Abstract
Heterotrimeric G proteins typically localize at the cytoplasmic face of the plasma membrane where they interact with heptahelical receptors. For G protein α subunits, multiple membrane targeting signals, including myristoylation, palmitoylation, and interaction with βγ subunits, facilitate membrane localization. Here we show that an additional membrane targeting signal, an N-terminal polybasic region, plays a key role in plasma membrane localization of non-myristoylated α subunits. Mutations of N-terminal basic residues in αs and αq caused defects in plasma membrane localization, as assessed through immunofluorescence microscopy and biochemical fractionations. In αs, mutation of four basic residues to glutamine was sufficient to cause a defect, whereas in αq a defect in membrane localization was not observed unless nine basic residues were mutated to glutamine or if three basic residues were mutated to glutamic acid. βγ co-expression only partially rescued the membrane localization defects; thus, the polybasic region is also important in the context of the heterotrimer. Introduction of a site for myristoylation into the polybasic mutants of αs and αq recovered strong plasma membrane localization, indicating that myristoylation and polybasic motifs may have complementary roles as membrane targeting signals. Loss of plasma membrane localization coincided with defects in palmitoylation. The polybasic mutants of αs and αq were still capable of assuming activated conformations and stimulating second messenger production, as demonstrated through GST-RGS4 interaction assays, cAMP assays, and inositol phosphate assays. Electrostatic interactions with membrane lipids have been found to be important in plasma membrane targeting of many proteins, and these results provide evidence that basic residues play a role in localization of G protein α subunits.
Keywords: heterotrimeric G protein, membrane targeting, subcellular localization, palmitoylation
INTRODUCTION
G protein signaling pathways are involved in a wide variety of physiological processes, and aberrant regulation of these pathways results in a multitude of pathological conditions such as cancer, heart disease, hypertension, endocrine disorders, and blindness [1–4]. Therefore understanding the mechanism of regulation of these pathways is of utmost importance. The inactive state of the G protein is a heterotrimer of α, β, and γ subunits, with the α subunit (Gα) bound to GDP. Upon activation by an extracellular agonist the heptahelical G protein-coupled receptor (GPCR) acts as a guanine nucleotide exchange factor (GEF) and catalyzes the exchange of GDP for GTP on Gα. The binding of GTP causes a conformational change in the α subunit, which decreases the affinity of Gα for the β and γ subunits (βγ) and results in at least partial dissociation of the heterotrimer. The separated subunits, Gα and βγ, are then able to elicit their own signaling cascades. For inactivation, a family of proteins known as Regulators of G Protein Signaling (RGS) proteins act as GTPase activating proteins (GAPs) for Gα and catalyze the hydrolysis of the GTP γ phosphate, thus returning Gα to the GDP-bound form. This restores the affinity of Gα for βγ and returns the heterotrimer to the inactive state [5–7].
In order for G proteins to couple the membrane-spanning GPCRs to their effectors, they must be localized at the cytoplasmic face of the plasma membrane (PM) [8–11]. There is a two-signal model for membrane targeting of peripheral membrane proteins, which has been well described for monomeric G proteins and members of the Src family [12–16]. This model states that two signals act synergistically to allow stable membrane binding, and one of these signals may dictate targeting to a specific cellular membrane or compartment. In the case of heterotrimeric G proteins, this second signal would dictate targeting to the PM rather than intracellular membranes [17]. These membrane targeting signals could be covalent lipid modifications, interactions with other membrane-binding proteins, or polybasic stretches of amino acids.
It is well established that lipid modifications are critical in anchoring the G protein heterotrimer to the membrane [9, 18]. The γ subunit is prenylated and the Gα subunit is fatty acylated. Members of the αi family are myristoylated and palmitoylated [19–22]. The myristate is attached co-translationally to the N-terminal glycine, after removal of the initiating methionine, through a stable amide bond catalyzed by a highly specific N-myristoyl-transferase (NMT) [23]. Palmitoylation occurs on the cysteine directly next to this glycine through a thioester bond [24]. Members of other Gα families, namely αs, αq, and α12, are not myristoylated, only palmitoylated [18]. Depending on the family member, palmitoylation could occur at 1, 2, or 3 cysteines in the N-terminus and there does not seem to be any similarities in the location of the cysteines or the surrounding sequences [9, 18, 25]. Moreover, while a family of palmitoyl protein acyl transferases (PATs) have been recently discovered [26], a PAT specific for Gα has yet to be identified, and at which organelle modification of Gα occurs has yet to be resolved [27–29]. Also, while there is evidence supporting the idea that the heterotrimer forms at an endomembrane prior to reaching the PM [28, 30], it is still unknown at which endomembrane this occurs.
With myristoylation being a co-translational event, it is the earliest step that promotes membrane targeting for members of the αi family, with palmitoylation as the second signal, as it is a post-translational modification [9, 18]. This is supported by mutational analysis of the modified glycine and cysteine. Mutation of the N-terminal glycine to alanine not only inhibits myristoylation, but palmitoylation as well, and causes a shift of the protein from the PM to the cytosol [22, 31–33]. On the other hand, mutating the cysteine next to the glycine abolishes palmitoylation, but the protein is still myristoylated and its localization is shifted to intracellular membranes as well as the PM [17, 24, 34]. Therefore, according to the two-signal model, it seems as though myristoylation can play the role as the initial membrane targeting signal for the αi family, with palmitoylation being the second signal that dictates localization specifically to the PM.
The situation is less clear for Gα families that are only palmitoylated and not myristoylated. It has been demonstrated that palmitoylation is required for PM binding and proper signaling [8, 11, 25], but according to the two-signal model, another initial membrane targeting signal is needed before this modification can occur. One possibility is interaction with βγ. Crystal structures of αi and αt, each in complex with βγ, reveal two surfaces of the α subunit that interact with βγ [35, 36]. The larger of these two contact surfaces involves residues in and adjacent to the switch 1 and 2 regions, where activation-induced conformational changes occur. The second contact surface is in the N-terminal α helix. When residues in the N-termini of αs and αq believed to directly contact βγ were mutated, defects in PM localization, palmitoylation, and signaling were observed [37]. Manipulating αq so that it could be myristoylated recovered PM binding and palmitoylation of the βγ-binding mutants [37].
While there is strong evidence that interaction with βγ acts as an initial membrane targeting signal for non-myristoylated Gα families, there is also evidence that additional targeting signals may exist in these subunits and the two signal model may be expanded to allow for multiple targeting signals [9]. A potential additional targeting signal is a prominent positively charged patch in the N-termini of non-myristoylated Gα subunits, as identified through homology modeling and electrostatic surface maps [38]. Positive charges in a protein can significantly enhance its affinity for the negatively charged interface of the PM via electrostatic interactions [39]. It has been demonstrated for the monomeric GTPase K-Ras that mutations in its C-terminal polybasic residues inhibit PM localization [14]. Also, given that this positively charged motif is much less pronounced in members of the αi family, it was proposed that the polybasic motif and myristoylation play complementary roles in PM targeting of Gα [38]. Moreover, the presence of an N-terminal polybasic region in Gα suggests a revised two-signal model, in which the polybasic motif or myristoylation act in conjunction with βγ-binding as two targeting signals to allow for palmitoylation and consequent PM localization [38].
Here we explore the importance of this polybasic motif in PM targeting of two non-myristoylated Gα subunits, αs and αq. We present evidence that mutation of residues in this region causes defects in PM localization of these subunits in HEK293 cells, which can be rescued by myristoylation and partially rescued by co-expression of βγ. Mutants defective in PM localization were also defective in palmitoylation. These results provide support for the proposal that N-terminal polybasic regions contribute to the PM localization of αs and αq.
EXPERIMENTAL PROCEDURES
Cell culture
HEK293 cells were obtained from the American Type Culture Collection (Manassas, VA) and grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin. Cells were maintained at 37°C and 5% CO2.
Transfection
Cells were seeded onto either wells of 6-well plates or 6-cm plates 1 day prior to transfection. Cells in 6-well plates were transfected with 0.6 µg αs or αq expression vector while cells in 6-cm plates were transfected with 1.8 µg αs or αq, along with an appropriate amount of empty vector pcDNA3 to give a final amount of 1 µg or 3 µg DNA, respectively. Slightly higher amounts of glutamic acid mutants were transfected for fractionation, palmitoylation, and GST pulldown assays due to expression differences. The αq9Q expression vector was transfected in 2 separate plates and the cell populations were combined for fractionation, palmitoylation, and GST pulldown assays. When βγ was co-expressed, cells were transfected at a ratio of 6:3:1 of α:β:γ expression vectors. Transfections were performed with the Fugene6 transfection reagent according to the manufacturer’s protocol (Roche Applied Sciences). Experiments were performed approximately 40 hours after transfection unless otherwise noted.
Expression Plasmids
The GST-RGS4 bacterial expression plasmid was provided by Dr. R. Neubig (Univ. Michigan). MycHis-β1pcDNA3, γ2pcDNA3, HA-αspcDNA3, HA-αqpcDNA3, and the HA-tagged βγ-binding mutant αqIE have been described previously [8, 37, 40, 41]. All constructs described here also contain the internal HA epitope. αs5Q was constructed by Stratagene QuikChange mutagenesis using αspcDNA3 as a template. αs4QpcDNA3 was prepared by mutating glutamine 34 of αs5QpcDNA3 back to lysine using a sequential PCR method [42]. αq2Q, αq3Q, αq3Qn, αq4E, αq2E, and αq3En were constructed in a similar fashion using wild type αqpcDNA3 as a template. αq7Q was made by the sequential PCR method using αq4Q as a template Similarly, αq9Q was constructed using αq7Q as a template. αq7E and αq9Q2 were generated using Stratagene QuikChange mutagenesis with αq4E and αq7Q as templates, respectively. Myr-αs5Q was made by Stratagene QuikChange mutagenesis with sense and antisense primers containing the mutations L4T, G5L, and N6S [43] using αs5Q as the template. Myr-αq9Q and myr-αq3En were made using Stratagene QuikChange mutagenesis with primers that mutate the codon for the initiating methionine along with alanine 8 to glycine [37] using αq9Q and αq3En as templates. The Q227L and Q209L constitutively activating mutations were also introduced by Stratagene QuikChange mutagenesis.
Immunofluorescence Microscopy
24 hours prior to transfection cells were seeded onto 6-well plates containing coverslips. 40 hours after transfection cells were fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS) for 20 minutes at room temperature and subsequently permeabilized by incubation with blocking buffer (2.5% nonfat milk and 1% Triton X-100 in Tris-buffered saline (TBS)) for 20 minutes. Coverslips were then incubated with the indicated primary antibodies for a minimum of 1 hour, after which cells were washed with blocking buffer. Subsequently cells were incubated for 30 minutes with a 1:100 dilution of a goat anti-mouse or a goat anti-rabbit secondary antibody conjugated to either Alexa 488 or Alexa 594 (Molecular Probes, Eugene, OR). The coverslips were then washed with 1% Triton X-100 in TBS and subsequently mounted on glass slides with Prolong Gold reagent (Molecular probes, Eugene, OR). Microscopy was performed with an Olympus BX61 microscope and an ORCA-ER (Hamamatsu, Bridgewater, NJ) cooled CCD camera controlled by Slidebook version 4.0 (Intelligent Imaging Innovations, Denver, CO). Images were transferred to Adobe Photoshop for digital processing.
Cell Fractionation Assay
Cells were seeded onto 6-cm dishes for transfection. 24 hours after transfection cells were reseeded onto 10-cm plates. 16 hours later cells were washed and harvested in ice-cold PBS, centrifuged and resuspended in 500 µl hypotonic lysis buffer (50 mM Tris-Hcl, pH 8, 2.5 mM MgCl2, 1mM EDTA, 1mM dithiothreitol) with protease inhibitors (0.5mM phenylmethonylsulfonylfluoride, 5 µg/ml leupeptin, 5 µg/ml aprotinin). Cells were passed through a 27-gauge needle 10 times to lyse. Lysates were centrifuged at 2,000 rpm for 5 minutes to remove nuclei and debri. The postnuclear supernatant was centrifuged at 60,000 rpm for 20 minutes at 4°C. The supernatant (soluble fraction) was saved and the pellet (particulate fraction) was resuspended in 500 µl hypotonic lysis buffer. 50 µl 2X SDS sample buffer was added to 50 µl each fraction and boiled for 5 minutes. 25 µl of these fractions were analyzed by SDS-PAGE and western blotting.
Palmitoylation assays
36 hours after transfection in 6-cm dishes, cells were labeled with 0.5 mCi/ml [3H] palmitate. After 3 hours of labeling cells were lysed in 500 µl radioimmune precipitation (RIPA) buffer (50 mM Hepes, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 1 mM EDTA, 2.5 mM MgCl2, 1 mM PMSF, 2 µg/ml leupeptin, and 2 µg/ml aprotinin). After incubation for 1 hour at 4°C lysates were centrifuged at full speed to pellet nuclei and debris. Lysates were then adusted to 0.1% SDS. For cells transfected with αq, 10 µl αq/11 antibody (Santa Cruz) was added to lysates and 4 µl αs antibody (Rockland Immunochemicals) was added to lysates of cells transfected with αs. After 3 hours, 50 µl Protein A/G (Santa Cruz) was added and the lysates were tumbled for 1 hour. Beads were then pelleted by centrifugation for 30 seconds and then washed three times with RIPA buffer. Protein was eluted from the beads with 50 µl 2X SDS PAGE sample buffer and 5 minutes of boiling. 20 µl were resolved by SDS PAGE and transferred to PVDF, which was sprayed with EnHance (Perkin-Elmer) and subjected to fluorography at −80°C for up to 1 week. Another 20 µl were analyzed by SDS and western blotting to confirm equal amounts of protein immunoprecipitated.
cAMP and inositol phosphate assays
24 hours after transfection in 6-well plates, cells were reseeded onto 4 wells of 24-well plates. 3 of these wells were labeled with 2 µCi/ml of [3H] adenine for cAMP assays or [3H] inositol for inositol phosphate (IP) production assays. After labeling for 16 hours cAMP and IP production were assayed. Calculations were performed using the averages of the 3 wells. The fourth well was used to assay protein expression. Cells in these wells were lysed in 100 µl 2X SDS sample buffer and boiled for 5 minutes. 25 µl of the lysates were analyzed by SDS PAGE and western blotting.
For cAMP assays, cells were washed with 0.5 ml assay media (20 mM Hepes-buffered Dulbecco’s modified eagle’s medium without bicarbonate) containing 1mM isobutylmethylxanthine (IBMX) after labeling. Cells were then incubated with assay media for 1 hour followed by lysing of the cells with 0.5 ml 5% trichloroacetic acid (TCA) containing 1mM cAMP and 1 mM ATP. The samples were loaded onto Dowex AG 50W-X4 columns and [3H] ATP was eluted from the columns with water. The Dowex columns were placed on top of Alumina columns and washed with water to elute [3H] cAMP from the Dowex columns onto the Alumina columns. [3H] cAMP was eluted from the alumina columns with immidazole. The radioactivity in each fraction was determined by liquid scintillation counting. Data are presented as [3H]cAMP/([3H]cAMP+[3H] ATP)*1000.
For IP assays, cells were washed with assay media contaning 5 mM lithium chloride after labeling. Cells were then incubated with assay media for 1 hour and the reaction was terminated by adding 0.75 ml 20 mM formic acid to the cells and incubating them at 4°C for 30 minutes. 0.1 ml 0.7 M ammonium hydroxide was then added to the samples, which were loaded onto AG1-X8 Dowex columns. 1 ml of 50 mM ammonium hydroxide was added to the columns and the eluate, which constituted the [3H] inositol fraction, was collected. Columns were then washed with 4 ml 40 mM ammonium formate, 0.1 M formic acid. The [3H] IP fraction was eluted with 1ml 4M ammonium formate, 0.2 M formic acid. The radioactivity in each fraction was determined by liquid scintillation counting. Data are presented as [3H]IP/([3H]IP+[3H] inositol)*1000.
GST-RGS4 Interaction Assay
GST-RGS4 was expressed in BL-21 cells and purified using glutathione-Sepharose 4B beads (Amersham, Biosciences) as previously described [44–46], and then GST-RGS4 pulldowns were performed as described [45]. Briefly, HEK293 cells were transfected with wild type or mutant αq. 24 hr after transfection cells were washed with ice cold PBS, harvested in 0.3 ml lysis buffer (20mM Tris HCl, pH 7.4, 1mM EDTA, 1 mM dithiothreitol, 100mM NaCl, 5 mM MgCl2, 0.7% Triton X-100, 1 mM phenylmethonylsulfonyl fluoride, and 5 µg/ml leupeptin and aprotinin), followed by incubation for 1 hour at 4°C for lysis. Cells were then centrifuged at full speed in a microcentrifuge to pellet nuclei and unbroken cells. 50µl of the cleared lysate was saved for analysis of total protein in cellular lysate (TCL). 50 µl of sample buffer was added to the TCL and boiled for 5 minutes. The remaining 250 µl of post-nuclear supernatant was equally split into two tubes and 25 µM AlCl3, 5 mM NaF, and 1 mM MgCl2 were added to one tube. Subsequently 8 µg GST-RGS4-bound beads were added to both sets of tubes and the lysates were then tumbled for 2 hr at 4°C. After this incubation beads were pelleted by centrifugation at low speed for 3 minutes at 4°C. Beads were then washed three times with lysis buffer. Protein was eluted from beads with 50 µl SDS sample buffer and 5 minutes of boiling. 20 µl each of TCL and the pulldowns were resolved by SDS PAGE analysis followed by western blotting.
Western Blotting
Samples were subjected to SDS-PAGE, transferred to PVDF membranes, and blocked with TBS/0.05%Tween/5%milk. Blots were then probed with 0.5 µg/ml of either anti-HA antibody 12CA5 (Roche Applied Sciences) or anti-myc antibody 9E10 (Roche Applied Sciences), followed by incubation with horseradish peroxidase-conjugated anti-mouse antibody (Promega, Madison, WI). Blots were visualized using SuperSignal West Pico (Pierce Chemical, Rockford, Il).
RESULTS
Mutations in the polybasic motif of αs and αq disrupt PM localization
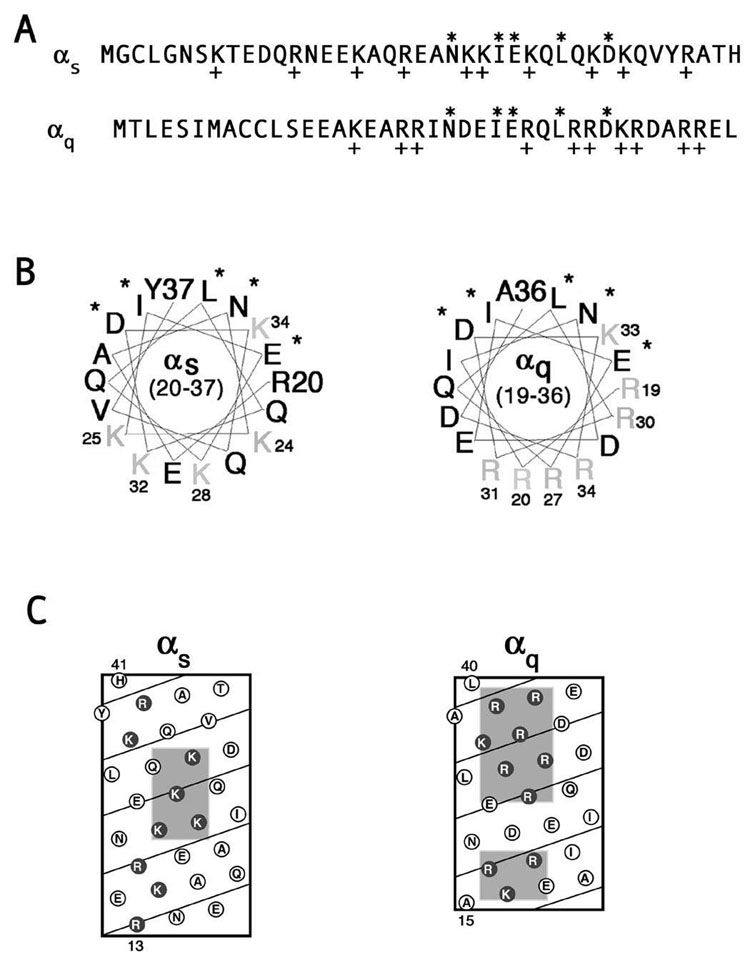
The N-terminal polybasic motif is not evident in the linear sequence of Gα (Figure 1A), but was initially identified in the N-termini of non-myristoylated Gα using homology modeling and electrostatic surface maps [38]. Indeed, helical wheel diagrams show that this motif maps to one face of the N-terminal helix of Gα, more specifically to the opposite side of the helix as residues that contact βγ (Figure 1B). Consequently these positively charged residues are free to interact with the negatively charged interface of the PM whether or not the α subunit is bound to βγ. Moreover, these basic residues are predicted to be clustered together in helical net diagrams of αs and αq (Figure 1C). In these helical net diagrams the N-terminus of αq contains two discrete basic patches while the basic residues of αs are more widespread throughout the N-terminus (Figure 1C).
Figure 1. N-termini of αs and αq.
A, Linear sequences of the N-termini of αs (amino acids 1–41) and αq (amino acids 1–40). Asterisks indicate amino acid side chains that contact βγ. Plus signs indicate basic residues. B, Helical wheel diagrams of the N-termini of αs (amino acids 20–37) and αq (amino acids 19–36). Asterisks indicate amino acid side chains that contact βγ. Basic residues are indicated in gray. C, Helical net diagrams of the N-termini of αs (amino acids 13–41) and αq (amino acids 15–40). Basic residues are indicated by the dark circles, and predicted clusters of basic residues are shaded in gray.
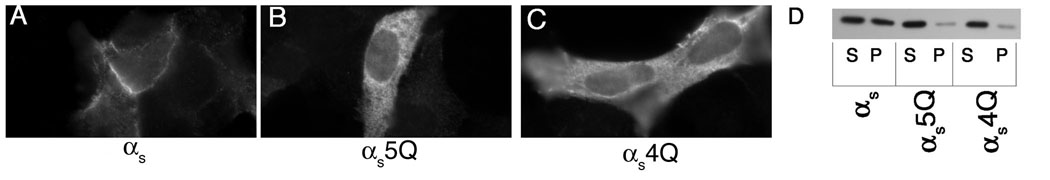
In an effort to determine the importance of the polybasic motif in PM targeting of non-myristoylated Gα subunits, we constructed mutants of αs and αq in which residues of these basic patches were mutated to glutamine, thus preserving the size of the side chain while removing the positive charge. In αs, there are five basic residues that map to one face, as shown in the helical wheel diagram of the N-terminus, namely lysines 24, 25, 28, 32, and 34 (Figure 1B). These residues were mutated to glutamine in an HA-tagged αs (αs5Q). Wild type αs and αs5Q were transiently transfected in HEK293 cells and their localization was assessed through immunofluorescence and cell fractionation assays, using an anti-HA antibody. Wild type αs exhibited strong PM localization in the majority of the cells when assessed through immunofluorescence microscopy (Figure 2A). In contrast, αs5Q displayed predominantly cytoplasmic staining (Figure 2B). When localization was assayed through subcellular fractionation, wild type αs was about evenly distributed between the soluble and particulate fractions, while αs5Q was mainly in the soluble fraction (Figure 2D). These results suggest that the polybasic motif is involved in targeting αs to the PM, as removal of positive charges of this motif changes the localization of αs from the PM to the cytosol. Since lysine 34 lies farther away from the other mutated lysines (Figure 1B), another construct (αs4Q) was created with only these four residues mutated. The localization of this mutant was indistinguishable from that of αs5Q (Figure 2C, D). Therefore, mutation of four basic residues was sufficient to cause a defect in PM localization of αs.
Figure 2. Localization of αs polybasic mutants.
HEK293 cells were transfected in 6-well plates containing coverslips with 0.4 µg pcDNA3 along with 0.6 µg αspcDNA3 (A), αs5QpcDNA3 (B), or αs4QpcDNA3 (C). 40 hours after transfection cells were fixed with 3.7% formaldehyde and visualized as described under “Experimental Procedures.” D, HEK293 cells were transfected in 6-cm plates with 1.2 µg pcDNA3 along with 1.8 µg pcDNA3 containing the indicated α subunit. Approximately 40 hours after transfection cells were lysed in hypotonic lysis buffer and soluble and particulate cell fractions were separated as described under “Experimental Procedures.” 2.5% of each fraction was resolved on 12% SDS-PAGE and the α subunits were visualized by immunoblotting with the 12CA5 antibody.
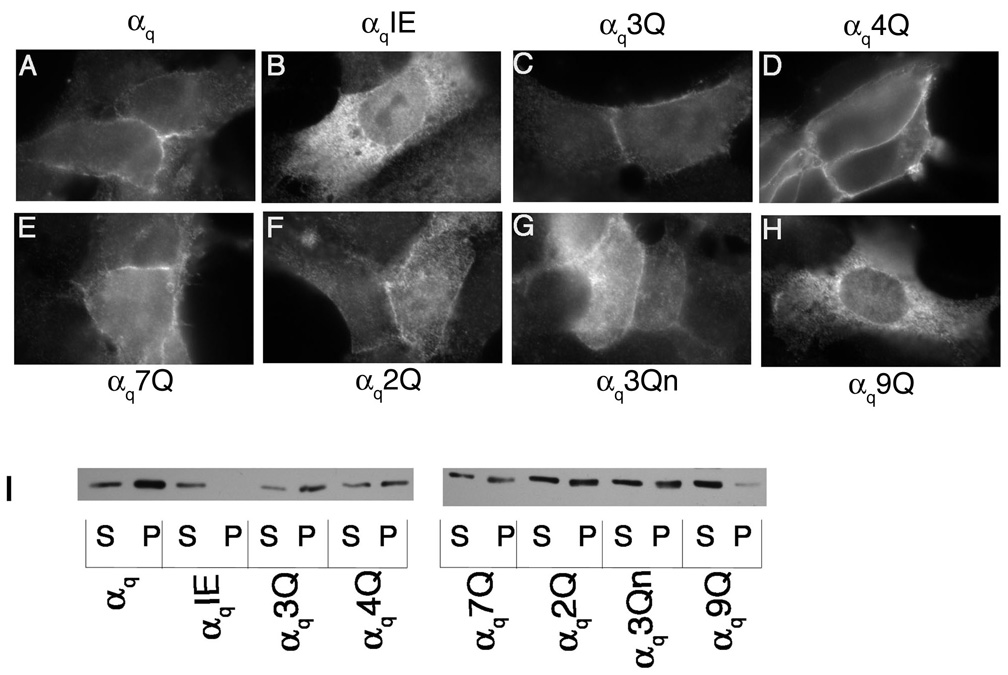
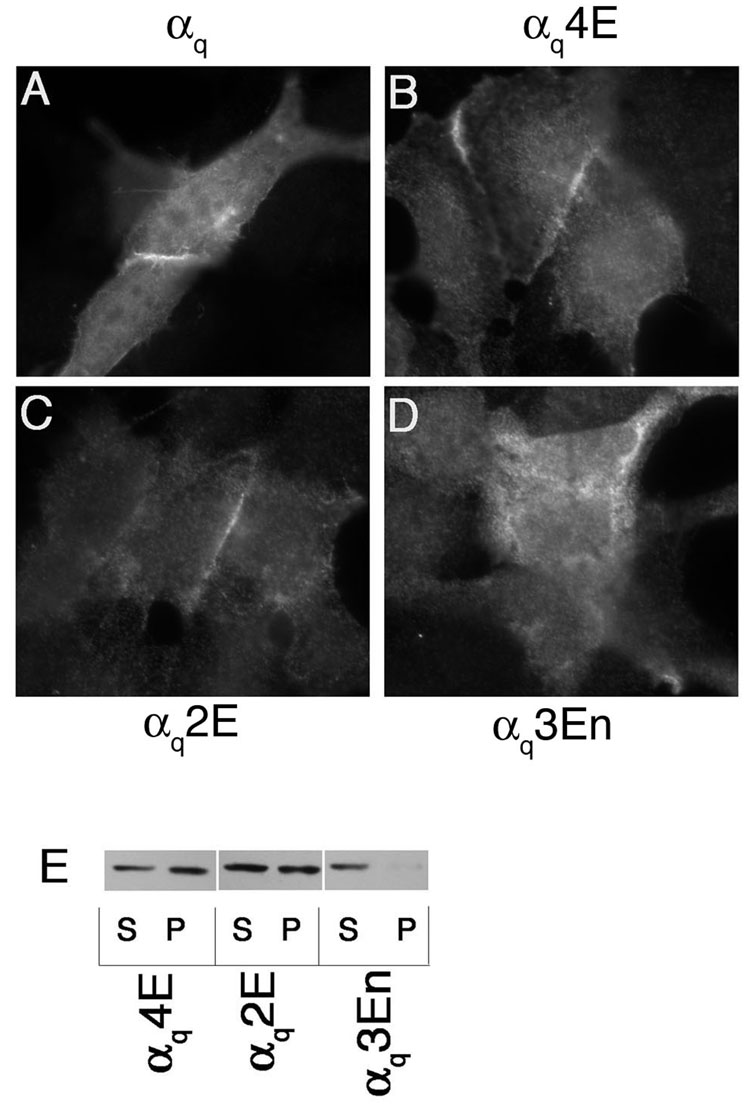
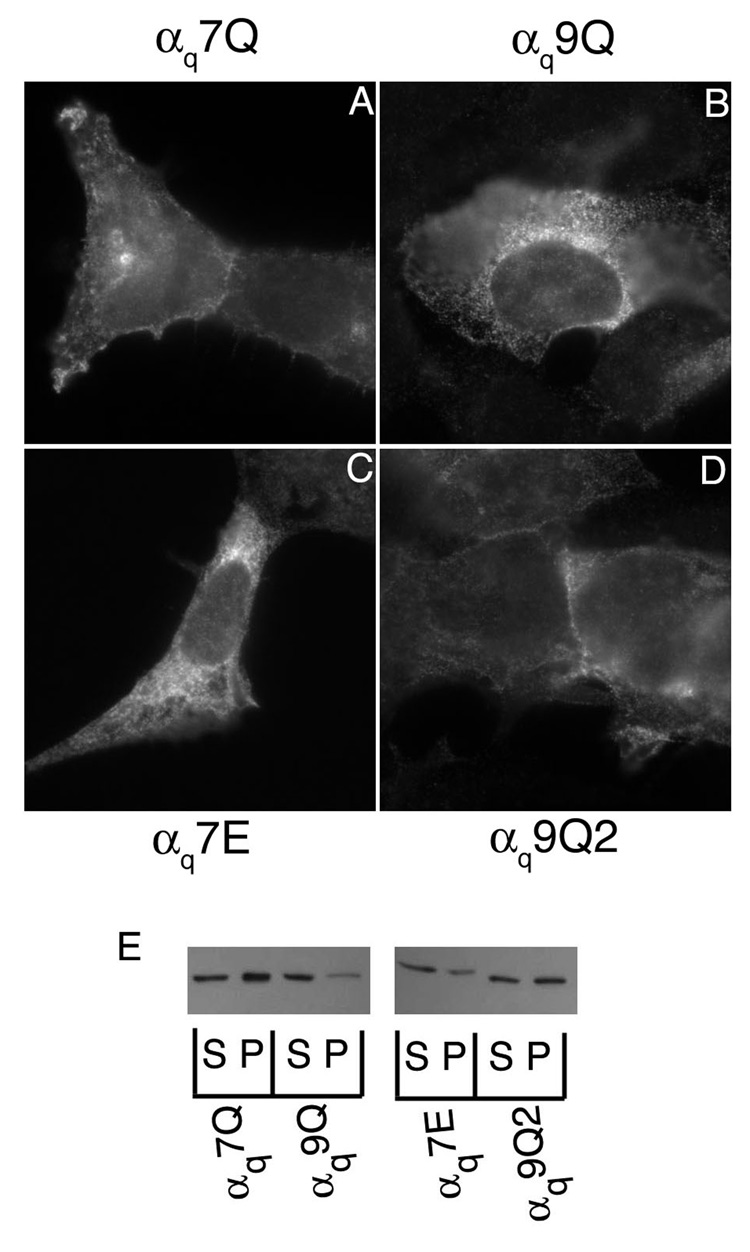
A mutant similar to αs4Q was constructed in an HA-tagged αq (αq4Q). In this mutant, arginines 27, 30, 31, and 34 were mutated to glutamine (αq4Q). These residues also map to one face of the αq N-terminal helical wheel diagram (Figure 1B) and are found clustered together in the helical net diagram of αq (Figure 1C). αq4Q and wild type αq were transiently transfected in HEK293 cells and their localization was assessed through immunofluorescence and cell fractionation assays, again using an anti-HA antibody. Wild type αq displays predominantly PM localization when assessed through immunofluorescence and is present mainly in the particulate fraction with a small portion in the soluble fraction (Figure 3A, I). As a negative control, the membrane localization of a βγ-binding mutant, αqIE, was also examined. As previously shown [37], this mutant displayed cytoplasmic staining and was present predominantly in the soluble fraction (Figure 3B, I). In contrast to αs4Q, immunofluorescence microscopy and subcellular fractionation studies revealed a pattern of localization of αq4Q that was similar to wild type αq (Figure 3D, I). Thus, mutation of these four basic residues was not sufficient to disrupt localization of αq. We next performed a more extensive mutagenesis of N-terminal basic residues of αq. A mutant was created in which arginines 37 and 38 along with lysine 33 were mutated (αq3Q). These residues are part of the large basic cluster, as seen in the helical net diagram, and are in close proximity to the residues that comprise the remainder of the large cluster and that were mutated in αq4Q (Figure 1C). All of the mutated residues of αq4Q and αq3Q were changed to glutamine in another mutant, αq7Q, where R27, R30, R31, K33, R34, R37, and R38 were all mutated to glutamine. The seven mutated residues of αq7Q along with arginines 19 and 20 were mutated to glutamine in αq9Q. A double mutant (αq2Q) with arginines 19 and 20 mutated to glutamine was also constructed. These arginines, together with lysine 16, form the second, smaller basic patch in the more extreme N-terminus of αq (Figure 1C). These three residues, lysine 16 and arginines 19 and 20, were mutated in another construct referred to as αq3Qn to determine whether the basic residues in the more extreme N-terminus were important for PM targeting. Immunofluorescence microscopy revealed that, of these mutants, only αq9Q displayed cytoplasmic staining (Figure 3H). The rest of the polybasic mutants were properly localized at the PM, similarly to wild type αq (Figure 3C–G). Consistent with the immunofluorescence images, only αq9Q displayed a drastic shift to the soluble fraction as assessed through subcellular fractionation assays (Figure 3I). Since αq2Q was present at the PM and in the particulate fraction (Figure 3F, I) this defect in membrane targeting of αq9Q cannot be attributed solely to arginines 19 and 20, the only extra residues mutated in αq9Q compared to αq7Q. We next investigated whether introducing negative charges into this basic region could cause a defect in localization of αq. To determine this we constructed mutants αq4E, αq2E, and αq3En, in which the mutated residues of αq4Q, αq2Q, and αq3Qn, respectively, were changed to glutamic acid instead of glutamine. Of these glutamic acid mutants, only αq3En had an observable defect in PM localization, as demonstrated through immunofluorescence and cell fractionations (Figure 4D, E). αq4E and αq2E were both localized at the PM (Figure 4B, C) and were still present to a significant degree in the particulate fraction (Figure 4E). Thus it seems as though mutations in the N-terminal polybasic region of αq do not have the same effects as they do in αs. A defect in αs could be observed upon mutation of only four lysines in the basic patch, whereas a defect in αq was only observed when 9 residues were mutated to glutamine or when residues 16, 19, and 20 were mutated to glutamic acid. Moreover, it seems as though mutation of the residues in the smaller basic patch in the more extreme N-terminus has more of an effect on the PM localization of αq, compared to the larger, more downstream basic patch.
Figure 3. Localization of αq glutamine mutants.
HEK293 cells were transfected in 6-well plates containing coverslips with 0.4 µg pcDNA3 along with 0.6 µg αqpcDNA3 (A), αqIEpcDNA3 (B), αq3QpcDNA3 (C), αq4QpcDNA3 (D), αq7QpcDNA3 (E), αq2QpcDNA3 (F), αq3QnpcDNA3 (G), or αq9QpcDNA3 (H). 40 hours after transfection cells were fixed with 3.7% formaldehyde and visualized as described under “Experimental Procedures.” I, HEK293 cells were transfected in 6-cm plates with 1.2 µg pcDNA3 along with 1.8 µg pcDNA3 containing the indicated α subunit. Approximately 40 hours after transfection cells were lysed in hypotonic lysis buffer and soluble and particulate cell fractions were separated as described under “Experimental Procedures.” 2.5% of each fraction was resolved on 12% SDS-PAGE and the α subunits were visualized by immunoblotting with the 12CA5 antibody.
Figure 4. Localization of αq glutamic acid mutants.
HEK293 cells were transfected in 6-well plates containing coverslips with 0.4 µg pcDNA3 along with 0.6 µg αqpcDNA3 (A), αq4EpcDNA3 (B), αq2EpcDNA3 (C), or αq3EnpcDNA3 (D). 40 hours after transfection cells were fixed with 3.7% formaldehyde and visualized as described under “Experimental Procedures.” E, HEK293 cells were transfected in 6-cm plates with 0.6 µg pcDNA3 along with 2.4 µg pcDNA3 containing a glutamic acid mutant. Approximately 40 hours after transfection cells were lysed in hypotonic lysis buffer and soluble and particulate cell fractions were separated as described under “Experimental Procedures.” 2.5% of each fraction was resolved on 12% SDS-PAGE and the α subunits were visualized by immunoblotting with the 12CA5 antibody.
To address the possibility that the introduction of multiple glutamines was causing the localization defect independent of the loss of positive charges, we examined two additional mutants. Although αq7Q did not display a membrane localization defect (Figure 3), we postulated that mutational replacement of the same residues with glutamic acid, to generate αq7E, rather than glutamine would decrease membrane localization if indeed charge of the N-terminal region of αq is critical. In agreement, with this idea αq7E displayed a pronounced shift to the cytoplasm (Figure 5C) and to the soluble fraction (Figure 5E). Next, we postulated that if indeed the loss of basic charge rather than simply a disrupting effect of multiple glutamines is important in loss of membrane binding of αq9Q, then introduction of glutamine substitutions at N-terminal sites other than the predicted critical arginines and lysines would not disrupt localization. Thus, we constructed a αq9Q2 mutant in which glutamic acid 17 and alanine 18 were mutated to glutamine in the context of αq7Q. These residues are proximal to arginines 19 and 20, the residues mutated in the context of αq7Q, to generate αq9Q. αq9Q2 displayed PM localization (Figure 5D) and an equal distribution between the soluble and particulate fractions (Figure 5E), whereas αq9Q shows a strong defect in localization at the PM and in the particulate fraction (Figure 3H and 3I, and Figure 5B and 5I), suggesting that it is specifically the loss of charge of arginines 19 and 20 that cause a defect in PM localization and not simply introduction of glutamine residues. Moreover, we compared the predicted helical content of the N-termini of the αq mutants using a secondary structure prediction algorithm [47]. αq7E is predicted to have a higher N-terminal helical content than αq7Q, although αq7E displays a membrane localization defect (Figure 5). Likewise, αq9Q2 is predicted to have a substantially decreased N-terminal helical content compared to αq9Q, but only αq9Q shows a prominent disruption of membrane localization (Figure 5). Taken together the results with the αq7E and αq9Q2 mutants support the conclusion that it is the loss of basic charge rather than simply a disruption of the N-terminal structure that leads to membrane localization defects.
Figure 5. Localization of αq7E and αq9Q2.
HEK293 cells were transfected in 6-well plate containing coverslips with 0.4 µg pcDNA3 along with 0.6 µg pcDNA3 containing αq7Q (A), αq9Q (B), αq7E (C), or αq9Q2 (D). 40 hours after transfection cells were fixed and stained with 12CA5 antibody as described under “Experimental Procedures.” E, HEK293 cells were transfected with 0.6 µg pcDNA3 along with 2.4 µg pcDNA3 containing an αq mutant. 40 hours after transfection cells were lysed in hypotonic lysis buffer and soluble and particulate cell fractions were separated as described under “Experimental Procedures.”
Myristoylation recovers PM localization of polybasic mutants of αs and αq
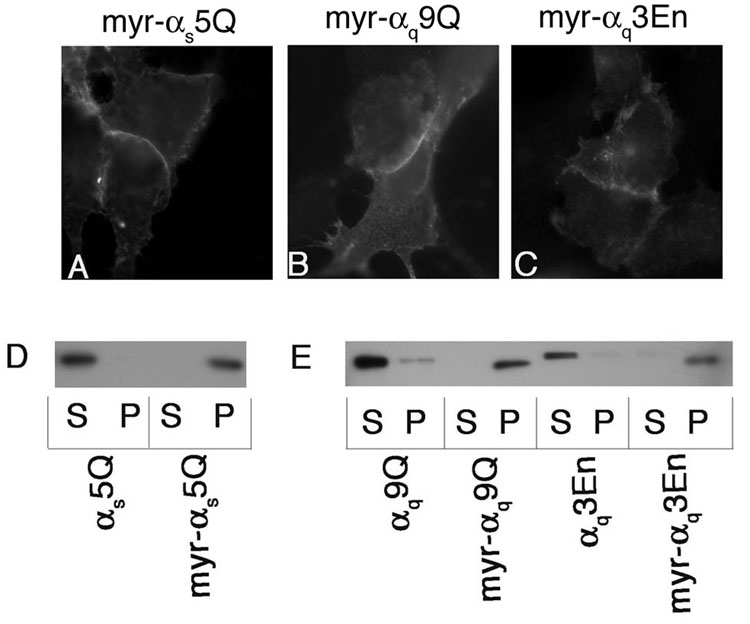
Since it has been proposed that myristoylation and this polybasic motif have complementary roles as an initial membrane targeting signal in Gα [38], we sought to determine whether myristoylaton could rescue PM localization of polybasic mutants. Replacing residues 4 through 6 of αs with residues 4 through 6 of αi results in myristoylation of αs [43]. This mutant (myr-αs) also exhibits increased palmitoylation and tighter membrane association compared to wild type αs [43]. When these mutations were introduced into αs5Q, strong PM localization was observed through immunofluorescence (Figure 6A) and a drastic shift to the particulate fraction was observed through cell fractionations (figure 6D). Myristoylation can be introduced into αq by mutating the codon for the initiating methionine, causing methionine 7 to become the initiating methionine. This mutation in conjunction with mutation of alanine 8 to glycine results in myristoylation, in addition to palmitoylation, of αq [37]. It was previously shown that introduction of these mutations into αq βγ-binding mutants rescued their PM localization and palmitoylation [37]. We thus introduced these mutations into the αq polybasic mutants that were deficient in PM localization, namely αq9Q (myr-αq9Q) and αq3En (myr-αq3En). Immunofluorescence microscopy revealed strong PM localization of these mutants (Figure 6B, C), and myr-αq9Q and myr-αq3En were also present exclusively in the particulate fraction (Figure 6E). These studies suggest that for αs and αq, the polybasic motif serves as the initial membrane targeting signal while for members of the αi family, myristoylation plays this role. These results are in accordance with the speculation that myristoylation and the polybasic motif have complementary roles as an initial membrane targeting signal [38].
Figure 6. Localization of myristoylated polybasic mutants.
HEK293 cells were transfected in 6-well plates containing coverslips with 0.4 µg pcDNA3 along with 0.6 µg pcDNA3 containing myr-αs5QpcDNA3 (A), myr-αq9QpcDNA3 (B), or myr-αq3EnpcDNA3 (C). 40 hours after transfection cells were fixed with 3.7% formaldehyde and visualized as described under “Experimental Procedures.” For fractionations, HEK293 cells were transfected in 6-cm plates with 1.2 µg pcDNA3 along with 1.8 µg pcDNA3 containing the indicated αs construct (D) or αq construct (E). 40 hours after transfection cells were lysed and fractionated as described under “Experimental Procedures.” Note that myr-αq9Q and myr-αq3En migrate slightly faster than αq9Q and αq3En, respectively. This is likely due to deletion of amino acids 1–6 in the myristoylated forms [37].
Expression of βγ rescues PM localization of αs and αq polybasic mutants
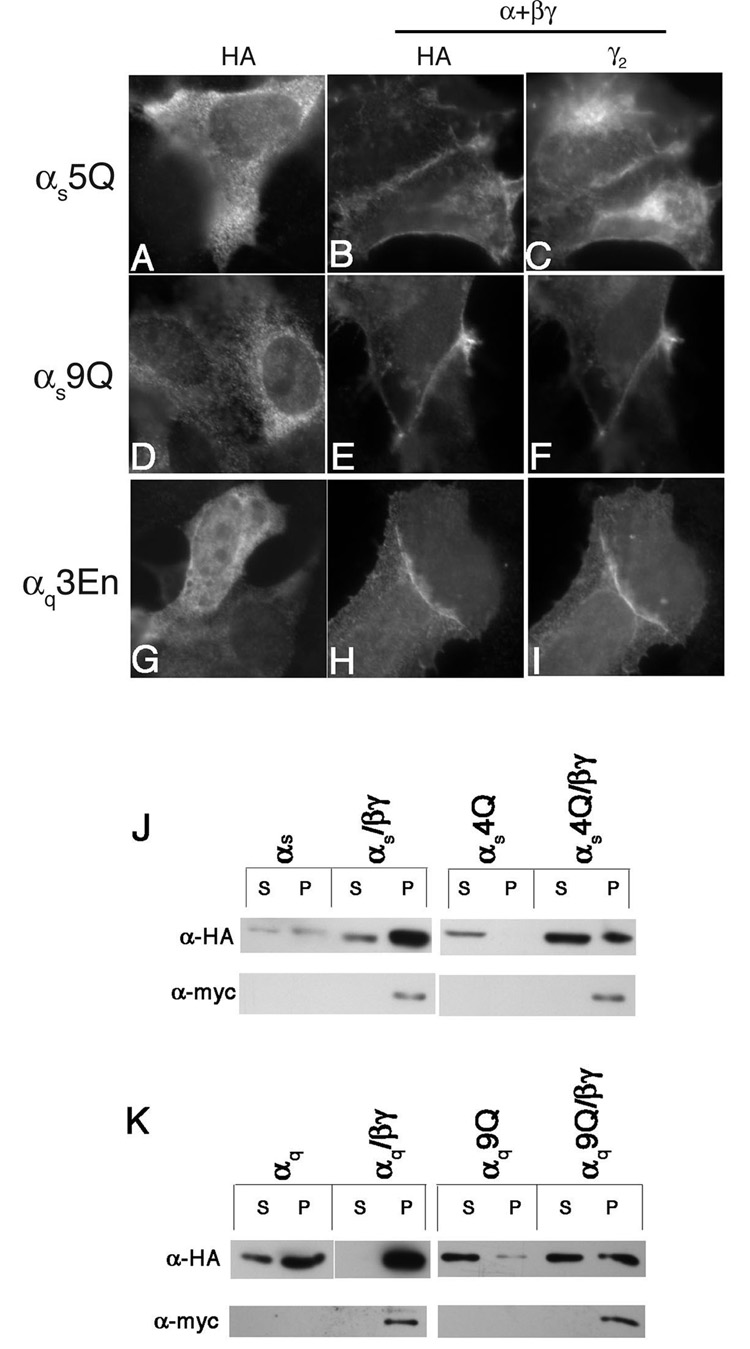
We next examined the effects of transient overexpression of β1γ2 on PM localization of the polybasic mutants. HEK293 cells were transfected with the α subunit constructs with or without co-transfection of MycHis-tagged β1 and γ2 subunits (β1γ2). Transient co-expression of β1γ2 restored PM localization of αs5Q, as determined through immunofluorescence microscopy with an anti-HA antibody (Figure 7A–C). Co-staining of the cells with an anti-γ2 antibody indicated that β1γ2 co-localized with αs5Q at the PM (Figure 7C). Similar results were observed for αs4Q (data not shown), αq9Q (Figure 7D–F), and αq3En (Figure 7G–I). The effects of β1γ2 co-expression were also assessed through cell fractionations. Co-expression of β1γ2 with wild type αs and αq resulted in an increased amount of each Gα in the particulate fraction relative to the soluble fraction (Figure 7J,K), such that 90% or more of wild type αs and αq were found in the particulate fraction when β1γ2 were also overexpressed. There was also a shift of the polybasic mutants to the particulate fraction when β1γ2 was co-expressed (Figure 7J, K). Interestingly, αs4Q and αq9Q were still not present in the particulate fraction to the same degree as wild type αs and αq. The polybasic mutants were distributed about 50:50 soluble:particulate when β1γ2 was co-expressed, while wild type αs and αq were present almost exclusively in the particulate fraction. Thus the effects of mutations in the polybasic motif can be observed even in the context of the heterotrimer.
Figure 7. Effects of coexpressing β1γ2 on the localization of polybasic mutants.
HEK293 cells were transfected in 6-well plates containing coverslips with 0.6 µg of either αs5Q pcDNA3 (A–C), αq9QpcDNA3 (D–F) or αq3EnpcDNA3 (G–I). α subunits were transfected along with either 0.4µg pcDNA3 (A, D, G) or 0.3 µg mycHis-β1pcDNA3 and 0.1 µg γ2pcDNA3 (B, C, E, F, H, I). 40 hours after transfection cells were fixed and stained with 12CA5 antibody (A, B, D, E, G, H) and anti-γ2 antibody (C, F, I) as described under “Experimental Procedures.” J, K, HEK293 cells were transfected with 1.8 µg pcDNA3 containing the indicated α subunit along with either 1.2 µg pcDNA3 or 0.9 µg mycHis-β1pcDNA3 plus 0.3 µg γ2pcDNA3. 40 hours after transfection cells were lysed and fractionated as described under “Experimental Procedures.” A portion of each fraction was resolved by SDS-PAGE and immunoblotted with either the 12CA5 antibody (upper panels) or 9E10 anti-myc antibody (lower panels).
Polybasic mutants defective in PM localization are also defective in palmitoylation
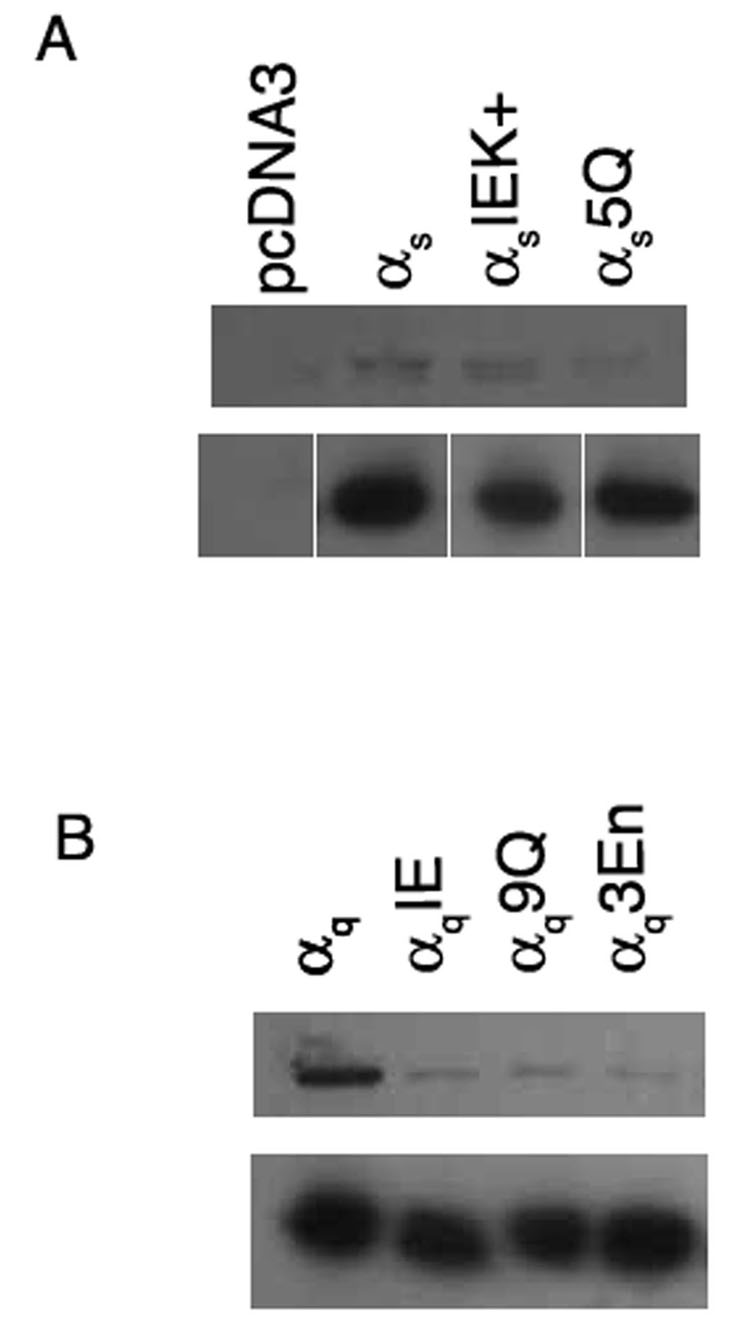
Next, we investigated whether defects in PM localization are linked with defects in palmitoylation. Gα was immunoprecipitated from lysates of cells labeled with [3H] palmitate, and immunoprecipitates were analyzed by SDS PAGE and fluorography. The βγ-binding mutant αsIEK+displayed a slightly weaker signal than wild type αs, as has been demonstrated previously [37]. αs5Q displayed a significantly weaker signal compared to wild type αs (Figure 8A). In accordance with these results, wild type αq displayed a strong signal, while αq9Q and αq3En displayed markedly reduced amounts of incorporated radiolabel (Figure 8B). These results imply that defects in PM localization of polybasic mutants are also indicative of defects in palmitoylation.
Figure 8. Palmitoylation of αs and αq polybasic mutants.
HEK293 cells were transfected in 6-cm plates with pcDNA3 containing either wild type or the indicated mutant αs (A) or wild type or the indicated mutant αq (B) in the amounts indicated under “Experimental Procedures.” αsIEK+ and αqIE are βγ-binding mutants described previously [37]. 40 hours after transfection, cells were labeled with 0.5 mCi/ml [3H] palmitate for 3 hours, after which cells were lysed and Gα was immunoprecipitated as described under “Experimental Procedures.” A portion of each immunoprecipitation was resolved by SDS-PAGE and [3H] palmitate incorporated into Gα was visualized by fluorography (upper panels) after a one-week exposure for αs and a two-day exposure for αq. A separate portion of each immunoprecipitation was analyzed by SDS-PAGE and immunoblotted with the 12CA5 antibody to confirm that similar amounts of wild type and mutant Gα were immunoprecipitated (lower panels). In each panel, samples were run together on the same gel. The samples in lower panel of A are separated to indicate that intervening lanes were removed.
Polybasic mutants can assume an activated conformation and elicit signaling responses
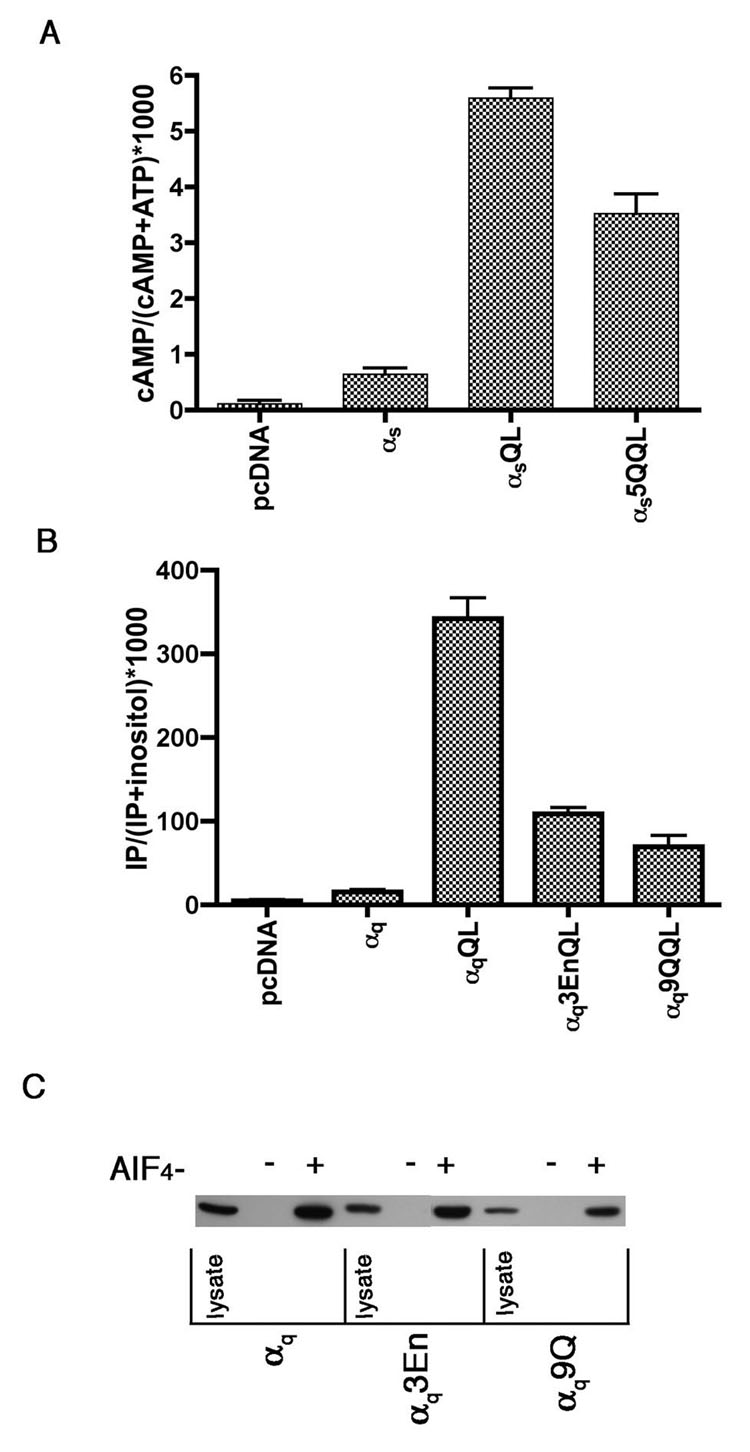
We next wanted to examine the effects of mutations in the polybasic motif on signaling abilities of αs and αq. For αs, we performed cAMP assays with constitutively active forms of the polybasic mutants. We introduced the glutamine 227 to leucine (QL) mutation into αs5Q (αs5Q QL). This mutation inhibits GTPase activity, rendering the Gα trapped in an active conformation and able to stimulate effectors independently of receptor activation [48]. HEK293 cells were transfected with wild type or mutant αs. Both αsQL and αs5Q QL were capable of stimulating cAMP (Figure 9A). This indicates that αs5Q is capable of effector activation, even though it is not present at the PM. This is not surprising, as a constitutively active palmitolyation mutant of αs, also not at the PM, was able to signal [8]. These results also indicate that the switch regions have not been perturbed, strongly suggesting that the mutant is structurally intact.
Figure 9. Signaling by αs and αq polybasic mutants.
A, HEK293 cells were transfected in 6-well plates with either 1 µg pcDNA3, 1 µg αspcDNA3 or 0.8 µg pcDNA3 plus 0.2 µg pcDNA3 containing the indicated mutant αs. 24 hours after transfection cells were labeled with [3H] adenine. 16 hours later cAMP accumulation was measured as described under “Experimental Procedures.” B, HEK293 cells were transfected in 6-well plates with either 1 µg pcDNA3 or 0.7 µg pcDNA3 plus 0.3 µg pcDNA3 containing the indicated αq construct. 24 hours after transfection cells were labeled with [3H] inositol. 16 hours later inositol phosphate was assayed as described under “Experimental Procedures.” C, HEK293 cells were transfected in 6-cm plates with the indicated αq construct. 24 hours after transfection cells were lysed and incubated in the presence or absence of AlF4 − for 2 hours at 4°C with GST-RGS4, purified as described under “Experimental Procedures.” Beads were then washed 3 times with lysis buffer. Protein was eluted from the beads by boiling in sample buffer for 5 minutes. 3.3% of the lysate and 40% of the pull downs were resolved by SDS-PAGE and visualized by immunoblotting with the 12CA5 antibody.
A similar constitutively activating mutation was also introduced at position 209 into the polybasic mutants of αq [49]. αq9Q QL and αq3En QL were capable of stimulating inositol phosphate production over basal levels, albeit to a much lesser extent than αqQL (Figure 9B), even though expression levels were similar (not shown). This could be due to the fact these mutants are presumably not present at the PM, and therefore less available to the effector, PLCβ; similarly, a constitutively active palmitolyation mutant of αq was strongly defective in its ability to stimulate inositol phosphate production in cells [8]. To rule out the possibility that this lowered activity results from altered structure of the mutants, we further confirmed the structural integrity of αq9Q and αq3En through examination of the ability of the mutants to interact with GST-RGS4 in the presence and absence of aluminum fluoride (AlF4−). AlF4 − binds to Gα-GDP in the position that would be filled by the γ phosphate of GTP, thereby promoting a Gα conformation that mimics the transition state of GTP-hydrolysis [50]. Typically, RGS proteins preferentially bind active GDP·AlF4 − -bound rather than the inactive GDP-bound Gα [5]; thus AlF4 − -dependent binding of an RGS protein and a Gα demonstrates the ability of the Gα to undergo activation-induced conformational changes. Wild type αq, αq9Q, and αq3En were expressed in HEK293 cells and cell lysates were prepared. The lysates were incubated with GST-RGS4-conjugated beads in the presence or absence of AlF4 −, followed by centrifugation to isolate interacting proteins. Western blot analysis of the pulldowns with an anti-HA antibody indicates that no wild type or mutant αq construct was pulled down in the absence of AlF4 −. On the other hand, the presence of AlF4 − caused similar amounts of wild type and mutant αq to be pulled down with GST-RGS4 (Figure 9C), indicating that the global structure and ability to undergo activation-induced conformational changes is not perturbed by N-terminal polybasic mutations in αq.
DISCUSSION
Overall, we have presented the first evidence that αs and αq contain N-terminal clusters of basic amino acids that contribute to PM localization. Mutational replacement of critical lysines and arginines with neutral glutamines strongly inhibited PM localization and palmitoylation of αs and αq. In addition, PM localization of N-terminal polybasic mutants of αs and αq could be rescued by another targeting signals, such as myristoylation. Importantly, the polybasic clusters of αs and αq also play a role in membrane localization in the context of the G protein heterotrimer; the N-terminal polybasic mutants of αs and αq showed a defect in membrane localization even when βγ was co-expressed. Thus, these results highlight a new membrane targeting signal for non-myristoylated Gα and are consistent with a revised model for membrane targeting of G proteins, in which multiple signals, including multiple lipid modifications, positively-charged patches of amino acids, and heterotrimer interactions, provide a complex combination of signals to direct G proteins to cellular membranes [9].
Although PM localization of both αs and αq could be disrupted by mutation of N-terminal basic amino acids, the studies reported here also indicate interesting differences in the N-terminal basic regions of αs and αq. Mutation of as few as four basic residues in the N-terminus of αs, as in αs4Q, was sufficient to cause a defect in PM localization, as observed by immunofluorescence microscopy and cell fractionation (Figure 2). These four critical amino acids in αs, lysines 24, 25, 28, and 32, are predicted to form a central basic patch in the N-terminus of αs (Figure 1). In contrast, a defect in PM localization was not observed for αq unless nine basic residues were mutated to glutamine (αq9Q) or three residues were mutated to glutamic acid (αq3En) (Figure 3–Figure 4). The αq9Q mutant contained substitutions in two out of the three basic residues in the first basic patch and all seven basic residues in the second, more extensive, basic patch (Figure 1). Surprisingly, mutational substitution of all seven basic residues of the second patch (αq7Q) failed to disrupt PM localization (Figure 3). The first basic patch in the N-terminus of αq thus plays an important role in PM localization, although it only consists of three basic amino acids (Figure 1). Accordingly, mutational replacement of the three basic residues of the first patch with glutamic acids (αq3En), to switch the charge of the side chains, strongly disrupted PM localization. Our extensive αq mutagenesis results (Figure 3–Figure 4) suggest that numerous basic residues in the N-terminus of αq contribute to membrane binding. Thus, PM localization of αs is more readily perturbed by N-terminal mutation of basic residues compared to αq. Interestingly, the opposite trend was observed in mutational analysis of βγ-contacting residues. A strong defect in βγ-binding, PM localization, and palmitoylation was not observed unless five βγ-contacting-residues in the N-terminus of αs were mutated [37]. On the other hand, mutation of only one βγ-interacting residue, isoleucine 25, was sufficient to cause a complete defect in βγ-binding, PM localization, and palmitoylation of αq [51]. These results could imply that the polybasic motif is more important for PM targeting of αs, whereas interaction with βγ is the more crucial PM-targeting signal for αq.
Not only did we observe differences in the importance of the N-terminal polybasic motifs in αs compared to αq, it appears that differences also exist within members of the αq subfamily. A recent report addressed mechanisms of PM localization of the αq subfamily members α14 and α16, and the data was consistent with little or no role for N-terminal basic clusters in PM localization of those two Gα [25]. The N-terminus of α14 contains nine basic residues at identical positions as nine of the ten basic residues in αq. Fusion of the N-terminal 34 amino acids of α14 to GFP was sufficient to target GFP to the PM. When all nine basic residues of the N-terminus of α14 were changed to glutamines, PM localization of the fusion protein was not disrupted, suggesting that the basic residues were not required for PM localization of α14. These mutations, however, were not examined in the context of full length α14. α16 contains eight N-terminal basic residues, although they are spread along the entire N-terminus and not predicted to comprise strong basic clusters, as in αq and α14. Mutation of five of the basic residues in the N-terminus of α16 did not cause a defect in PM localization of a GFP fusion construct and resulted in little or no change in the particulate/soluble partitioning of full-length α16 [25]. Those results combined with our results presented here suggest that the role of polybasic motifs can differ among members of the same Gα subfamily. These differences may be due to the fact that α14 and α16 have three cysteines that can serve as sites of palmitoylation [25], while αq has two [9]. This could render the polybasic motif more important for αq than it is for α14 and α16. Likewise αs, for which PM localization is disrupted by mutation of only four basic residues, has only one cysteine site of palmitoylation, consistent with the possibility that the presence of mutiple palmitoylated cysteines decreases the relative importance of polybasic motifs. It will be important to determine the role of polybasic motifs in other non-myristoylated Gα subunits, such as α12 and α13.
In view of the fact that this N-terminal polybasic motif is only present in the non-myristoylated Gα families, Kosloff, et al. hypothesized that this motif and myristoylation have complementary roles as a targeting signal. This group also proposed a revised two signal model in which for αi, myristoylation and interaction with βγ act in conjunction to allow for palmitoylation and PM localization, whereas for the other Gα subunit families, the polybasic motif and interaction with βγ act synergistically to result in palmitoylation and PM localization [38]. Our results demonstrating that the αs and αq polybasic mutants defective in PM localization are also defective in palmitoylation (Figure 8) support this model, as well as our results demonstrating that myristoylation of αs and αq can restore localization of PM-defective polybasic mutants (Figure 6). We were able to introduce N-terminal myristoylation signals into αs5Q, αq9Q and αq3En, and introduction of such myristoylation motifs into the background of these cytoplasmic αs and αq polybasic mutants resulted in strong PM localization and drastic shifts to the particulate fractions (Figure 6).
Basic motifs are present in a multitude of PM-targeted proteins. For example, while H-Ras and N-Ras are farnesylated and palmitoylated, K-Ras is farnesylated and has a cluster of basic residues in its C-terminal hypervariable region as its additional PM targeting signal [52]. When the localization of 125 CFP-tagged constitutively active small GTPases was examined, 37 out of 48 PM-localized small GTPases had C-terminal polybasic clusters consisting of four or more lysine or arginine residues at positions 5 to 20 from the C-terminus [53, 54]. Basic residues have also been implicated in the PM-localization of growth-associated protein 43 (GAP43), Src, myristoylated alanine-rich C kinase substrate (MARCKS), G-protein-coupled receptor kinases (GRKs), and other signaling proteins [16, 39, 55, 56]. In some cases, extensive polybasic domains are sufficient for PM targeting, while in many cases polybasic motifs function together with other membrane targeting signals, such as lipid modifications, to mediate membrane localization. Here we provide evidence that N-terminal basic residues are required for proper PM localization of heterotrimeric Gα subunits, thus adding to the growing list of proteins that utilize basic patches on their surfaces to bind membranes.
Regions of positive charges on the surface of a protein function as membrane binding signals via electrostatic interactions with negatively charged headgroups of membrane lipids [16, 39, 56]. Potential lipid-binding partners for basic clusters could be the monovalent acidic phosphatidylserine (PS) or the more negatively charged phosphatidylinositol 4,5-bisphosphate (PIP2) [57]. Moreover, these acidic lipids are concentrated at the PM compared to other intracellular membranes [56, 58], thus providing a simple mechanism for basic clusters directing a protein specifically to the inner surface of the PM. MARCKS has been shown to interact strongly with membranes containing physiological fractions of PS [59]. However, it was demonstrated recently that combined depletion of PIP2 and phosphatidylinositol 3,4,5-trisphosphate (PIP3) at the PM affects PM localization of several peptides containing basic clusters, such as the C-terminal tails of Rit, Rin, K-Ras, as well as the effector domain of MARCKS [54], suggesting that the phosphoinositides may be more important than PS for binding basic surfaces of proteins. It will be important in future work to define which membrane lipids are interacting with the polybasic motifs of Gα.
Polybasic-mediated protein binding to acidic lipids is not merely a static event but can be dynamic and subject to regulation through changes in the membrane lipids and changes in the electrostatics of the polybasic region. For example, decreases in PM content of phosphatidylinositol phosphates can be affected by phospholipases and lipid phosphatases, and this would cause a decrease in the acidic nature of the PM and a consequent decrease in the ability of polybasics to interact with the PM [54]. The polybasic regions themselves can be regulated through binding of other proteins to the polybasics, which would serve to sequester the polybasic region and prevent it from interacting with the acidic lipids. Ca2+/calmodulin (Ca2+/CaM) is an abundant cellular protein that often binds with high affinity to basic regions of proteins. Indeed, changes in cellular Ca2+ can promote Ca2+/CaM binding to MARCKS and induce the translocation of MARCKS from the PM [56]. Another way to disrupt the positive charge nature of a polybasic surface is through phosphorylation within the polybasic region [60]. As an example, a recent report showed that phosphorylation of K-Ras within its C-terminal polybasic region by PKC causes K-Ras to translocate from the PM to the mitochondria, where it induces apoptosis [61]. For Gα, there is some evidence for N-terminal phosphorylation [62]; it will be interesting to determine whether N-terminal phosphorylation plays a physiological role in regulating Gα interaction with the PM. In addition, it is tempting to speculate that differences in polybasic-mediated PM binding may somehow account for why αs translocates to the cytosol upon activation while other Gα subunits do not [9].
CONCLUSION
In conclusion, N-terminal basic residues can promote PM targeting of αs and αq. These studies showed similarities between the N-terminal polybasic regions of αs and αq, namely the ability of other targeting signals such as myristoylation or βγ co-expression or to restore or partially restore, respectively, PM localization of polybasic mutants. There were also some critical differences between αs and αq, such as the difference in sensitivity to these mutations of N-terminal basic residues and the ability of polybasic mutants to stimulate second messenger production. Thus, the role of basic residues in PM targeting may vary amongst Gα subunits, as has been shown through studies of the polybasic motifs of α14 and α16 [25]. Our results support the hypothesis that myristoylation and interaction with βγ lead to palmitoylation and PM localization of αi family members, whereas for the non-myristoylated Gα families, the polybasic motif compensates for myristoylation and acts in conjunction with βγ and palmitoylation to target the heterotrimer to the PM [38]. These studies shed light on mechanisms of trafficking of the G protein heterotrimer, and future studies to further discern where the heterotrimer forms and where in the cell palmitoylation occurs will provide additional insight into how the polybasic motif is targeting the heterotrimer to the PM.
ACKNOWLEDGEMENTS
This work was supported by NIH grants GM56444 (P.W.) and DK07705 (M.C.)
The abbreviations used are
- G protein
guanine nucleotide-binding protein
- PM
plasma membrane
- HEK293 cells
human embryonic kidney cells
- HA
hemagglutinin
- PAGE
polyacrylamide gel electrophoresis
- PVDF
polyvinylidene difluoride
- RGS
regulator of G protein signaling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Malbon CC. Nat Rev Mol Cell Biol. 2005;6(9):689–701. doi: 10.1038/nrm1716. [DOI] [PubMed] [Google Scholar]
- 2.Melien O. Methods Mol Biol. 2007;361:119–144. doi: 10.1385/1-59745-208-4:119. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel AM, Weinstein LS. Annu Rev Med. 2004;55:27–39. doi: 10.1146/annurev.med.55.091902.103843. [DOI] [PubMed] [Google Scholar]
- 4.Spiegelberg BD, Hamm HE. Curr Opin Genet Dev. 2007;17(1):40–44. doi: 10.1016/j.gde.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Watson N, Linder ME, Druey KM, Kehrl JH, Blumer KJ. Nature (London) 1996;383:172–175. doi: 10.1038/383172a0. [DOI] [PubMed] [Google Scholar]
- 6.Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Endocr Rev. 2003;24(6):765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- 7.Neer EJ. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 8.Wedegaertner PB, Chu DH, Wilson PT, Levis MJ, Bourne HR. The Journal of Biological Chemistry. 1993;268(33):25001–25008. [PubMed] [Google Scholar]
- 9.Marrari Y, Crouthamel M, Irannejad R, Wedegaertner PB. Biochemistry. 2007;46(26):7665–7677. doi: 10.1021/bi700338m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taussig R, Iniguez-Lluhi JA, Gilman AG. Science. 1993;261(5118):218–221. doi: 10.1126/science.8327893. [DOI] [PubMed] [Google Scholar]
- 11.Wise A, Parenti M, Milligan G. FEBS Lett. 1997;407(3):257–260. doi: 10.1016/s0014-5793(97)00300-1. [DOI] [PubMed] [Google Scholar]
- 12.Cadwallader KA, Paterson H, Macdonald SG, Hancock JF. Mol Cell Biol. 1994;14(7):4722–4730. doi: 10.1128/mcb.14.7.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hancock JF, Cadwallader K, Paterson H, Marshall CJ. Embo J. 1991;10(13):4033–4039. doi: 10.1002/j.1460-2075.1991.tb04979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock JF, Patterson H, Marshall CJ. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- 15.Kwong J, Lublin DM. Biochem Biophys Res Commun. 1995;207(2):868–876. doi: 10.1006/bbrc.1995.1266. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin S, Aderem A. Trends Biochem Sci. 1995;20(7):272–276. doi: 10.1016/s0968-0004(00)89042-8. [DOI] [PubMed] [Google Scholar]
- 17.Morales J, Fishburn CS, Wilson PT, Bourne HR. Mol Biol Cell. 1998;9(1):1–14. doi: 10.1091/mbc.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wedegaertner PB. Biol Signals Recept. 1998;7(2):125–135. doi: 10.1159/000014538. [DOI] [PubMed] [Google Scholar]
- 19.Buss JE, Mumby SM, Casey PJ, Gilman AG, Sefton BM. Proc Natl Acad Sci U S A. 1987;84(21):7493–7497. doi: 10.1073/pnas.84.21.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neubert TA, Johnson RS, Hurley JB, Walsh KA. J Biol Chem. 1992;267(26):18274–18277. [PubMed] [Google Scholar]
- 21.Schultz AM, Tsai SC, Kung HF, Oroszlan S, Moss J, Vaughan M. Biochem Biophys Res Commun. 1987;146(3):1234–1239. doi: 10.1016/0006-291x(87)90780-7. [DOI] [PubMed] [Google Scholar]
- 22.Mumby SM, Heukeroth RO, Gordon JI, Gilman AG. Proc Natl Acad Sci U S A. 1990;87(2):728–732. doi: 10.1073/pnas.87.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farazi TA, Waksman G, Gordon JI. J Biol Chem. 2001;276(43):39501–39504. doi: 10.1074/jbc.R100042200. [DOI] [PubMed] [Google Scholar]
- 24.Parenti M, Vigano MA, Newman CMH, Milligan G, Magee AI. Biochemical Journal. 1993;291:349–353. doi: 10.1042/bj2910349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedone KH, Hepler JR. J Biol Chem. 2007;282(35):25199–25212. doi: 10.1074/jbc.M610297200. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ. J Lipid Res. 2006;47(6):1118–1127. doi: 10.1194/jlr.R600007-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Dunphy JT, Greentree WK, Manahan CL, Linder ME. Journal of Biological Chemistry. 1996;271(12):7154–7159. doi: 10.1074/jbc.271.12.7154. [DOI] [PubMed] [Google Scholar]
- 28.Michaelson D, Ahearn I, Bergo M, Young S, Philips M. Mol Biol Cell. 2002;13(9):3294–3302. doi: 10.1091/mbc.E02-02-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takida S, Wedegaertner PB. FEBS Lett. 2004;567(2–3):209–213. doi: 10.1016/j.febslet.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 30.Takida S, Wedegaertner PB. J Biol Chem. 2003;278(19):17284–17290. doi: 10.1074/jbc.M213239200. [DOI] [PubMed] [Google Scholar]
- 31.Jones TL, Simonds WF, Merendino JJ, Jr, Brann MR, Spiegel AM. Proc Natl Acad Sci U S A. 1990;87(2):568–572. doi: 10.1073/pnas.87.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallak H, Brass LF, Manning DR. J Biol Chem. 1994;269(6):4571–4576. [PubMed] [Google Scholar]
- 33.Wilson PT, Bourne HR. J Biol Chem. 1995;270(16):9667–9675. doi: 10.1074/jbc.270.16.9667. [DOI] [PubMed] [Google Scholar]
- 34.Grassie MA, McCallum JF, Guzzi F, Magee AI, Milligan G, Parenti M. Biochem J. 1994;302(Pt 3):913–920. doi: 10.1042/bj3020913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. Nature. 1996;379(6563):311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 36.Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. Cell. 1995;83(6):1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 37.Evanko DS, Thiyagarajan MM, Wedegaertner PB. J Biol Chem. 2000;275(2):1327–1336. doi: 10.1074/jbc.275.2.1327. [DOI] [PubMed] [Google Scholar]
- 38.Kosloff M, Elia N, Selinger Z. Biochemistry. 2002;41(49):14518–14523. doi: 10.1021/bi026729x. [DOI] [PubMed] [Google Scholar]
- 39.Murray D, Hermida-Matsumoto L, Buser CA, Tsang J, Sigal CT, Ben-Tal N, Honig B, Resh MD, McLaughlin S. Biochemistry. 1998;37(8):2145–2159. doi: 10.1021/bi972012b. [DOI] [PubMed] [Google Scholar]
- 40.Levis MJ, Bourne HR. J Cell Biol. 1992;119(5):1297–1307. doi: 10.1083/jcb.119.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snow BE, Betts L, Mangion J, Sondek J, Siderovski DP. Proc Natl Acad Sci U S A. 1999;96(11):6489–6494. doi: 10.1073/pnas.96.11.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ausubel FM, Brent RE, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Short Protocols in Molecular Biology. New York: John Wiley and Sons; 1992. [Google Scholar]
- 43.Thiyagarajan MM, Bigras E, Van Tol HH, Hebert TE, Evanko DS, Wedegaertner PB. Biochemistry. 2002;41(30):9470–9484. doi: 10.1021/bi025533u. [DOI] [PubMed] [Google Scholar]
- 44.Sterne-Marr R, Tesmer JJ, Day PW, Stracquatanio RP, Cilente JA, O'Connor KE, Pronin AN, Benovic JL, Wedegaertner PB. J Biol Chem. 2003;278(8):6050–6058. doi: 10.1074/jbc.M208787200. [DOI] [PubMed] [Google Scholar]
- 45.Day PW, Wedegaertner PB, Benovic JL. Methods Enzymol. 2004;390:295–310. doi: 10.1016/S0076-6879(04)90019-5. [DOI] [PubMed] [Google Scholar]
- 46.Day PW, Carman CV, Sterne-Marr R, Benovic JL, Wedegaertner PB. Biochemistry. 2003;42(30):9176–9184. doi: 10.1021/bi034442+. [DOI] [PubMed] [Google Scholar]
- 47.Garnier J, Gibrat JF, Robson B. Methods Enzymol. 1996;266:540–553. doi: 10.1016/s0076-6879(96)66034-0. [DOI] [PubMed] [Google Scholar]
- 48.Graziano MP, Gilman AG. J Biol Chem. 1989;264(26):15475–15482. [PubMed] [Google Scholar]
- 49.Qian NX, Winitz S, Johnson GL. Proc Natl Acad Sci U S A. 1993;90(9):4077–4081. doi: 10.1073/pnas.90.9.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coleman DE, Berghuis AM, Lee E, Linder ME, Gilman AG, Sprang SR. Science. 1994;265(5177):1405–1412. doi: 10.1126/science.8073283. [DOI] [PubMed] [Google Scholar]
- 51.Evanko DS, Thiyagarajan MM, Takida S, Wedegaertner PB. Cell Signal. 2005;17(10):1218–1228. doi: 10.1016/j.cellsig.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Hancock JF, Magee AI, Childs JE, Marshall CJ. Cell. 1989;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 53.Heo WD, Meyer T. Cell. 2003;113(3):315–328. doi: 10.1016/s0092-8674(03)00315-5. [DOI] [PubMed] [Google Scholar]
- 54.Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. Science. 2006;314(5804):1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thiyagarajan MM, Stracquatanio RP, Pronin AN, Evanko DS, Benovic JL, Wedegaertner PB. J Biol Chem. 2004;279(17):17989–17995. doi: 10.1074/jbc.M310738200. [DOI] [PubMed] [Google Scholar]
- 56.McLaughlin S, Murray D. Nature. 2005;438(7068):605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 57.Ghomashchi F, Zhang X, Liu L, Gelb MH. Biochemistry. 1995;34(37):11910–11918. doi: 10.1021/bi00037a032. [DOI] [PubMed] [Google Scholar]
- 58.Mulgrew-Nesbitt A, Diraviyam K, Wang J, Singh S, Murray P, Li Z, Rogers L, Mirkovic N, Murray D. Biochim Biophys Acta. 2006;1761(8):812–826. doi: 10.1016/j.bbalip.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Taniguchi H, Manenti S. J Biol Chem. 1993;268(14):9960–9963. [PubMed] [Google Scholar]
- 60.Kim J, Shishido T, Jiang X, Aderem A, McLaughlin S. J Biol Chem. 1994;269(45):28214–28219. [PubMed] [Google Scholar]
- 61.Bivona TG, Quatela SE, Bodemann BO, Ahearn IM, Soskis MJ, Mor A, Miura J, Wiener HH, Wright L, Saba SG, Yim D, Fein A, Perez de Castro I, Li C, Thompson CB, Cox AD, Philips MR. Mol Cell. 2006;21(4):481–493. doi: 10.1016/j.molcel.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 62.Kozasa T, Gilman AG. J Biol Chem. 1996;271(21):12562–12567. doi: 10.1074/jbc.271.21.12562. [DOI] [PubMed] [Google Scholar]