively. The solid curves in Fig. 1 show the model fits. These analyses used I0 values based upon Rushton’s (1963b, 1965) reflection densitometry. There is uncertainty in the optimal values of I0. Miller (1972) used heterochromatic flicker photometry to estimate I0 in two dichromats and measured photopigment OD based on Rushton’s I0 values and 4.1 log Tds. Different I0 values might account for the discrepancy in M- and L-cone photopigment OD values found between individual studies. It should be noted, however, that for this study a different I0 would not affect the analysis of age-related changes in photopigment OD provided I0 does not change with age. This has been evaluated in a population of 50 trichromats and no significant change in I0 was found across the age range tested, 30–69 years (Elsner et al., 1988).
Fig. 1.
Log Δ sensitivity vs. background illuminance (log Tds) for individual dichromats. Left panels show the sensitivity differences between a 615-nm and 545-nm test light for younger and older protanopes at 0 (fovea), 2, 4, and 8 deg temporal. Right panels show the sensitivity differences between a 650-nm and 565-nm test light for younger and older deuteranopes at 0, 2, 4, and 8 deg temporal. The solid curves in each panel are model fits according to Eq. (1). Error bars denote ±1 S.E.M.
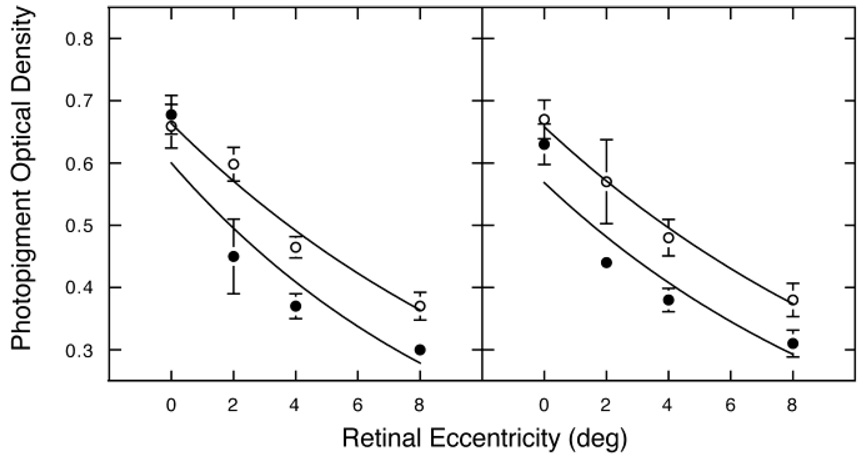
Photopigment OD and normal aging
For all subjects, photopigment OD was highest in the fovea and decreased with retinal eccentricity. This observation is valid regardless of age, genotype or phenotype. Fig. 2 shows mean photo-pigment OD as a function of retinal eccentricity for all protanopes and deuteranopes, plotted separately for younger and older observers as filled and unfilled symbols respectively. An exponential equation [Eq. (2)], following Pokorny et al. (1976), was used to describe photopigment OD as a function of retinal eccentricity for each subject. Excellent fits were obtained for all but two subjects (mean r2 = 0.88 for all dichromats for whom at least three retinal loci were measured). Comparisons of the slope of the exponential indicate that there were no significant differences between protanopes and deuteranopes, or between single- and multi-gene dichromats (unpaired t-tests, P > 0.05). As a general rule, the slopes of the exponential curve were not as steep as originally reported by Pokorny et al. (1976), a result also found by others (Picotte et al., 1994; Viénot, 2001). The smooth curves in Fig. 2 were fitted with an exponential function following Pokorny et al. (1976). Curve-fitting parameters are
| (2a) |
| (2b) |
| (2c) |
| (2d) |
where OD is photopigment optical density, M is M-cone, L is L-cone, K is OD at 8-deg temporal (M-cone = 0.30 and 0.37 for younger and older protanopes, respectively; L-cone = 0.31 and 0.38 for younger and older deuteranopes, respectively), K + OD at 8 deg is the maximum OD in the fovea, and x is retinal eccentricity in degrees.
Fig. 2.
Mean photopigment OD as a function of retinal eccentricity (deg temporal) for M-cones (protanopes) and L-cones (deuteronopes) in the left and right panels, respectively. Filled and unfilled symbols represent younger and older observers, respectively. Curves are the best-fitting exponential functions [Eq. (2)]. Error bars denote ±1 S.E.M.
Although our parameters for the best-fitting functions are different from Pokorny et al. (1976), the fit of the exponential is excellent (r2 = 0.88–0.99). Pokorny et al. obtained a steeper slope notwithstanding the fact that our mean foveal photopigment OD was substantially higher than they obtained with larger stimuli. It should be noted that all previous studies typically used 1–2 deg test stimuli. Initially, the photopigment OD value of 0.65 for foveal cones seems surprisingly high. However, because photopigment OD changes exponentially with retinal eccentricity, by extrapolation, our high foveal photopigment OD values are not unexpected for a stimulus diameter of only 0.3 deg. This has not been emphasized in the literature, although peaked distributions have been demonstrated in young observers with reflection densitometry (e.g. Elsner et al., 1993).
There is no significant difference in foveolar OD for younger and older observers nor did separate regression analysis reveal a significant correlation between photopigment OD of cones in the foveola and age. The mean photopigment OD outside the foveal center of older subjects is higher than for younger subjects. Foveal measurements were made for all subjects; however, because multiple sessions were required it was not possible to obtain measurements at more eccentric retinal locations for all subjects. The number of subjects contributing to each datum is presented in Table 1 along with means and standard deviations for each subgroup. The fewest number of data points were obtained for the 2-deg retinal eccentricity. To obtain sufficient power to test the hypothesis that photopigment OD in the para/perifovea increases with age, the data from the 4- and 8-deg measurements were pooled (by averaging) for all dichromatic subjects for whom measurements at both eccentricities had been made. Twenty-seven subjects fell into this category. There was a significant correlation between age and photopigment OD for this group (r = 0.47; P = 0.010). The best-fit line has a slope of 0.013 per decade. In a second analysis, an unpaired t-test was used to compare younger and older groups. The older group tested as having a significantly higher variance than the younger group. Thus, Welch’s correction for unequal variances was applied. The test yields a significant difference between younger and older groups (P = 0.0010;Welch’s approximate t = 3.743, 24 degrees of freedom). For the same two groups, the photopigment OD measurements at the foveola were very similar; 0.642 versus 0.646 for young and old, respectively. Overall, the range of 0.36–0.97 log unit photopigment OD foveal for all protanopes and 0.29–0.91 for all deuteranopes reveals substantial interindividual variability that was larger than seen for the results at 2, 4, and 8 deg in our data, but not larger than expected from previous literature.
Table 1.
Photopigment optical density from 0–8 deg for protanopes and deuteranopes
| Foveola |
2 (deg) |
4 (deg) |
8 (deg) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | ||
| Protanopes | |||||||||||||
| 12–27 years | Single gene | 0.66 | — | 1 | — | — | — | — | — | — | — | — | — |
| Multi-gene | 0.68 | 0.095 | 7 | 0.45 | 0.085 | 2 | 0.37 | 0.028 | 2 | 0.30 | 0.014 | 2 | |
| Total | 0.68 | 0.088 | 8 | 0.45 | 0.085 | 2 | 0.37 | 0.028 | 2 | 0.30 | 0.014 | 2 | |
| 55–83 years | Single gene | 0.72 | 0.205 | 8 | 0.61 | 0.080 | 3 | 0.44 | 0.059 | 5 | 0.35 | 0.075 | 4 |
| Multi-gene | 0.62 | 0.104 | 12 | 0.58 | 0.007 | 2 | 0.48 | 0.054 | 6 | 0.39 | 0.025 | 3 | |
| Total | 0.66 | 0.157 | 20 | 0.60 | 0.061 | 5 | 0.47 | 0.057 | 11 | 0.37 | 0.059 | 7 | |
| All | Single gene | 0.72 | 0.193 | 9 | 0.61 | 0.080 | 3 | 0.44 | 0.059 | 5 | 0.35 | 0.075 | 4 |
| Multi-gene | 0.64 | 0.103 | 19 | 0.51 | 0.087 | 4 | 0.45 | 0.070 | 8 | 0.36 | 0.055 | 5 | |
| Total | 0.66 | 0.140 | 28 | 0.56 | 0.094 | 7 | 0.45 | 0.063 | 13 | 0.35 | 0.060 | 9 | |
| Deuteranopes | |||||||||||||
| 13–29 years | Single gene | 0.63 | 0.141 | 11 | 0.44 | 0.038 | 4 | 0.39 | 0.041 | 6 | 0.29 | 0.050 | 5 |
| Multi-gene | 0.64 | 0.153 | 8 | 0.44 | 0.000 | 2 | 0.37 | 0.085 | 4 | 0.36 | — | 1 | |
| Total | 0.63 | 0.142 | 19 | 0.44 | 0.029 | 6 | 0.38 | 0.059 | 10 | 0.31 | 0.052 | 6 | |
| 56–77 years | Single gene | 0.67 | 0.142 | 21 | 0.54 | 0.150 | 3 | 0.48 | 0.125 | 14 | 0.37 | 0.091 | 11 |
| Multi-gene | 0.65 | 0.242 | 4 | 0.65 | — | 1 | 0.52 | 0.028 | 2 | 0.48 | — | 1 | |
| Total | 0.67 | 0.155 | 25 | 0.57 | 0.135 | 4 | 0.48 | 0.117 | 16 | 0.38 | 0.092 | 12 | |
| All | Single gene | 0.66 | 0.141 | 32 | 0.48 | 0.104 | 7 | 0.45 | 0.114 | 20 | 0.35 | 0.087 | 16 |
| Multi-gene | 0.64 | 0.176 | 12 | 0.51 | 0.121 | 3 | 0.42 | 0.104 | 6 | 0.42 | 0.085 | 2 | |
| Total | 0.65 | 0.149 | 44 | 0.49 | 0.103 | 10 | 0.45 | 0.110 | 26 | 0.36 | 0.087 | 18 | |
Photopigment OD in M- and L-cones
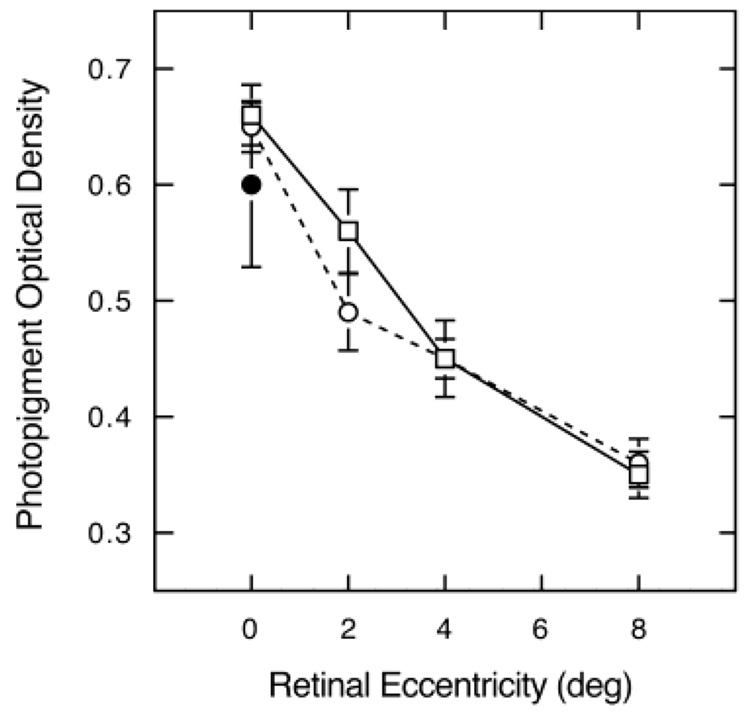
Fig. 3 shows photopigment OD for all protanopes and deuteronopes plotted as a function of retinal eccentricity. The overlapping S.E.M.s for protanopes and deuteranopes are consistent with the optical densities in M- and L-cones being the same. Unpaired t-tests did not reveal significant differences in photopigment OD between deuteranopes and protanopes or between single- and multi-gene dichromats at any retinal location.
Fig. 3.
Photopigment OD plotted as a function of retinal eccentricity for M-cones (protanopes; squares) and L-cones (deuteranopes; unfilled circles). Filled circle shows foveal values for trichromats. Error bars denote + 1 S.E.M.
Berendschot et al. (1996) reported similar optical densities for ten protanopes (0.39) and seven deuteranopes (0.42). These values were significantly lower than obtained for trichromats (0.57) which might be explained by the fact that some dichromats are missing an entire subpopulation of cones (Carroll et al., 2004). Such cone losses would not influence OD measured with our psychophysical methods. However, in view of the densitometry results, Fig. 3 presents foveal data from eight trichromats (5 younger, 12–36 years; 3 older, 69–73 years) who were tested for comparison with dichromats using our methods. These data are also relevant to many earlier studies (and all aging studies) performed exclusively with color-normal subjects. One has to bear in mind that here it is not obvious which cone type is responsible for detection. It is even possible that the responsible cone type changes as a function of bleaching level. The mean foveal photopigment OD of trichromats was 0.60, close to the mean of 0.65 for dichromats. All groups demonstrated large interindividual variability, but there were no significant differences between trichromats and protanopes or deuteranopes.
Individual variability in photopigment OD
Because of the large intersubject variability in the foveal data, it is difficult to evaluate whether or not there are differences in photopigment OD associated with differences in amino acid sequence. However, the variance for the pooled perifoveal data is much lower. Among the protanopes for whom both 4- and 8-deg results were available, three subjects (13, 26, & 36) have exon 2 encoded amino acid sequences that are typically associated with L-pigments, while three others (16, 19, & 30) have typical M-pigment exon 2 encoded sequences. The polymorphic amino acid positions are 65, 111, 116, 153, 171, 174, 178, 180, 230, 233, and 236, and positions 65, 111, and 116 are specified by exon 2. The amino acid at each of the polymorphic positions, in order and using the single letter amino acid code was TISMVAIATSV for subject 13, TISMVVISTSV for subject 26, and TISLVAIATSV for subject 36. (The single letter amino acid code is: A = alanine, L = leucine, M = methionine, I = isoleucine; T = threonine, S = serine, Y = tyrosine, V = valine.) Subjects 16, 19, and 30 all had the same M-pigment sequence at the polymorphic positions, which was IVYMVAISTSV. The differences at positions 65, 111, and 116 were previously proposed to be involved in producing changes in photopigment OD but not in λmax (Neitz et al., 1999). These two types of protanopes do have slightly different average photopigment OD values (0.388 vs. 0.448) with the higher photopigment OD associated with the normal M exon 2 sequence. An unpaired t-test shows this difference to be statistically significant but not highly so (P = 0.0495). With small differences and a limited number of subjects in each category strong claims cannot be made, but this result is consistent with the previous results from protanomalous individuals.
Discussion
Photopigment OD and normal aging
The hypothesis that reductions in photopigment OD are responsible for age-dependent sensitivity losses in M- and L-cone pathways may be rejected. Quite to the contrary, in the perifovea photopigment OD increases significantly with age. This corresponds to an approximately 14% increase in light absorption by the cones. In the absence of other changes, this would be expected to result in a measurable increase in visual sensitivity with age. Previous studies using color matching to estimate photopigment OD in relation to age have reported reductions in the fovea, but they were not as large outside the fovea (Eisner et al., 1987; Swanson & Fish, 1996). Alpern (1979), however, found a (statistically significant) increase in photopigment OD for six subjects 19 to 75 years of age. Our conclusions are based on a larger age range than previous studies (seven age decades) and is the only aging study to use X-linked dichromats (n = 72) to separate M- and L-cones. The punctate test stimulus (0.3-deg diameter) also differs from previous studies and allowed us to measure photopigment OD for discrete retinal areas, where photoreceptors may be reasonably assumed to have the same size, length, and photopigment concentration.
When response of early retinal processes is probed with the multifocal electroretinogram using achromatic stimuli (hence modulating primarily M- and L-cones), there is an age-related reduction in amplitude and increases in implicit time at all retinal eccentricities, but these changes are greater in the fovea than in the perifovea/parafovea (Gerth et al., 2002). This variation with retinal eccentricity could be related to the different age-related changes in photopigment OD across the retina, but other factors must outweigh the age-related changes in photopigment OD. Psychophysically, changes in M- and L-cone sensitivity are similar across the range of retinal eccentricities at which photopigment OD was measured in this study (Werner et al., 2000). Age-related sensitivity losses in M- and L-cone pathways must thus be due to changes other than photopigment OD, and these changes must be large since they overwhelm the paradoxical increase in perifoveal photopigment OD with age. The contribution of other factors to sensitivity losses is not surprising in view of substantial senescent changes in inner and outer segment morphology which may reduce quantal efficiency despite stable or increasing perifoveal photopigment OD (Marshall, 1978; Tucker, 1986).
Several studies have suggested that there may be age-related change in foveal architecture, and that could be relevant to the changes in photopigment OD distribution that we have observed (Elsner et al., 1998; Gorrand & Delori, 1999). Alpern (1979) suggested that perhaps “cone outer segments may be growing longer as subjects grow older” (p. 99). One possibility is that photopigment OD increases with age to compensate for light losses associated with reduced retinal illuminance resulting from lenticular senescence and reductions of pupillary area. It is possible that this occurs only in the perifovea/parafovea because the outer segment length of receptors of the foveola has reached a ceiling.
An alternative interpretation of the elevated perifovea/parafoveal photopigment OD with age might be that older subjects had difficulty maintaining fixation during eccentric threshold measurements causing their perifoveal values to be more similar to those in the fovea. To sustain this interpretation, it must be assumed that at 8 deg subjects had incredible fixation errors. We think this is unlikely because fixation was monitored in the initial test sessions and found to be stable. Also, in a previous study using similar tasks (Werner et al., 1987), fixation was monitored by an eye tracker using the pupil-cornea reflection method at a 60-Hz sampling rate. 94% of fixation time was within 0.5 deg of the foveal stimulus while engaged in a parafoveal detection task and there were no differences in fixation accuracy between younger and older subjects. Inaccuracy of fixation thus cannot account for elevations in photopigment OD beyond the fovea for elderly observers.
To maintain high quantal efficiency in the fovea with aging, the cones must remain correctly oriented to retain their waveguide properties. Longitudinal measurements of the Stiles-Crawford effect across more than 40 years (Rynders et al., 1995; DeLint et al., 1997) imply that this is the case. The foveal outer segments may be lengthening with age but gains in length may be offset by disruptions in orientation that could come from squeezing the longer outer segments in the constrained subretinal space. Other mechanisms might also contribute to changes in perifovea/parafovea photopigment OD with age as it has been shown that in rat rod photoreceptors, outer segment length, cell diameter, rhodopsin packing per disk, and regeneration rate may all vary so that photon capture is relatively constant across a range of ambient intensities (Schremser & Williams, 1992).
Photopigment OD in M- and L-cones
There is disagreement in the literature as to whether photopigment OD differs between M- and L-cones, although only limited data on photopigment OD in dichromats are available in the literature. Two psychophysical studies (Miller, 1972; Smith & Pokorny, 1973) report higher L-cone than M-cone photopigment OD, but the sample sizes were small. More recently, reflection densitometry did not reveal a difference between M- and L-cones of protanopes and deuteranopes, respectively (Berendschot et al., 1996). Our mean values of 0.65 for L-cones (range 0.29–0.91) and 0.66 for M-cones (range 0.36–0.97) are higher than the values from most previous studies, but not unexpected in view of the small field size (0.3 deg). Importantly, the number of subjects is sufficient to be confident that photopigment OD does not differ for M- and L-cones across the population.
Individual variability in photopigment OD
Individual differences in foveal photopigment OD using a 0.3-deg stimulus are substantial. The range is 0.29–0.91 for deuteranopes and 0.36–0.97 for protanopes. Large individual differences were also reported in previous work using different methods (e.g. King-Smith, 1973a,b; Swanson & Fish, 1996). Some studies also found that photopigment OD of older subjects varied more than those of younger subjects (Elsner et al., 1988, 1998).
While the molecular basis of the spectral tuning of the photo-pigments is well understood for M- and L-pigments (Neitz et al., 1991; Merbs & Nathans, 1993; Asenjo et al., 1994), less is known about how amino acid variation may affect photopigment OD. Neitz et al. (1999) reported that photopigment OD differences among the photopigments of protanomalous men are correlated with the presence of exon 2 encoded amino acid differences. Variations in pigment sequences may regulate the photopigment OD of cones and contribute to interindividual variability, more evident in the perifoveal measurements where the variance is lower. The data from protanopes presented here are consistent with the hypothesis that substitutions in exon 2 of the M genes produce pigments with lower photopigment OD.
Acknowledgments
This research was supported by the National Institute on Aging (AG04058), National Eye Institute (EY09620 and EY09303), National Eye Institute Core Grants (EY12576 and EY01931), a Jules and Doris Stein RPB Professorship, a Lew Wasserman RPB Merit Award, and unrestricted RBP funds.
References
- Alpern M. Lack of uniformity in colour matching. Journal of Physiology (London) 1979;288:85–105. [PMC free article] [PubMed] [Google Scholar]
- American Conference of Governmental Industrial Hygienists (ACGIH) TLV’s, Threshold Limit Values and Biological Exposure Indices for 2003; American Conference of Governmental Industrial Hygienists; Cincinnati, OH. 2003. [Google Scholar]
- Asenjo AB, Rim J, Oprian DD. Molecular determinants of human red/green color discrimination. Neuron. 1994;12:1131–1138. doi: 10.1016/0896-6273(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Berendschot TTJM, van de Kraats J, van Norren D. Foveal cone mosaic and visual pigment density in dichromats. Journal of Physiology (London) 1996;492:307–314. doi: 10.1113/jphysiol.1996.sp021310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker JK, Dartnall HJA. Visual pigments of rods and cones in a human retina. Journal of Physiology (London) 1980;298:501–511. doi: 10.1113/jphysiol.1980.sp013097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SA, Elsner AE. Color matching at high illuminances: Photopigment optical density and pupil entry. Journal of the Optical Society of America A. 1993;10:221–230. doi: 10.1364/josaa.10.000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J, Neitz M, Hofer H, Neitz J, Williams DR. Functional photoreceptor loss revealed with adaptive optics: An alternate cause of color blindness. Proceedings of the National Academy of Sciences of the U.S.A. 2004;101(22):8461–8466. doi: 10.1073/pnas.0401440101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coile DC, Baker HD. Foveal dark adaptation, photopigment regeneration, and aging. Visual Neuroscience. 1992;8:27–39. doi: 10.1017/s0952523800006465. [DOI] [PubMed] [Google Scholar]
- DeLint PJ, Vos JJ, Berendschot TTJM, Van Norren D. On the Stiles–Crawford effect with age. Investigative Ophthalmology and Visual Science. 1997;38:1271–1274. [PubMed] [Google Scholar]
- Eisner A, Fleming SA, Klein ML, Mauldin WM. Sensitivities in older eyes with good acuity: cross-sectional norms. Investigative Ophthalmology and Visual Science. 1987;28:1824–1831. [PubMed] [Google Scholar]
- Elsner AE, Berk L, Burns SA, Rosenberg PR. Aging and human cone photopigments. Journal of the Optical Society of America A. 1988;5:2106–2112. doi: 10.1364/josaa.5.002106. [DOI] [PubMed] [Google Scholar]
- Elsner AE, Burns SA, Webb RH. Mapping cone photo-pigment optical density. Journal of the Optical Society of America A. 1993;10:52–58. doi: 10.1364/josaa.10.000052. [DOI] [PubMed] [Google Scholar]
- Elsner AE, Burns SA, Beausencourt E, Weiter JJ. Foveal cone pigment distribution: small alterations associated with macular pigment distribution. Investigative Ophthalmology and Visual Science. 1998;39:2394–2404. [PubMed] [Google Scholar]
- Enoch JM, Stiles WS. The colour change of monochromatic light with retinal angle of incidence. Optica Acta. 1961;8:329–358. doi: 10.1080/713826396. [DOI] [PubMed] [Google Scholar]
- Gerth C, Garcia SM, Ma L, Keltner JL, Werner JS. Multifocal electroretinogram: Age-related changes for different luminance levels. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2002;240:202–208. doi: 10.1007/s00417-002-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrand J-M, Delori FC. Reflectance and curvature of the inner limiting membrane at the foveola. Journal of the Optical Society of America A. 1999;6:1229–1237. doi: 10.1364/josaa.16.001229. [DOI] [PubMed] [Google Scholar]
- Keunen JEE, van Norren D, van Meel GJ. Density of foveal cone pigments at older age. Investigative Ophthalmology and Visual Science. 1987;28:985–991. [PubMed] [Google Scholar]
- Kilbride PE, Hutman LP, Fishman M, Read JS. Foveal cone pigment density difference in the aging human eye. Vision Research. 1986;26:321–325. doi: 10.1016/0042-6989(86)90029-5. [DOI] [PubMed] [Google Scholar]
- King-Smith PE. The optical density of erythrolable determined by retinal densitometry using the self-screening method. Journal of Physiology (London) 1973a;230:535–549. doi: 10.1113/jphysiol.1973.sp010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Smith PE. The optical density of erythrolable determined by a new method. Journal of Physiology (London) 1973b;230:551–560. doi: 10.1113/jphysiol.1973.sp010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch K, Vital-Durand F, Barbur JL. Variation of chromatic sensitivity across the life span. Vision Research. 2001;41:23–36. doi: 10.1016/s0042-6989(00)00205-4. [DOI] [PubMed] [Google Scholar]
- Marcos S, Tornow R-P, Elsner AE, Navarro R. Foveal cone spacing and cone photopigment density difference: Objective measurements in the same subjects. Vision Research. 1997;37:1909–1915. doi: 10.1016/s0042-6989(96)00295-7. [DOI] [PubMed] [Google Scholar]
- Marshall J. Aging changes in human cones. In: Shimzu K, Oosterhuis JA, editors. XXIII Concilium Ophthalmologicum, Kyoto. Elsevier North-Holland: Amsterdam; 1978. pp. 375–378. [Google Scholar]
- Merbs SL, Nathans J. Role of hydroxyl-bearing amino acids in differentially tuning the absorption spectra of the human red and green cone pigments. Photochemistry and Photobiology. 1993;58:716–710. doi: 10.1111/j.1751-1097.1993.tb04956.x. [DOI] [PubMed] [Google Scholar]
- Miller SS. Psychophysical estimates of visual pigment densities in red–green dichromats. Journal of Physiology (London) 1972;223:89–107. doi: 10.1113/jphysiol.1972.sp009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neitz J, Neitz M, He JC, Shevell SK. Trichromatic color vision with only two spectrally distinct photopigments. Nature Neuroscience. 1999;2:884–888. doi: 10.1038/13185. [DOI] [PubMed] [Google Scholar]
- Neitz M, Neitz J, Jacobs GH. Spectral tuning of pigments underlying red–green color vision. Science. 1991;252:971–974. doi: 10.1126/science.1903559. [DOI] [PubMed] [Google Scholar]
- Neitz M, Carroll J, Renner A, Knau H, Werner JS, Neitz J. Variety of genotypes in males diagnosed as dichromatic on a conventional clinical anomaloscope. Visual Neuroscience. 2004;21:205–216. doi: 10.1017/s0952523804213293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picotte CJ, Stromeyer CF, III, Eskew RT., Jr The foveal color-match-area-effect. Vision Research. 1994;34:1605–1608. doi: 10.1016/0042-6989(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Smith VC, Starr SJ. Variability of color mixture data–II. The effect of viewing field size on the unit coordinates. Vision Research. 1976;16:1095–1098. doi: 10.1016/0042-6989(76)90248-0. [DOI] [PubMed] [Google Scholar]
- Polyak SL. The Retina. Chicago, Illinois: University of Chicago Press; 1941. [Google Scholar]
- Rushton WAH. The density of chlorolable in the foveal cones of the protanope. Journal of Physiology (London) 1963a;168:360–373. doi: 10.1113/jphysiol.1963.sp007197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton WAH. Cone pigment kinetics in the protanope. Journal of Physiology (London) 1963b;168:374–388. doi: 10.1113/jphysiol.1963.sp007198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton WAH. Cone pigment kinetics in the deuteranope. Journal of Physiology (London) 1965;176:38–45. doi: 10.1113/jphysiol.1965.sp007533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynders MC, Grosvenor T, Enoch JM. Stability of the Stiles–Crawford function in a unilateral amblyopic subject over a 38-year period: A case study. Optometry and Vision Science. 1995;72:177–185. doi: 10.1097/00006324-199503000-00005. [DOI] [PubMed] [Google Scholar]
- Schefrin BE, Werner JS, Plach M, Utlaut N. Sites of age-related sensitivity loss in a short-wave cone pathway. Journal of the Optical Society of America A. 1992;9:355–363. doi: 10.1364/josaa.9.000355. [DOI] [PubMed] [Google Scholar]
- Schefrin BE, Shinomori K, Werner JS. Contributions of neural pathways to age-related losses in chromatic discrimination. Journal of the Optical Society of America A. 1995;12:1233–1241. doi: 10.1364/josaa.12.001233. [DOI] [PubMed] [Google Scholar]
- Schremser J-L, Williams TP. Photoreceptor plasticity in the albino rat retina following unilateral optic nerve section. Experimental Eye Research. 1992;55:393–399. doi: 10.1016/0014-4835(92)90111-5. [DOI] [PubMed] [Google Scholar]
- Smith VC, Pokorny J. Psychophysical estimates of optical density in human cones. Vision Research. 1973;13:1199–1202. doi: 10.1016/0042-6989(73)90156-9. [DOI] [PubMed] [Google Scholar]
- Stockman A, Sharpe LT. The spectral sensitivities of the middle-and long-wavelength-sensitive cones derived from measurements in observers of known genotype. Vision Research. 2000;40:1711–1737. doi: 10.1016/s0042-6989(00)00021-3. [DOI] [PubMed] [Google Scholar]
- Swanson WH, Fish GE. Age-related changes in the color-match-area effect. Vision Research. 1996;36:2079–2085. doi: 10.1016/0042-6989(95)00280-4. [DOI] [PubMed] [Google Scholar]
- Tucker GS. Refractile bodies in the inner segments of cones in the aging human retina. Investigative Ophthalmology and Visual Science. 1986;27:708–715. [PubMed] [Google Scholar]
- Viénot F. Retinal distributions of the macular pigment and the cone effective optical density from colour matches of real observers. Color Research and Application. 2001;26:264–268. [Google Scholar]
- Walraven PL, Bouman MA. Relation between directional sensitivity and spectral response curves in human vision. Journal of the Optical Society of America. 1960;50:780–784. doi: 10.1364/josa.50.000780. [DOI] [PubMed] [Google Scholar]
- Werner JS, Donnelly SK, Kliegl R. Aging and human macular pigment density; appended with translations from the work of Max Schultze and Ewald Hering. Vision Research. 1987;27:257–268. doi: 10.1016/0042-6989(87)90188-x. [DOI] [PubMed] [Google Scholar]
- Werner JS, Bieber ML, Schefrin BE. Senescence of foveal and parafoveal cone sensitivities and their relations to macular pigment density. Journal of the Optical Society of America A. 2000;17:1918–1932. doi: 10.1364/josaa.17.001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G. The Maxwellian view. Vision Research. 1966;6:669–682. doi: 10.1016/0042-6989(66)90078-2. [DOI] [PubMed] [Google Scholar]