Abstract
Background
Pancreatic insufficiency in cystic fibrosis (CF), even with replacement pancreatic enzyme therapy, is often associated with decreased carotenoid absorption. Because the macular pigment of the retina is largely derived from 2 carotenoids, lutein and zeaxanthin, the decreased serum concentrations seen in CF may have consequences for ocular and retinal health
Objectives
Our aims were to determine plasma carotenoid concentrations, determine absorption and distribution of macular pigment, and assess retinal health and visual function in CF patients.
Design
In 10 adult CF patients (ages 21–47 y) and 10 age- and sex-matched healthy control subjects, we measured macular pigment density in vivo, measured serum lutein and zeaxanthin concentrations, and comprehensively assessed visual performance (including contrast sensitivity, color discrimination, and retinal function) under conditions of daylight illumination.
Results
Serum lutein and zeaxanthin were significantly reduced (P< 0.005) in CF patients (x̄± SD:87 ± 36.1 and 27 ± 15.8 nmol/L, respectively) compared with control subjects (190 ± 72.1 and 75 ± 23.6 nmol/L, respectively). Although macular pigment optical density was significantly lower (P < 0.0001) in the CF group (0.24 ± 0.11) than in the control group (0.53 ± 0.12), no significant differences in visual function were observed.
Conclusions
Adults with CF have dramatically low serum and macular concentrations of carotenoids (lutein and zeaxanthin), but their ocular status and visual function are surprisingly good. The clinical implications of low plasma concentrations of carotenoids in CF are yet to be clarified.
Keywords: Carotenoids, cystic fibrosis, lutein, zeaxanthin, macular pigment, color vision, contrast sensitivity, electroretinogram
INTRODUCTION
Cystic fibrosis (CF) is an autosomal recessively inherited genetic disorder caused by mutations of the CFTR gene. It is most notably expressed in epithelial cells of the respiratory tract and pancreatic ducts, causing abnormalities of the airway environment and pancreatic electrolyte and water secretion and transport, which result in more viscous and elastic secretions from these organs(1). In the respiratory tract, these processes provide a favorable environment for infection and secondary exuberant inflammation, resulting in progressive lung damage and ultimately respiratory failure. Pancreatic duct obstruction leads to autolysis with resultant pancreatic exocrine and often endocrine deficiencies. Despite pancreatic enzyme replacement therapy, pancreatic insufficiency often leads to deficient absorption of fat-soluble vitamins. Although supplements of the major lipophilic micronutrients (eg, vitamins A, D, E, and K) are usually prescribed for patients with CF(2), less attention has been directed toward routine replacement of the carotenoids. Not surprisingly, carotenoid concentrations are almost uniformly low in CF patients(3–8).
The persistent infection and inflammation at airway surfaces continuously subjects CF patients to oxidative stress(9–11), especially during pulmonary exacerbations(12, 13). These oxidative processes can be expected to increase antioxidant micronutrient consumption. Because carotenoids are antioxidant micronutrients(14, 15), an increased carotenoid consumption caused by chronic respiratory inflammation can be expected to compound the deficiencies.
Two carotenoids, lutein and zeaxanthin, specifically accumulate in the macula(16, 17), the area of the retina responsible for maximal visual resolution. These molecules, which give the macula a yellow-orange color because of their absorption of short-wavelength light, are believed to play a major role in protecting retinal constituents from free radicals, both by the absorption of the highly phototoxic effects of short-wavelength light and by their free radical scavenging protective antioxidant properties(16–19). Decreased plasma concentrations of these pigments are associated with an increased incidence of macular degeneration(20), although it is unclear whether concentrations of these carotenoids in the eye directly relate to the risk of retinal disease(21). More subtle than frank macular degeneration are the losses in sensitivity of cone photoreceptors associated with normal aging(22). A high amount of macular pigment might protect the retina from age-related loss in cone sensitivity(23), but alternative interpretations have been offered(22). If macular pigment does forestall the effects of normal aging, one might expect that CF patients with chronically reduced macular pigment optical density (MPOD) would exhibit an acceleration of normal age-related changes in visual function.
The major objective of this study was to assess plasma and retinal concentrations of lutein and zeaxanthin, the main macular carotenoids, and to evaluate the status of visual performance in a group of young adult CF patients. It was hypothesized that the expected low serum concentrations of carotenoids would cause a decreased retinal carotenoid content that, in turn, could cause sub-clinical manifestations of visual abnormalities as have been reported in previous studies of ocular health in CF(24, 25).
SUBJECTS AND METHODS
Study population
Ten adult CF patients (ages 21–47 y) were recruited from a university-based CF outpatient clinic. Ten control subjects were collectively matched for age (20–51 y), sex, and ethnicity. All CF patients were clinically stable and receiving the usual medical and nutritional management as detailed in Table 1. In addition, all of the CF patients had moderate-to-severe decrements of pulmonary function and most were nutritionally sufficient on the basis of their body mass index (BMI; in kg/m2) values; only 2 patients were underweight, with BMIs < 18.5(26). All patients were exocrine pancreatic insufficient and 3 were endocrine insufficient (diabetic). All patients were taking nutritional supplements, but only one subject was taking a commercial multivitamin containing lutein. Most of the patients had low β-carotene but normal α-tocopherol concentrations on the basis of a routine chemistry panel for CF. One subject had undergone a lung transplantation. None of the subjects had symptoms of visual dysfunction other than refractive errors and presbyopia. After the initial eye examination, the patients chose one test eye to be used for more extensive testing; in most cases, the right eye was chosen.
TABLE 1.
Characteristics of the cystic fibrosis patients1
| Subject no. | Age | Sex | FEV1 | DM | BMI | Supplements | Serum α-tocopherol2 | Serum β-carotene3 | Plasma lycopene4 |
|---|---|---|---|---|---|---|---|---|---|
| Y | % of predicted | kg/m2 | µmol/L | nmol/L | nmol/L | ||||
| 1 | 27 | M | 70 | No | 24 | ADEK | 24 | 74 | 29 |
| 25 | 47 | M | 18 | Yes | 22 | ADEK | 25 | 19 | <10 |
| 3 | 21 | M | 58 | No | 20 | ADEK | ND | 37 | <10 |
| 4 | 43 | M | 30 | No | 20 | ADEK, vitamin E | 23 | 80 | 226 |
| 5 | 20 | M | 40 | No | 17 | ADEK | 6 | 24 | <10 |
| 6 | 39 | M | 30 | No | 26 | ADEK, MV | 44 | 46 | 26 |
| 76 | 40 | M | 85 | No | 21 | MV, lutein | 60 | 59 | 111 |
| 8 | 23 | F | 25 | Yes | 19 | MV, vitamin K, vitamin E | 21 | 16 | <10 |
| 9 | 26 | M | 18 | Yes | 16 | ADEK, vitamin C, iron | 11 | 52 | 61 |
| 10 | 26 | M | 78 | No | 20 | ADEK | 40 | 71 | 33 |
| x̄ ± SD | 31.2 ± 10.0 | — | 45 ± 25.4 | — | 21 ± 3.0 | — | 28 ± 16.9 | 48 ± 23.4 | 53 ± 68.7 |
FEV1, forced expiratory volume in 1 s; DM, diabetes mellitus; MV, multivitamins; ND, not determined. The ADEK preparation includes 150 IU α-tocopherol and 3 mg β-carotene.
Laboratory reference range for healthy persons: 12–46 µmol/L.
Laboratory reference range for healthy persons: 110–370 nmol/L.
Laboratory reference range for healthy persons: 150–300 nmol/L.
This patient had a history of smoking.
This patient had a lung transplantation 5 y before the study and was taking a multivitamin preparation containing 250 µg lutein/capsule.
The control subjects were volunteers recruited through the University of California, Davis, Department of Ophthalmology. They had no ocular or systemic conditions, such as diabetes, and all were nonsmokers. These subjects denied taking any supplements containing lutein. After approval by the University of California, Davis, Office of Human Research Protection, consecutive patients seen in the adult CF clinic were offered half-day eye examinations; those who accepted were enrolled. Approximately 50% volunteered for the study. Of those who chose not to participate, lack of time and the often-considerable distance to the clinic were the 2 major reasons for refusal to participate.
Measurement of carotenoid concentrations
Serum concentrations of β-carotene and α-tocopherol were routinely available as part of our yearly assessments of CF patients and are as reported in Table 1. Lycopene, as an additional indicator of carotenoid uptake, was measured on the day of eye examination, as were lutein and zeaxanthin as the major components of the macular pigment. Blood and plasma samples were covered to reduce light exposure during handling and were frozen at − 70° within 30 min of collection until analyzed.
For these latter 3 carotenoid determinations, liquid-liquid carotenoid extraction of plasma samples was performed with ethanol n-hexane containing 0.05% butylated hydroxytoluene as previously described(27). After evaporation of the hexane layers, the residue was redissolved in 160 µL methanol: THF (50:50) and analyzed by reversed-phase HPLC. The carotenoids were separated by gradient elution with the use of a C30 YMC carotenoid column (5 FM, 250 × 4.6 mm; Waters, Milford, MA) and detected at 450 nm. The column was calibrated for retention times and quantification with the use of external calibration plots prepared from crystalline lutein (Sigma, St Louis), all-trans zeaxanthin, and all-trans lycopene.
Eye examination
All subjects received a complete optometric or ophthalmologic examination to detect the presence of retinal disease and abnormal ocular media. This included a slit-lamp examination and direct and indirect ophthalmoscopy. Color stereo fundus photographs of the macula and optic disc (Early Treatment Diabetic Retinopathy Study fields 1 and 2) were evaluated by a retinal specialist using a stereo viewer. Intraocular pressure was ≤22 mm Hg.
Optical density and distribution of macular pigment
The macular pigment acts as a filter of short-wave (blue) light. By quantifying this filter effect, it is possible to assess the amount and distribution of macular pigment. The absorption and distribution of the macular pigment (at 15 min, 30 min, 1°, 1.75°, and 7° retinal eccentricity) were assessed by the psychophysical method of heterochromatic flicker photometry(22, 28). For this study we used the densitometer by Macular Metrics, Providence, RI(29). The stimulus consisted of a 10°, 3.0-cd/m2, 470-nm adapting background. Superimposed on the background was 1 of 5 test stimuli: 2 test spots of 15 and 30 min diameter, 2 circular rings of 1° and 1.75°, and a 2° spot for the peripheral measurement. The test stimuli were composed of 2 narrow-band lights (from light-emitting diodes) having dominant wavelengths of 460 and 550 nm, which were alternated in square-wave counter-phase at 15–25 Hz for the 4 central stimuli and 7–12 Hz for the peripheral one. The flicker frequency was optimized for each subject on the basis of preliminary tests of flicker sensitivity. The subject’s task was to eliminate or minimize the flicker by turning a dial that changed the ratio of light of the 2 wavelengths while maintaining a constant overall luminance. The subject viewed the stimulus in free view from a chin and forehead rest and with habitual refractive correction. Before each session, the light intensity from each wavelength was calibrated with a photodiode.
Spatial contrast sensitivity
Photopic contrast sensitivity was measured to provide an assessment of spatial vision under conditions of daylight illumination (photopic vision). This measure quantifies pattern vision for objects of any size including small objects as more traditionally measured by visual acuity. The subjects’ eyes were dilated and cyclopleged with 2.5% Phenylephrine and 0.5% Tropicamide (both from Bausch & Lomb Pharmaceuticals, Inc (Tampa, FL). Measurements of the contrast sensitivity function were obtained with vertically oriented sinusoidal gratings at 6 spatial frequencies (0.55, 1.125, 2.25, 4.5, 9, and 18 cycles/degree) and a spaceaverage luminance of 45 cd/m2. The stimuli were presented on a computer monitor and viewed at optical infinity through an astronomical telescope designed so that the effective pupil are a was constant across all subjects. Details of the apparatus and calibration are described by Schefrin et al(30). Thresholds, defined in terms of Michelson contrast, were measured by using a maximum-likelihood(31), 2-alternative, temporalforced-choice procedure in which subjects pressed a button to indicate in which of 2 intervals (defined by a computer voice) the stimulus was presented. Contrast thresholds for the test gratings corresponded to a detection probability of 75%, based on a logistic psychometric function.
Color discrimination
The perception of color is mediated by parallel neural pathways, which begin with the 3 cone types maximally sensitive at short, middle, or long wavelengths. Discrimination of colors dominated by each of the 3 cone types is expressed as the protan, deutan, and tritan axes of the Cambridge Colour Test (Cambridge Research Systems, Cambridge, United Kingdom). Losses in the sensitivity of cone pathways has been associated with normal aging(22), and selective losses have been demonstrated in retinal and optic nerve disorders(32, 33). Unspecified color vision defects have been reported for CF patients(24). For this study, the Cambridge Colour Test was used to present Landolt C patterns (2–16 cd/m2) on a calibrated computer monitor by using a video board with 15-bit resolution. The subject’s task was to detect the location of the gap in a Landolt C with the use of a 4-alternative forced choice based on the principles of pseudo-isochromatic plates(34). Discrimination thresholds were defined by the minimum chromatic contrast required for detection of stimuli varied in terms of the stimulation of short-wave (tritan), middle-wave (deutan), and long-wave (protan) sensitive cones.
Multifocal electroretinogram
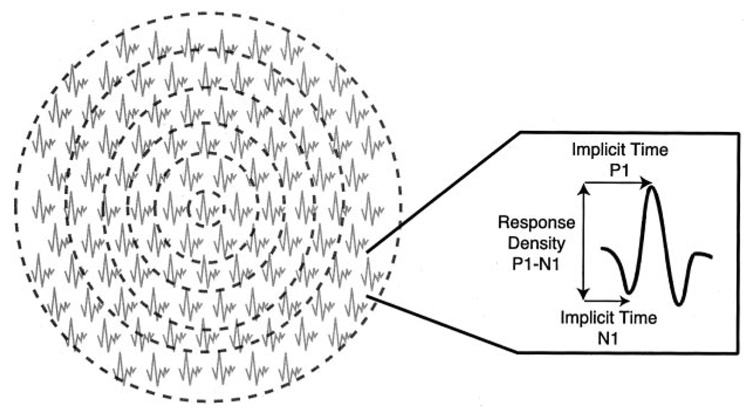
Retinal function was assessed electrophysiologically by the multifocal electroretinogram (mfERG). As illustrated in Figure 1, this technique permits the response at discrete retinal locations to be isolated and to detect retinal dysfunction that has not yet led to visible changes in the fundus or global decreases in visual performance. This technique is based on a cross-correlation between hexagonal black-and-white stimulus changes and the local response of the retina, thus reflecting the first stage of retinal processing(35). The stimulus consisted of 103 scaled hexagons (flashed pseudorandomly at intervals of 13.3 ms) and extended to a retinal area of ≈50° in diameter. The recording procedure was performed as published previously(36). First-order kernel responses were evaluated in each of the patient’s mfERGs. These responses were grouped into 6 concentric rings. Implicit times (latencies) of N1 (first negative trough) and P1 (first positive peak) and amplitudes P1-N1 (difference between P1 and N1) were analyzed for each of the 6 areas as indicated in Figure 1. Amplitudes were measured on the response-density-scaled regional averages. Data were not compared with those of the control subjects, but rather with data from a more extensive agematched control group of 10 subjects in each age decade(36), which formed the standard forour laboratory. Latencies within or <2 SDs of the control group were considered normal.
FIGURE 1.
Multifocal electroretinogram responses for a control subject. The 103 responses were grouped into 6 concentric rings (ring 1: area stimulated by the central hexagon, 1° radius; ring 2: 1–5°; ring 3: 5–10°; ring 4: 10–15°; ring 5: 15–20°; ring 6: 20–25°). The response extracted from each ring was analyzed in terms of the parameters illustrated in the right panel. P1, first positive peak; N1, first negative trough; P1-N1, difference between P1 and N1.
Statistics
The plasma tocopherol and carotenoid concentrations are reported as means ± 1 SD. The analysis of the data was conducted with the use of parametric tests, analysis of variance, t tests, and regression [STATVIEW 5.0.1 (SAS Institute, Cary, NC) and Excel X (Microsoft, Redmond, WA)]. Unless stated, the differences between groups were considered significant if the P values were < 0.05.
RESULTS
Carotenoid concentrations
As shown in Table 1, the CF patients represented a group of young, mostly male adults with various degrees of lung impairment. All of the patients were taking vitamins and pancreatic enzyme supplements, and 3 were taking insulin. Most of the subjects had normal tocopherol concentrations. However, as in other studies(3–8), all of the subjects had low β-carotene and lycopene concentrations. As shown in Table 2, lutein and zeaxanthin concentrations in the CF patients were typically less than one-half those of the healthy control subjects. Only CF patient 7 was taking a lutein supplement, and only this patient had received a lung transplant. This fact is important because this patient no longer had a chronically infected lung that could conceivably represent a site for antioxidant micronutrient overconsumption.
TABLE 2.
Plasma carotenoid concentrations in the cystic fibrosis (CF) patients and the healthy control subjects1
| CF patients | Control subjects | |||
|---|---|---|---|---|
| Lutein | Zeaxanthin | Lutein | Zeaxanthin | |
| nmol/L | nmol/L | |||
| 1 | 85 | 34 | 182 | 81 |
| 2 | 67 | <10 | 134 | 66 |
| 3 | 69 | 35 | 256 | 83 |
| 4 | 105 | 38 | 110 | 60 |
| 5 | 69 | 33 | 202 | 77 |
| 6 | 83 | 33 | 223 | 77 |
| 7 | 183 | 55 | 127 | 55 |
| 8 | 64 | <10 | 136 | 43 |
| 9 | 66 | <10 | 347 | 131 |
| 10 | 76 | <10 | 182 | 81 |
| x̄ ± SD | 87 ± 36.1 | 27 ± 15.8 | 190 ± 72.1 | 75 ± 23.6 |
Plasma lutein and zeaxanthin concentrations were measured on the same day as the macular pigment densities were measured and the visual performance tests were conducted. Laboratory reference ranges for healthy persons are 105–370 nmol/L for lutein and 34–117 nmol/L for zeaxanthin 37). There was an overall significant difference in plasma concentrations of lutein (unpaired t test, P < 0.001) and zeaxanthin (unpaired t test, P < 0.001) between the 2 groups.
Clinical eye examinations
The clinical eye examination showed that one patient had a corneal scar as the result of prior trauma. Another patient had suffered a retinal vein occlusion in the eye that was not used in testing. All other patients were found to be in good ocular health and to have a maximum refractive error of − 2.0 diopters and a best-corrected visual acuity of 20/20 or better.
Macular pigment optical density
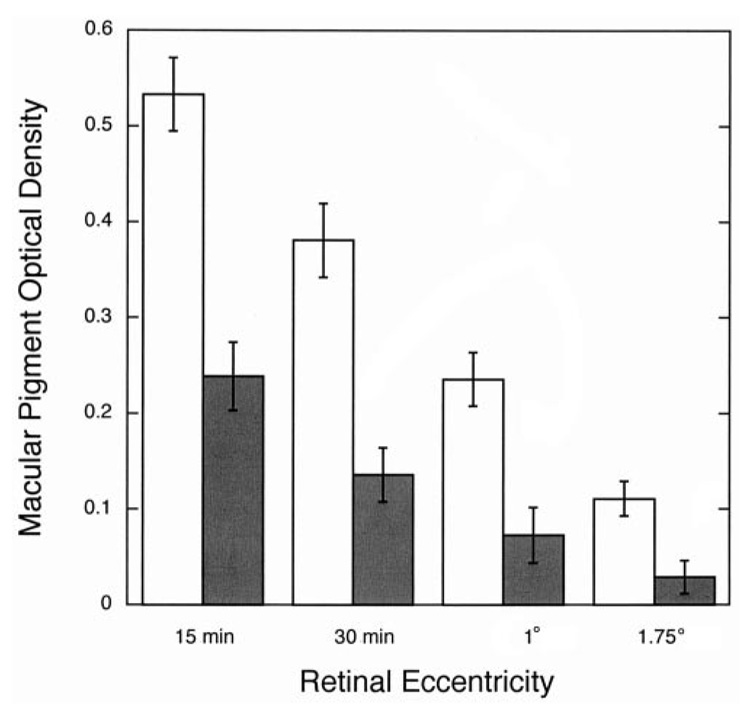
As shown in Figure 2, MPOD was lower at all retinal locations in the CF patients than in the control group. In the central 15 min, where MPOD is highest, optical density was 0.24 ± 0.11 in the CF patients compared with 0.53 ± 0.12 in the control group. The spatial distribution of macular pigment is also shown in Figure 2. A mixed between-within analysis of variance verified that there was a significant difference in MPOD between the CF patients and age-matched control subjects (P < 0.0001) and a significant difference in MPOD among the 4 retinal eccentricities. However, no significant interaction between the groups and retinal eccentricity was observed (P > 0.05).
FIGURE 2.
Mean (± 1 SEM) macular pigment optical density at 4 retinal eccentricities (15 and 30 min, 1°, and 1.75°) in cystic fibrosis (CF) patients (■; n = 10) and age-matched control subjects (□; n = 10). Analysis of variance showed a significant difference (P < 0.05) in overall macular pigment optical density between the CF patients and the control subjects but no significant group × retinal eccentricity interaction.
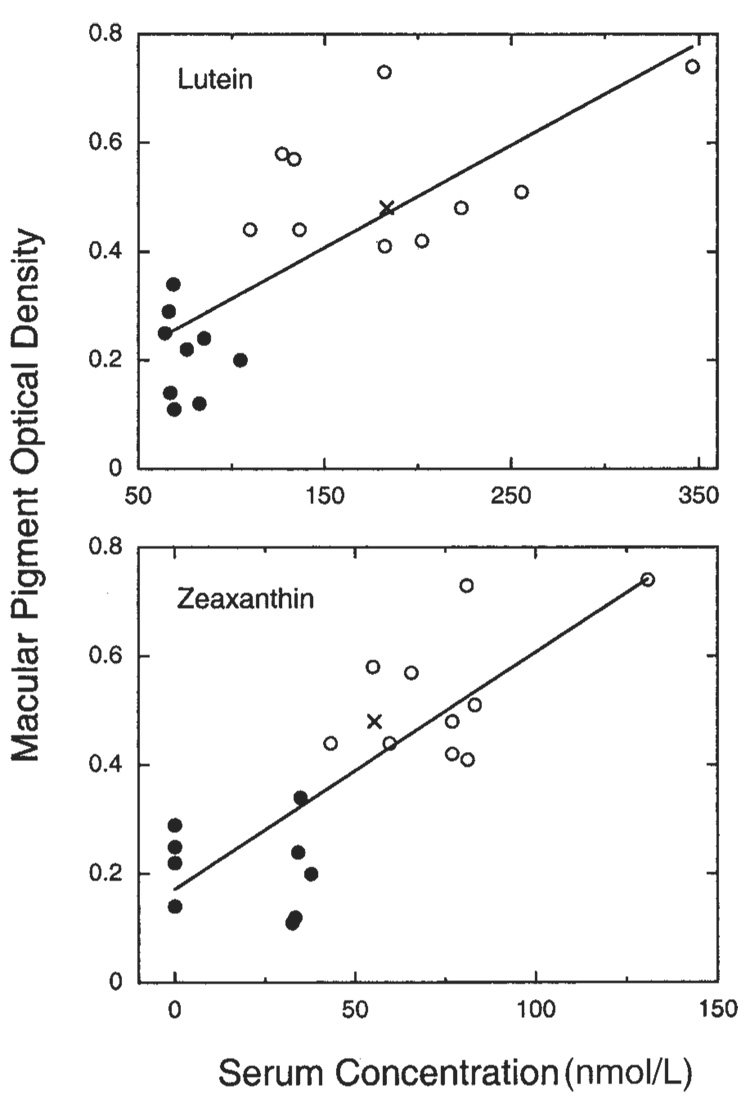
MPOD plotted as a function of serum concentration of lutein and zeaxanthin is illustrated in Figure 3. The regression lines were fitted to all subjects, but the data for the CF patients and healthy control subjects are denoted separately by different symbols. The correlation between MPOD and lutein was significant (r= 0.76, P < 0.001), as was the correlation between MPOD and zeaxanthin (r = 0.80, P < 0.001). Three of the 4 CF patients aged > 35 y had unusually low MPOD values (x̄: 0.15 at 15 min). However, there was one outlier—the one patient who had been taking a lutein-containing multivitamin supplement. As shown in Figure 3, this patient’s MPOD and serum lutein and zeaxanthin concentrations were uncharacteristically high for a CF patient.
FIGURE 3.
Mean macular pigment optical density as a function of serum lutein and zeaxanthin concentrations in cystic fibrosis (CF) patients (●; n = 10; the “x” represents one CF patient who was taking a lutein supplement) and age-matched control subjects (○; n = 10). The linear regression equations fitted to all subjects were as follows: lutein, y = 0.126 + 0.0019x (r = 0.76); zeaxanthin, y = 0.171 + 0.0044x (r = 0.80).
Contrast sensitivity
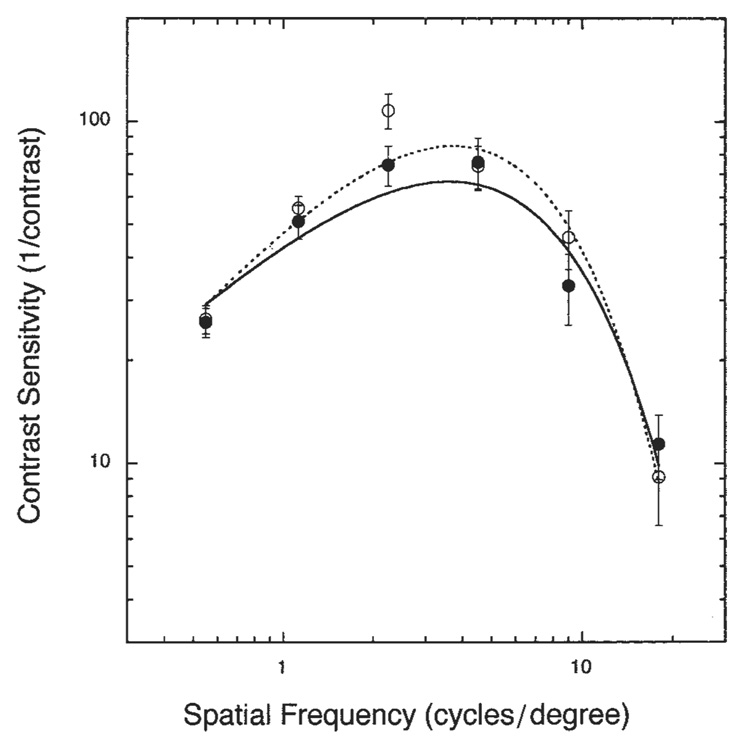
Photopic contrast sensitivity plotted as a function of spatial frequency for CF patients and age-matched control subjects is shown in Figure 4. Error bars signify ± 1 SEM. The data were fit with a double-exponential function(38). Although there was a main effect of spatial frequency (P < 0.05), we found no significant difference between the groups (P = 0.2428) and no significant group × spatial frequency interaction (P = 0.5753).
FIGURE 4.
Mean (± 1 SEM) contrast sensitivity as a function of spatial frequency for cystic fibrosis (CF) patients (●; n = 10) and age-matched control subjects (○; n = 10). Smooth curves fitted to the data are double exponentials (solid and dashed curves for the CF and control subjects, respectively) as described in the text. Analysis of variance showed no significant difference between groups and no significant group × spatial frequency interaction.
Color discrimination
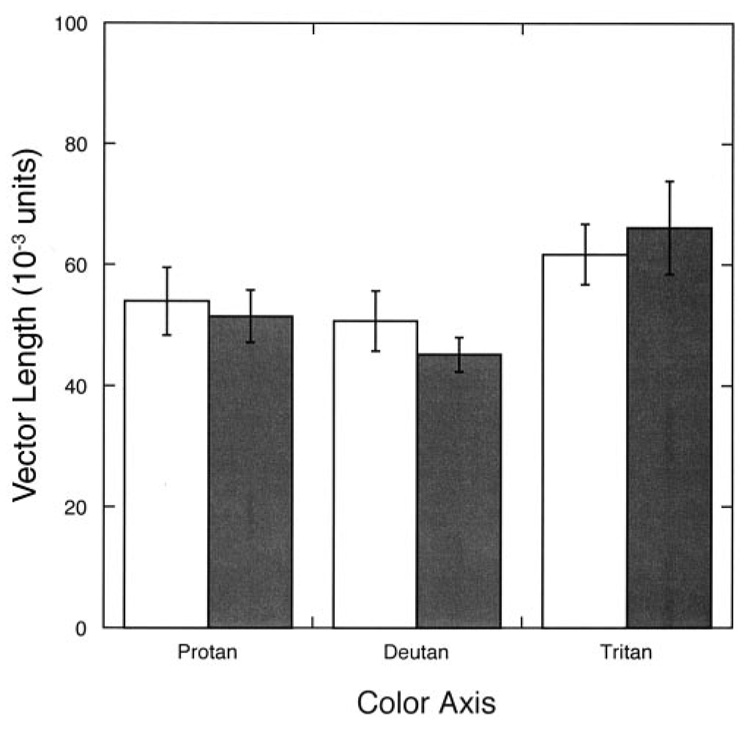
As shown in Figure 5, the CF patients had color discrimination values well within the normal range as defined by the designers of the Cambridge Colour Test (vector lengths ≤ 100 for the protan and deutan axes and ≤ 150 for the tritan axis)(39). Control subject no. 1 was excluded from the statistical analysis of the test results because he was known to be deuteranomalous. The unpaired Student’s t test showed no significant difference between CF patients and control subjects for any of the 3 axes (protan axis: P= 0.729; deutan axis: P = 0.331; tritan axis: P = 0.645).
FIGURE 5.
Mean (± 1 SEM) vector length (a measure of color discrimination) in Commission International d’Eclairage u’v’ chromaticity space for cystic fibrosis (CF) patients (■; n = 10) and age-matched control subjects (□; n = 9) for protan, deutan, and tritan color confusion axes. Unpaired t tests showed no significant differences between the CF patients and the control subjects.
Multifocal electroretinogram
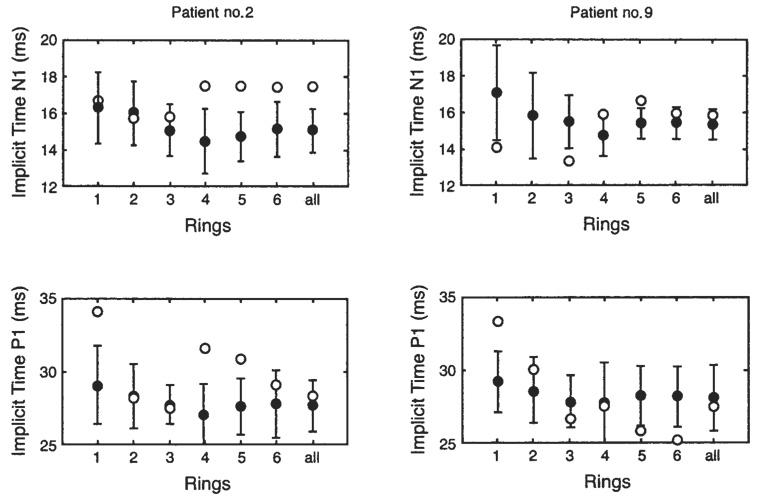
Nine of the 10 recorded mfERGs were analyzed. One of the mfERGs could not be analyzed because of low signal-to-noise-ratio. The concentric ring analysis showed normal responses in 7 of the 9 CF patients analyzed. Two CF patients showed delayed latencies but normal response densities. In CF patient no. 2, implicit times N1 and P1 were delayed in rings 4 to 6 and in rings 1, 4, and 5, respectively, as shown in Figure 6. In CF patient no. 9, implicit time N1 was delayed in ring 5 and implicit time P1 was delayed in the central area.
FIGURE 6.
Delay in N1 (first negative trough) and P1 (first positive peak) implicit times for 2 cystic fibrosis patients (E) and for the respective age-matched control subjects (●; n = 10). The error bars for the control subjects represent ± 2 SDs. The 6 concentric rings are numbered from the center (ring 1) to the periphery (ring 6) as illustrated in Figure 1.
DISCUSSION
CF patients have to cope with a high level of lung oxidative stress due to chronic infection and inflammation. They often also suffer from a lack of fat-soluble antioxidant vitamins because of chronic malabsorption. Our study aimed to investigate whether the expected reductions of carotenoids in CF patients would manifest in a reduction in retinal lutein and zeaxanthin and whether this loss of presumably protective carotenoids affects ocular function, retinal health, and visual performance. The data verified that serum concentrations of carotenoids, including lutein and zeaxanthin, were low in adult CF patients. This low concentration was correlated with considerably low retinal concentrations of the 2 carotenoids. The severe degree of retinal carotenoid depletion was not associated with any visual abnormalities by the testing procedures used.
Plasma and retinal carotenoids
The mean serum concentrations of lutein and zeaxanthin (87 and 27 nmol/L, respectively) in CF patients represented 47% and 36%, respectively, of those in the collectively matched control subjects and were lower than the values found in the lowest quintile of concentrations in the third National Health and Nutrition Examination Survey(20). We also found a remarkably strong correlation between serum carotenoids and macular pigment for the patients and control subjects, which was consistent with the findings of an epidemiologic study of 280 adult volunteers whose serum concentrations of lutein and zeaxanthin correlated with MPOD values(40). Of special interest in this context are CF patient no. 7, who had been taking a lutein supplement and thus had high normal serum carotenoid concentrations and MPOD values, and CF patient no. 2, who had a smoking history and who consistently had very low carotenoid concentrations and a very low MPOD. Interestingly, smokers represent another group of patients that are known to have a high incidence of airway inflammation and reduced carotenoid concentrations(41).
The starting point of this investigation was the hypothesis that CF patients with known low carotenoid concentrations would have low ocular concentrations of the antioxidants lutein and zeaxanthin. These carotenoids have largely been thought to protect the retina from oxidative damage(21, 42, 43), so the question arose as to whether the lack of macular pigment would produce symptoms of increased oxidative stress such as those found in the degenerative retinal diseases. Although we found the low values for MPOD that we had expected, only one CF patient had retinal abnormalities, and this patient’s retinal responses were normal as assessed with the mfERG. The retinal vein occlusion observed in CF patient no. 7 was thought by retinal specialists to be due to a vascular rather than to an ocular incident and was probably caused by this patient’s uncontrolled hypertension secondary to the side effects of the transplantation medications being taken. This finding is in marked contrast with the findings of previous studies of CF patients, which reported loss of contrast sensitivity and color vision deficiencies(25) and abnormal electrophysiological findings(24, 44, 45).
The discrepancy between the findings of the current study and those of previous studies might be due to several factors. First, the clinical management of exocrine pancreatic insufficiency has improved in the past 2 decades. The focus on pancreatic enzyme replacement and high-dose supplements of vitamins such as ADEK clearly has helped prevent ocular symptoms of vitamin A deficiency such as night blindness and severe forms of xerophthalmia, as has been shown in previous studies(25). One study(45) reported an improvement in contrast sensitivity after high-dose vitamin A supplementation. Second, use of the broad-spectrum antibiotic chloramphenicol in CF patients has been reduced to almost nothing. This drug has long been known to cause intermittent and permanent optic nerve damage in CF patients(46) and might have contributed to the loss of contrast sensitivity and the unspecified color vision deficiencies reported in a previous study(24). Although we could not eliminate the possibility that our patients had been treated with chloramphenicol at some point, it seems reasonable to assume that they may have been exposed to less of it than were the adult participants of previous studies. A third factor contributing to the ocular health of our adult CF patients is the management of CF-related diabetes. Good blood sugar regulation might delay the onset of diabetic ocular manifestations that lead to cataract and diabetic retinopathy.
Methodologic limitations
Our results need to be interpreted with some caution because we only studied a small number of CF patients, and there was little ethnic and socioeconomic variance in this group. Our studies were cross-sectional and focused on an age group that seldom exhibits age-related maculopathy. Moreover, the CF patients had few risk factors for age-related maculopathy, such as elevated blood pressure (except for CF patient no. 7) or a severe degree of atherosclerosis, although 1 smoked and 3 had diabetes(47). Our previous work shows that the protocol used in this study included several measures that are sensitive to deficits associated with early development of age-related macular degeneration(48). On the other hand, the plasma and macular pigment concentrations of lutein and zeaxanthin were largely different between the CF patients and the control subjects in the current study. If inverse relations between concentrations of these 2 carotenoids and ocular and visual abnormalities were causal and strong, we hypothesized that with sensitive testing methods we might have been able to detect them.
Clinical implication
Although there is accumulating evidence that the consumption of high amounts of dietary antioxidant micronutrients is positively associated with both respiratory(49) and eye(50) health, and that carotenoid intakes (including lutein and zeaxanthin) contribute to this evidence, it is interesting to note that both the recent Institute of Medicine guidelines(50) and the consensus CF nutritional guidelines(2) issue no specific recommendations for healthy persons or CF patients regarding the consumption of carotenoid supplements or foods high in lutein and zeaxanthin. As the life span of CF patients continues to increase as a function of improved nutrition, better strategies for handling airway clearance, respiratory tract infections, and lung transplantation, it is predictable that CF patients may increasingly manifest the consequences of age-related diseases, including macular degeneration.
We concluded that serum and retinal lutein and zeaxanthin concentrations were dramatically low but there was no evidence of a loss in visual performance or compromised retinal health in adult CF patients receiving standard care for CF. It would be useful to conduct the same protocol used in this study in a larger group of CF patients studied longitudinally over a broader spectrum of the life span and with a focus on CF patients aged > 50 y.
Footnotes
Supported by the Cystic Fibrosis Foundation, Hoffmann-La Roche Inc, the National Institute on Aging (AG04058), the Research to Prevent Blindness Jules and Doris Stein Professorship, Klee-Stiftung, Frankfurt aM, and the Studienstiftung des deutschen Volkes (German Scholarship Foundation). The all-trans zeaxanthin used was a gift from NI Krinsky (Tufts University, USDA, Boston), and the all-trans lycopene used was a gift from Hoffmann-La Roche (Basel, Switzerland).
All of the authors contributed to the study design, data collection, data analysis, and writing of the manuscript. None of the authors had a known conflict of interest.
REFERENCES
- 1.Welsh MJ, Tsui IT, Boat TF, Beaudet AL. The metabolic basis of inherited disease. New York: McGraw Hill; 1995. [Google Scholar]
- 2.Ramsey BW, Farrell PM, Pencharz P. Nutritional assessment and management in cystic fibrosis: a consensus report. The Consensus Committee. Am J Clin Nutr. 1992;55:108–116. doi: 10.1093/ajcn/55.1.108. [DOI] [PubMed] [Google Scholar]
- 3.Homnick DN, Spillers CR, Cox SR, et al. Single- and multiple-dose-response relationships of beta-carotene in cystic fibrosis. J Pediatr. 1995;127:491–494. doi: 10.1016/s0022-3476(95)70089-7. [DOI] [PubMed] [Google Scholar]
- 4.Winklhofer-Roob BM, Puhl H, Khoschsorur G, van’t Hof MA, Esterbauer H, Shmerling DH. Enhanced resistance to oxidation of low density lipoproteins and decreased lipid peroxide formation during beta-carotene supplementation in cystic fibrosis. Free Radic Biol Med. 1995;18:849–859. doi: 10.1016/0891-5849(94)00203-v. [DOI] [PubMed] [Google Scholar]
- 5.Winklhofer-Roob BM, Schlegel-Haueter SE, Khoschsorur G, van’t Hof MA, Suter S, Shmerling DH. Neutrophil elastase/alpha 1-proteinase inhibitor complex levels decrease in plasma of cystic fibrosis patients during long-term oral beta-carotene supplementation. Pediatr Res. 1996;40:130–134. doi: 10.1203/00006450-199607000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Lepage G, Champagne J, Ronco N, et al. Supplementation with carotenoids corrects increased lipid peroxidation in children with cystic fibrosis. Am J Clin Nutr. 1996;64:87–93. doi: 10.1093/ajcn/64.1.87. [DOI] [PubMed] [Google Scholar]
- 7.Rust P, Eichler I, Renner S, Elmadfa I. Long-term oral beta-carotene supplementation in patients with cystic fibrosis—effects on antioxidative status and pulmonary function. Ann Nutr Metab. 2000;44:30–37. doi: 10.1159/000012818. [DOI] [PubMed] [Google Scholar]
- 8.Renner S, Rath R, Rust P, et al. Effects of beta-carotene supplementation for six months on clinical and laboratory parameters in patients with cystic fibrosis. Thorax. 2001;56:48–52. doi: 10.1136/thorax.56.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown RK, Kelly FJ. Evidence for increased oxidative damage in patients with cystic fibrosis. Pediatr Res. 1994;36:487–493. doi: 10.1203/00006450-199410000-00013. [DOI] [PubMed] [Google Scholar]
- 10.van der Vliet A, Eiserich JP, Marelich GP, Halliwell B, Cross CE. Oxidative stress in cystic fibrosis: does it occur and does it matter? Adv Pharmacol. 1997;38:491–513. doi: 10.1016/s1054-3589(08)60996-5. [DOI] [PubMed] [Google Scholar]
- 11.Cobanoglu N, Ozcelik U, Gocmen A, Kiper N, Dogru D. Antioxidant effect of beta-carotene in cystic fibrosis and bronchiectasis: clinical and laboratory parameters of a pilot study. Acta Paediatr. 2002;91:793–798. doi: 10.1080/08035250213212. [DOI] [PubMed] [Google Scholar]
- 12.Hull J, Vervaart P, Grimwood K, Phelan P. Pulmonary oxidative stress response in young children with cystic fibrosis. Thorax. 1997;52:557–560. doi: 10.1136/thx.52.6.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood LG, Fitzgerald DA, Gibson PG, Cooper DM, Garg ML. Increased plasma fatty acid concentrations after respiratory exacerbations are associated with elevated oxidative stress in cystic fibrosis patients. Am J Clin Nutr. 2002;75:668–675. doi: 10.1093/ajcn/75.4.668. [DOI] [PubMed] [Google Scholar]
- 14.Burton GW. Antioxidant action of carotenoids. J Nutr. 1989;119:109–111. doi: 10.1093/jn/119.1.109. [DOI] [PubMed] [Google Scholar]
- 15.Krinsky NI, Yeum KJ. Carotenoid-radical interactions. Biochem Biophys Res Commun. 2003;305:754–760. doi: 10.1016/s0006-291x(03)00816-7. [DOI] [PubMed] [Google Scholar]
- 16.Bone RA, Landrum JT, Cains A. Optical density spectra of the macular pigment in vivo and in vitro. Vision Res. 1992;32:105–110. doi: 10.1016/0042-6989(92)90118-3. [DOI] [PubMed] [Google Scholar]
- 17.Schalch W, Dayhaw-Barker P, Barker FM, editors. Nutritional and environmental influences on the eye. Boca Raton, FL: CRC Press; 1999. [Google Scholar]
- 18.Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995;62 suppl:1448S–1461S. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell P, Wang JJ, Smith W, Leeder SR. Smoking and the 5-year incidence of age-related maculopathy: the Blue Mountains Eye Study. Arch Ophthalmol. 2002;120:1357–1363. doi: 10.1001/archopht.120.10.1357. [DOI] [PubMed] [Google Scholar]
- 20.Mares-Perlman JA, Fisher AI, Klein R, et al. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am J Epidemiol. 2001;153:424–432. doi: 10.1093/aje/153.5.424. [DOI] [PubMed] [Google Scholar]
- 21.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 22.Werner JS, Bieber ML, Schefrin BE. Senescence of foveal and parafoveal cone sensitivities and their relations to macular pigment density. J Opt Soc Am A. 2000;17:1918–1932. doi: 10.1364/josaa.17.001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammond BR, Jr, Wooten BR, Snodderly DM. Preservation of visual sensitivity of older subjects: association with macular pigment density. Invest Ophthalmol Vis Sci. 1998;39:397–406. [PubMed] [Google Scholar]
- 24.Spaide RF, Diamond G, D’Amico RA, Gaerlan PF, Bisberg DS. Ocular findings in cystic fibrosis. Am J Ophthalmol. 1987;103:204–210. doi: 10.1016/s0002-9394(14)74228-x. [DOI] [PubMed] [Google Scholar]
- 25.Ansari EA, Sahni K, Etherington C, et al. Ocular signs and symptoms and vitamin A status in patients with cystic fibrosis treated with daily vitamin. A supplements. Br J Ophthalmol. 1999;83:688–691. doi: 10.1136/bjo.83.6.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korner J, Aronne LJ. The emerging science of body weight regulation and its impact on obesity treatment. J Clin Invest. 2003;111:565–570. doi: 10.1172/JCI17953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obermueller-Jevic UC, Espiritu I, Corbacho AM, Cross CE, Witschi H. Lung tumor development in mice exposed to tobacco smoke and fed beta-carotene diets. Toxicol Sci. 2002;69:23–29. doi: 10.1093/toxsci/69.1.23. [DOI] [PubMed] [Google Scholar]
- 28.Werner JS, Wooten BR. Opponent chromatic mechanisms: relation to photopigments and hue naming. J Opt Soc Am. 1979;69:422–434. doi: 10.1364/josa.69.000422. [DOI] [PubMed] [Google Scholar]
- 29.Wooten BR, Hammond BR, Jr, Land RI, Snodderly DM. A practical method for measuring macular pigment optical density. Invest Ophthalmol Vis Sci. 1999;40:2481–2489. [PubMed] [Google Scholar]
- 30.Schefrin BE, Tregear SJ, Harvey LO, Jr, Werner JS. Senescent changes in scotopic contrast sensitivity. Vision Res. 1999;39:3728–3736. doi: 10.1016/s0042-6989(99)00072-3. [DOI] [PubMed] [Google Scholar]
- 31.Harvey LO. Efficient estimation of sensory thresholds. Behav Res Methods Instr Computers. 1986;18:623–632. [Google Scholar]
- 32.Krastel H, Moreland D. Colour vision deficiencies in ophthalmic diseases. In: Foster DH, editor. Inherited and acquired colour vision deficiencies. Boston: CRC Press, Inc; 1991. pp. 115–172. [Google Scholar]
- 33.Sperling HG. Vulnerability of the blue-sensitive mechanism. In: Foster DH, editor. Inherited and acquired colour vision deficiencies. Boston: CRC Press, Inc; 1991. pp. 72–87. [Google Scholar]
- 34.Reffin JP, Astell S. Trials of a computer-controlled colour vision test that preserves the advantages of pseudoisochromatic plates. In: Drum B, Moreland D, Serra A, editors. Colour vision deficiencies. London: Kluwer Academic Publishers; 1991. pp. 69–76. [Google Scholar]
- 35.Sutter EE. The fast m-transform: a fast computation of cross-correlations with binary m-sequences. SIAM J Comput. 1991;20:686–694. [Google Scholar]
- 36.Gerth C, Garcia SM, Ma L, Keltner JL, Werner JS. Multifocal electroretinogram: age-related changes for different luminance levels. Graefes Arch Clin Exp Ophthalmol. 2002;240:202–208. doi: 10.1007/s00417-002-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rock CL, Thornquist MD, Neuhouser ML, et al. Diet and lifestyle correlates of lutein in the blood and diet. J Nutr. 2002;132:525S–530S. doi: 10.1093/jn/132.3.525S. [DOI] [PubMed] [Google Scholar]
- 38.Movshon JA, Kiorpes L. Analysis of the development of spatial contrast sensitivity in monkey and human infants. J Opt Soc Am A. 1988;5:2166–2172. doi: 10.1364/josaa.5.002166. [DOI] [PubMed] [Google Scholar]
- 39.Regan BC, Reffin JP, Mollon JD. Luminance noise and the rapid determination of discrimination ellipses in colour deficiency. Vis Res. 1993;34:1279–1299. doi: 10.1016/0042-6989(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 40.Curran-Celentano J, Hammond BR, Jr, Ciulla TA, Cooper DA, Pratt LM, Danis RB. Relation between dietary intake, serum concentrations, and retinal concentrations of lutein and zeaxanthin in adults in a Midwest population. Am J Clin Nutr. 2001;74:796–802. doi: 10.1093/ajcn/74.6.796. [DOI] [PubMed] [Google Scholar]
- 41.Cross CE, Traber M, Eiserich J, van der Vliet A. Micronutrient antioxidants and smoking. Br Med Bull. 1999;55:691–704. doi: 10.1258/0007142991902565. [DOI] [PubMed] [Google Scholar]
- 42.Werner JS, Donnelly SK, Kliegl R. Aging and human macular pigment density. Appended with translations from the work of Max Schultze and Ewald Hering. Vision Res. 1987;27:257–268. doi: 10.1016/0042-6989(87)90188-x. [DOI] [PubMed] [Google Scholar]
- 43.Stahl W, Junghans A, de Boer B, Driomina ES, Briviba K, Sies H. Carotenoid mixtures protect multilamellar liposomes against oxidative damage: synergistic effects of lycopene and lutein. FEBS Lett. 1998;427:305–308. doi: 10.1016/s0014-5793(98)00434-7. [DOI] [PubMed] [Google Scholar]
- 44.Suttle CM, Harding GF. The VEP and ERG in a young infant with cystic fibrosis. A case report. Doc Ophthalmol. 1998;95:63–71. doi: 10.1023/a:1001772327537. [DOI] [PubMed] [Google Scholar]
- 45.Leguire LE, Pappa KS, McGregor ML, Rogers GL, Bremer DL. Electrooculogram in vitamin A deficiency associated with cystic fibrosis. Short communication. Ophthalmic Paediatr Genet. 1992;13:187–189. doi: 10.3109/13816819209046488. [DOI] [PubMed] [Google Scholar]
- 46.Keith CG, De Haller J, Young WF. Side-effects to antibiotics in cystic fibrosis. I: ocular changes in relation to antibiotic administration and to severity of pulmonary involvement. Arch Dis Child. 1966;41:262–266. doi: 10.1136/adc.41.217.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein R, Klein BEK, Tomany SC, Moss SE. Ten-year incidence of age-related maculopathy and smoking and drinking: the Beaver Dam eye study. Am J Epidemiol. 2002;156:589–598. doi: 10.1093/aje/kwf092. [DOI] [PubMed] [Google Scholar]
- 48.Gerth C, Hauser D, Delahunt PB, Morse LS, Werner JS. Assessment of multifocal electroretinogram abnormalities and their relation to morphologic characteristics in patients with large drusen. Arch Ophthalmol. 2003;121:1404–1414. doi: 10.1001/archopht.121.10.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schunemann HJ, McCann S, Grant BJ, Trevisan M, Muti P, Freudenheim JL. Lung function in relation to intake of carotenoids and other antioxidant vitamins in a population-based study. Am J Epidemiol. 2002;155:463–471. doi: 10.1093/aje/155.5.463. [DOI] [PubMed] [Google Scholar]
- 50.Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]