Summary
Molecular Breast Imaging (MBI) is a nuclear medicine technique used to image the breast. In this review we discuss our experience with this technique and clinical applications for the use of MBI.
Background
Molecular Breast Imaging (MBI) depicts functional uptake of targeted radiotracers in the breast using dedicated gamma cameras.
Methods
MBI studies were performed under several institutional protocols evaluating the use of MBI in screening and diagnosis.
Results
Using a single head system, sensitivity for breast cancer detection was 85% (57/67) overall and 29% for tumors ≤5mm in diameter. Sensitivity improved to 91% (117/128) overall and 69% for tumors ≤5mm.using a dual head system. In 650 high risk patients undergoing breast cancer screening, MBI detected 7 cancers, 5 which were missed on mammography. In 24/149 (16%) breast cancer patients MBI detected additional disease not seen on mammography. Sensitivity of MBI was 88% (83/94) for IDC, 79% (23/29) for ILC, 89% (25/28) for DCIS.
Conclusion
MBI can detect IDC, DCIS, and ILC. It has a promising role in evaluating extent of disease and multifocal disease in the breast for surgical treatment planning.
Keywords: molecular breast imaging, scintimammography, breast cancer, detection
Introduction
Breast cancer is one of the most common cancers, with over 180,000 new cases estimated in 2008.1 Early detection of breast cancer can allow treatment at an earlier stage and therefore minimize morbidity and mortality. Mammography is currently the gold-standard for early breast cancer detection and several analyses have shown that regular screening mammography can significantly reduce the mortality rate due to breast cancer.2, 3 While reported overall sensitivities of mammography are high (ranging from 71–96%),4 the sensitivity of mammography is significantly reduced in certain subsets of women, particularly for women with radiographically dense breasts.5,6,7 To overcome the limitations of mammography, other modalities for breast imaging such as digital mammography, ultrasound and magnetic resonance imaging (MRI) are being evaluated to determine their role in both screening and diagnostic applications.
Another type of imaging that may serve as an adjunct to mammography is nuclear medicine-based breast imaging, which detects the functional uptake of radiotracers in breast disease. Scintimammography (SMM) has been in use since the 1990’s, when it was discovered that 99mTc-sestamibi, a radiotracer commonly used for cardiac perfusion imaging, was also taken up by breast cancers. An advantage to this functional imaging method is that uptake appears to be independent of mammographic breast density.8 Studies evaluating SMM for the diagnosis of breast cancer reported an average sensitivity and specificity of 75% and 83% respectively9, however, the sensitivity of SMM was poor for lesions less than 1 cm in size,10 and therefore its prospects for clinical applications appeared to be limited.
The poor sensitivity of SMM techniques to identify small lesions is primarily due to limitations in the detector technology. SMM uses conventional large field-of-view gamma cameras which are designed for whole-body imaging. Because these large cameras are unable to be positioned close to the breast, spatial resolution is significantly degraded. Over the last decade, advances in detector technology have allowed the development of small field-of-view breast imaging systems dedicated to imaging the breast, including both positron emission mammography (PEM) technology and a variety of single-photon gamma camera technologies.11,12,13,14,15,16,17 These dedicated systems offer improved spatial resolution due to the use of pixilated detectors and by permitting optimal breast positioning analogous to views acquired with mammography.
Over the last six years, we have been studying the use of dedicated semiconductor-based gamma cameras for breast imaging. In order to distinguish this technique from the scintillating detector technology used in SMM, we have named it Molecular Breast Imaging (MBI). In this paper, we present a review of MBI studies performed at the Mayo Clinic and discuss the potential clinical role of MBI in the management of breast cancer patients.
Methods
MBI Technology
In 2001, our first prototype MBI system was constructed. The system comprised a single small field-of-view semiconductor-based gamma camera (G.E. Medical Systems, Haifa, Israel) mounted on a modified mammography gantry. The detector, previously described in detail,17,18,19 was made up of an array of 80 × 80 Cadmium Zinc Telluride (CZT) elements, each 2.5 mm × 2.5 mm, providing a 20 cm × 20 cm field of view. This CZT prototype offered improved energy resolution and spatial resolution relative to conventional SMM systems. The detector had only 2–3 mm of dead space between the edge and the active area of the detector, allowing optimal positioning of the breast on the detector in a manner similar to a conventional mammography unit.
The next step in the development of MBI technology was the construction of a dual-head MBI system, in which the breast could be positioned between two opposing dedicated detectors. Addition of a second detector head ensures that the distance between a breast lesion and one of the detectors does not exceed half of the breast thickness, which should improve resolution, thereby improving the sensitivity of MBI. In addition, the collimation for this system was optimized20 in order to provide higher counts per pixel in the MBI images.
The dual-head system (shown in Figure 1) comprises two CZT detectors mounted on a modified mammographic gantry. Two such systems are currently in use at Mayo; one utilizes two of the same detectors as described in the single-head MBI, and the other dual-head system utilizes the LumaGem CZT detectors (Gamma Medica-Ideas, Northfield, CA). The LumaGem detectors also offer superior spatial and energy resolution to a conventional SMM system and are comprised of 96 × 128 array of CZT elements with pixel size of 1.6 × 1.6 mm2, giving a total detector area of 15 cm × 20 cm.
Figure 1.

Dual-head Molecular Breast Imaging system comprising two cadmium zinc telluride detectors mounted on a modified mammographic gantry.
Pre-biopsy studies
In order to evaluate the ability of this MBI system to detect small breast lesions, MBI studies were performed in women prior to biopsy of suspicious breast lesions identified by mammography and/or ultrasound. An initial study of 100 women was performed using the single head MBI system and a second study of 150 women was performed with the dual-head MBI system.
The single-head pre-biopsy study was performed in 100 patients with suspicious breast lesions less than 2 cm in size identified by mammography and/or ultrasound and scheduled for biopsy21. MBI was performed 10 minutes after the injection of 740MBq 99mTc-sestamibi. Craniocaudal (CC) and mediolateral oblique (MLO) views of each breast were obtained with the breast lightly compressed between the detector and a compression paddle for 10 minutes/view. MBI studies were examined by a radiologist for the presence of focal uptake, and results were compared with pathology from core needle biopsy or surgical excision. To evaluate the incremental value of a second detector head, a second study, comparable to that described above for the single-head MBI system, was performed in 150 women with suspicious breast lesions (BI-RADs 4 and 5) using the dual head MBI system22.
Screening study
In order to evaluate the potential role of MBI in screening for breast cancer, we are currently performing a study in which women presenting for screening mammography also undergo screening MBI. To be eligible for the study, patients are required to be asymptomatic, have either heterogeneously dense or extremely dense breast parenchyma and have an increased risk for breast cancer as defined by at least one of the following risk factors: a personal history of breast cancer; a family history of one first-degree relative with breast cancer; a family history of two second-degree relatives with breast cancer; a known BRCA mutation; a personal history of lobular carcinoma in situ, atypical ductal hyperplasia, or atypical papilloma; an estimated five-year risk of breast cancer ≥ 1.7% or an estimated lifetime risk of breast cancer ≥ 20% as calculated by the Gail model.
MBI was performed using the dual-head system following injection of 740MBq 99mTc-sestamibi. Both CC and MLO views of each breast were acquired for 10 minutes/view. MBIs were interpreted in isolation by two radiologists who were blinded to the interpretation of the screening mammogram and all other ancillary patient information. Radiologists reading the screening mammograms were not aware of the MBI findings.
Results
Single head pre-biopsy study
Of the 100 women in the study, 53 patients had breast cancer confirmed at surgery, and 47 patients had benign findings. In the 53 patients with breast cancer, 59 tumors were initially identified with mammography and/or ultrasound and 8 additional tumors were identified with MBI, for a total of 67 tumors in the study. MBI detected 57 of the 67 tumors for a sensitivity of 85%. Average tumor size was 1.3cm. Thirty five tumors were ≤10 mm in diameter, of which 26 were detected on MBI (sensitivity 74%). As given in Table 1, sensitivity was 29%, 86% and 97%, for tumors ≤5, 6–10, and >10mm respectively. In 7 patients with breast cancer there were discordant findings between MBI and mammography, with MBI indicating 8 additional tumors not seen on mammography. Four of these were in the contralateral breast. In all 8 cases, the MBI findings were in agreement with breast MRI and confirmed as true positives at surgery (6 cases of DCIS, 1 invasive ductal cancer and 1 invasive lobular cancer).
Table 1.
Sensitivity of MBI as a function of tumor size.
| Tumor Size (mm) | Single-head pre-biopsy study | Dual-head pre-biopsy study | Screening study | All studies combined |
|---|---|---|---|---|
| 0–5 | 0.29 (2/7) | 0.69 (11/16) | 0.00 (0/1) | 0.54 (13/24) |
| 6–10 | 0.86 (24/28) | 0.91 (41/45) | 1.00 (3/3) | 0.89 (68/76) |
| >10 | 0.97 (31/32) | 0.97 (65/67) | 0.90 (4/5) | 0.96 (100/104) |
| All tumors | 0.85 (57/67) | 0.91 (117/128) | 0.91 (117/128) | 0.89 (181/204) |
MBI gave false negative results for 10 tumors in 7 patients. Further analysis of these 7 false negative patients showed that 5 of the patients had very small tumors (< 5 mm), all 7 patients had very large breasts (compressed breast thickness of > 5 cm), 5 patients had technically poor breast positioning during the MBI and 5 patients had a low number of counts in the MBI images. In larger breasts, it is more likely that a lesion could be located farther from the detector. Because the resolving power of any gamma camera diminishes with distance from the face of the collimator, small lesions located far from the detector (>5cm away) appear fainter and may not be distinguishable above the background activity.
Dual head pre-biopsy study
Of the 150 women in the study, 88 patients had breast cancer and 62 patients had benign findings. In the 88 patients with breast cancer, 119 were initially identified by mammography and/or ultrasound, and 9 additional tumors were found with MBI, for a total of 128 tumors detected in the study. Dual-head MBI detected 117/128 cancers for an overall sensitivity of 91%. The sensitivity of MBI for cancers ≤5 mm, 6–10 mm, and > 10 mm was 69% (11/16), 91% (41/45), and 97% (65/67) respectively (Table 1). Two of the 11 false negative cancers were not visible on MBI due to inadequate positioning of the patient. Of the 9 other false negatives, 5 were less than 5 mm in size. In 9 patients, 9 cancers that were occult on mammography and ultrasonography were found with MBI and confirmed on MRI and at surgery.
These results with the dual-head MBI system demonstrate high sensitivity for detection of small breast cancers and an improvement in sensitivity compared to the single head system. In particular, the sensitivity for the detection of tumors ≤5 mm improved from 29% to 69%.
Screening study
Six hundred and fifty women (of an eventual 2000) have been evaluated with screening dual-head MBI thus far. A total of nine cancers were detected from blinded interpretation of the MBIs and mammograms. Five cancers were detected with MBI alone, 1 cancer was detected by mammography alone, 2 cancers were detected by both mammography and MBI, and 1 cancer was not detected by either modality. The one cancer undetected by both mammography and MBI occurred in a patient in whom innumerable bilateral clusters of microcalcifications on mammography were considered probably benign and both MBI and MRI exams performed on this patient were also interpreted as benign. At a recommended 6 month follow up mammogram, biopsy was performed and extensive DCIS was found. The one cancer detected by mammography but not by MBI was a < 5 mm area of DCIS. At this interim analysis, MBI has detected more than twice as many cancers as mammography.
Histological subtypes detected with MBI
From all MBI studies performed in our laboratory as described above, a total of 181 / 204 tumors have been detected with MBI in 149 patients for an overall sensitivity of 89%. MBI has detected invasive ductal carcinoma (IDC), invasive lobular carcinoma (ILC) as well as ductal carcinoma in situ (DCIS). Sensitivity of MBI for each histological subtype was 88% (83/94) for IDC, 79% (23/29) for ILC, 89% (25/28) for DCIS, 97% (31/32) for IDC with DCIS, 93% (14/15) for IDC with ILC and 83% (5/6) for other invasive cancers. Table 2 lists the sensitivity of MBI for each reported study as a function of histological subtype.
Table 2.
Sensitivity of MBI as a function of histological subtype.
| Histological subtype | Single-head pre-biopsy study | Dual-head pre-biopsy study | Screening study | All studies combined |
|---|---|---|---|---|
| IDC | 0.84 (37/44) | 0.91 (41/45) | 1.00 (5/5) | 0.88 (83/94) |
| ILC | 0.71 (5/7) | 0.81 (17/21) | 1.00 (1/1) | 0.79 (23/29) |
| DCIS | 1.00 (8/8) | 0.94 (16/17) | 0.33 (1/3) | 0.89 (25/28) |
| IDC with DCIS | 0.67 (2/3) | 1.00 (29/29) | — | 0.97 (31/32) |
| Mixed IDC/ILC | 1.00 (4/4) | 0.91 (10/11) | — | 0.93 (14/15) |
| Other subtypes | 1.00 (1/1) | 0.80 (4/5) | — | 0.83 (5/6) |
IDC = invasive ductal carcinoma, ILC = invasive lobular carcinoma, DCIS = ductal carcinoma in situ
Additional Cancers Detected with MBI
Our composite data indicate that in 149 breast cancer patients studied thus far, additional disease of greater extent that seen on mammography or ultrasound, was detected with MBI in 24/149 (16%) of patients. In all cases, these additional cancers have been confirmed by either biopsy or surgical resection. Figure 2 shows an MBI study in a patient with a known 20 mm invasive ductal carcinoma (IDC) seen on mammography. The MBI study demonstrated this lesion and an additional 10 mm IDC that was occult on mammography.
Figure 2.
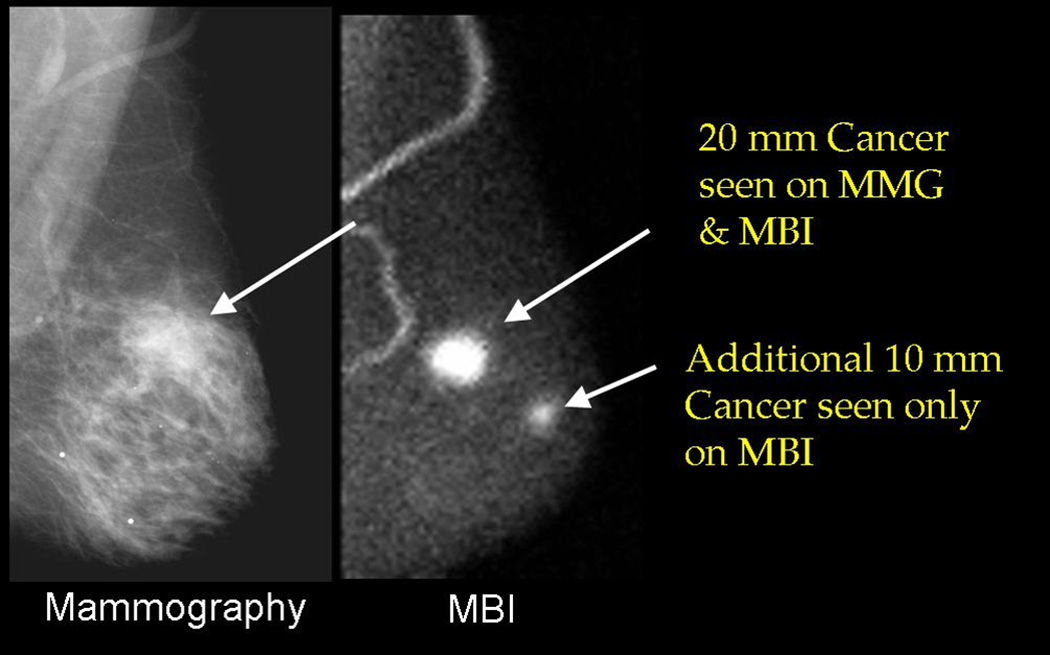
Mammogram (MLO view at left) demonstrating a 20 mm invasive ductal carcinoma (IDC). The corresponding MBI study (MLO view at right) demonstrated an additional 10 mm IDC occult on mammogram.
Discussion
MBI has been studied in over 900 patients at the Mayo Clinic during the last six years. In our experience, we have found the dedicated semiconductor-based MBI technology to offer optimal breast positioning analogous to mammography and superior spatial and energy resolution compared with conventional SMM cameras. From the body of work performed at Mayo and at other laboratories23 it is clear that MBI has a potentially important role as an adjunct to mammography in the imaging workup of breast cancer patients.
Because the tracer uptake with MBI is independent of breast density, MBI may serve as a valuable imaging technique for women with mammographically dense breasts. In both screening and diagnostic settings, we have found that MBI consistently detects additional cancers in the breast that are occult on mammography, while maintaining a reasonable false positive rate that is equal to or less than that of mammography. However, at present the ability of MBI to detect microcalcifications is unclear and hence MBI must be viewed as an adjunct technique to mammography rather than an alternative.
In MBI, images obtained of the breast are analogous to those in mammography (craniocaudal and mediolateral oblique), facilitating interpretation and comparison with mammography. One advantage of MBI over mammography is that heavy compression of breast tissues is not required. During MBI, light pain-free compression about 1/3 the pressure of mammography is applied, primarily to limit patient movement. The MBI procedure is generally well tolerated with a lower pain score than mammography21, 24
MRI is increasingly being used in breast diagnostic evaluation and has been shown to have excellent sensitivity (90% or better). However, its variable specificity, high cost, and the high level of interpretive expertise are deterrents to its widespread use. In a retrospective analysis of 48 patients that have undergone imaging with both MBI and MRI at Mayo, there has been 97% (47/48) concordance in imaging findings. In one patient, two cancers were undetected on MBI that were detected with MRI. This concordance has led us to postulate that MBI may be equally sensitive but more cost-effective than MRI in breast imaging. Currently, a prospective comparison of MBI with MRI is underway at Mayo Clinic. Figure 3 shows a comparison of concordant MBI and MRI imaging findings.
Figure 3.
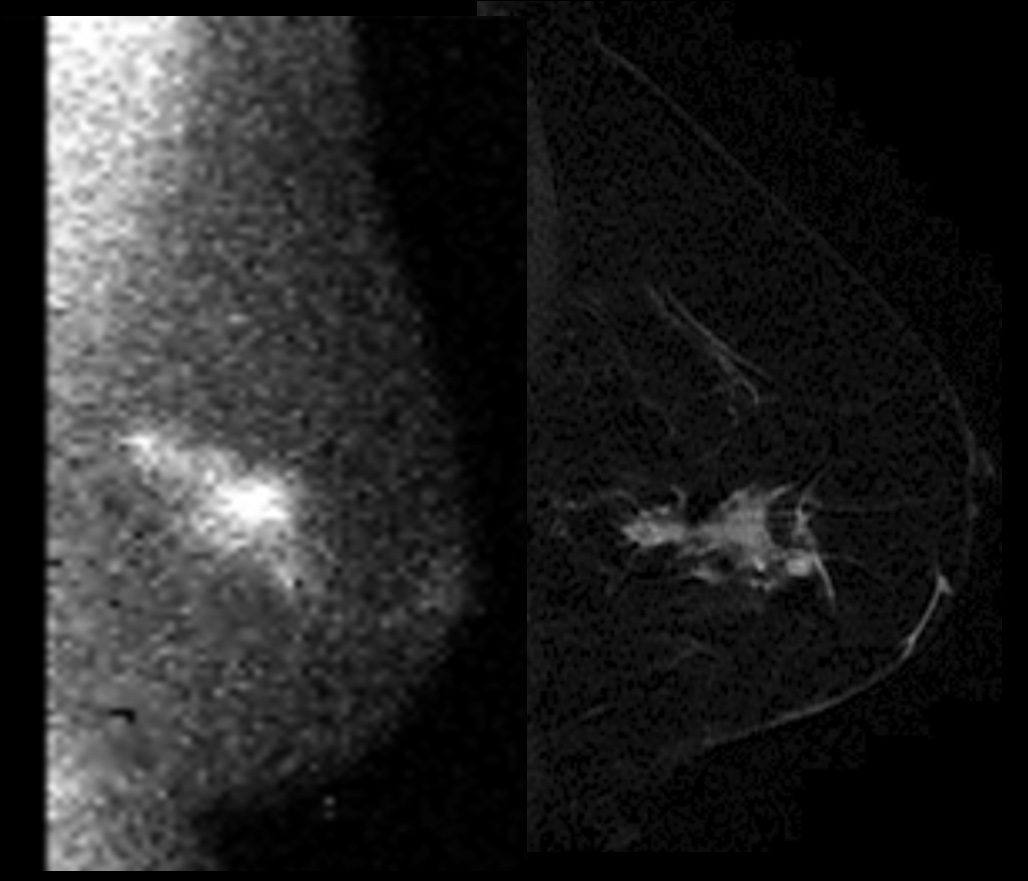
An example of a patient with concordant MBI (left) and breast MRI (Right) findings. MBI shows linear focal abnormal tracer uptake in the posterolateral left breast with orientation towards the nipple. There are 3 foci of intense tracer uptake suggesting a combination of invasive breast cancer and DCIS. MRI shows an irregular enhancing mass in the outer breast measuring 4.6cm × 1.8cm × 2.2cm.
An advantage of MBI over MRI is that it can be utilized in patients with contraindications to MRI, such as allergy to gadolinium, claustrophobia, cardiac pacemakers and implanted defibrillator, cochlear implants, metal such as aneurysm clips etc. Interpretation of MBI images is also much less intensive than interpretation of MRI, as bilateral dual-head MBI generates 8 images in comparison to upwards of 1000 images generated by bilateral breast MRI. The most striking difference between the two modalities is cost; MRI is approximately 10–15 times the cost of mammography. The cost of MBI study has not been established but is estimated to be about 3–4 times that of mammography. Medicare charges have been estimated at $1029.69 and $292.12 for Breast MRI and MBI respectively, making MBI approximately 3 times less expensive than MRI.
A potential application of MBI is in the evaluation of extent of disease (number of foci, size of disease, and detection of contralateral breast lesions) for preoperative planning. Identification of additional lesions is critical to preoperative planning and can alter surgical recommendations between breast conservation and mastectomy. The occurrence of multifocal, multicentric, or contralateral breast cancers is not uncommon; however additional cancers other than the index lesion are often missed or underestimated by mammography. While many studies have indicated that MRI performs particularly well in the preoperative evaluation of the breast,25 MRI is not routinely used or available in many institutions at this time. Currently, a study is under development at our institution to evaluate the role of MBI in delineating the extent of disease prior to surgical resection.
We have also recently begun evaluating the role of MBI in patients undergoing neoadjuvant chemotherapy or neoadjuvant hormone therapy. The goal of this work is to determine whether MBI can accurately assess tumor size pre- and post-neoadjuvant therapy and to see if MBI can predict response to therapy based on radiotracer uptake and washout in the tumor. Prior studies examining the ability of SMM to predict the response to neoadjuvant chemotherapy have given contrasting results, with some authors documenting that a rapid tumor clearance of 99mTc-sestamibi in early images may predict a lack of breast tumor response to neoadjuvant chemotherapy,26, 27 whereas others have shown no association between tumor uptake and washout with respect to response to therapy.28 It remains to be seen whether MBI performed with the new dedicated technology will allow better response prediction. Preliminary MBI studies performed in patients undergoing neoadjuvant therapy have indicated that MBI may also have a role in delineating the extent of disease prior to therapy, as seen in Figure 4.
Figure 4.
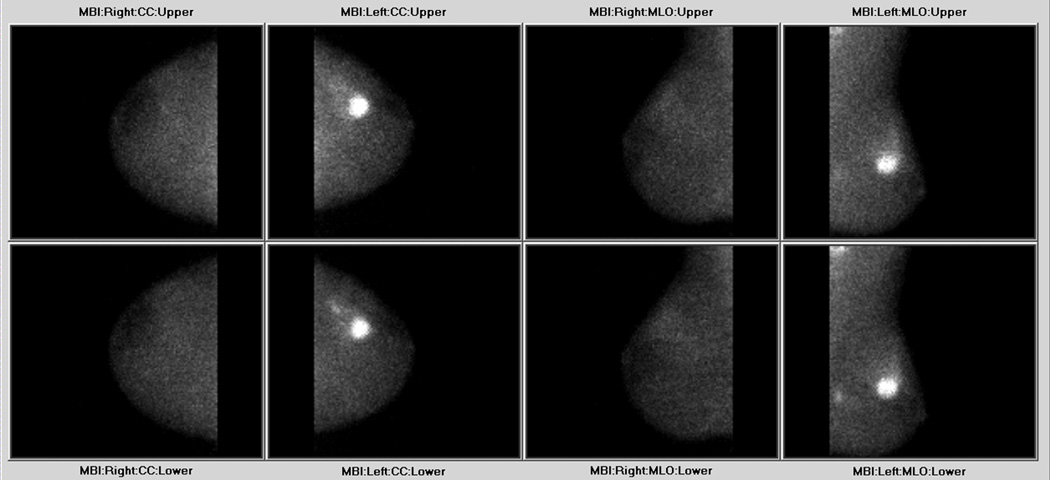
A 38-year-old female presented with palpable left breast mass. Biopsy revealed IDC. Shown are the dual-head MBI images, which demonstrate a focal area of intense tracer uptake, with an associated tail of segmental tracer uptake extending posteriorly away from the mass, consistent with IDC and associated DCIS. Additionally a second focus of biopsy proven IDC is seen by the moderate focal uptake in the posterior lower outer quadrant of the left breast.
Another important area of investigation in our laboratory is the study of dose reduction techniques for MBI. The radiation dose to the breast from MBI is less than that from mammography (0.13 rad/ 20 mCi for MBI vs. 0.75 rad for a screening mammogram). However, unlike mammography, the administration of a systemic radiotracer during MBI also presents a radiation dose to the entire body. The 20 mCi dose of Tc-99m sestamibi for diagnostic purposes presents a reasonable risk with an extremely low probability of harmful effects to the patient. As a reference, this dose is approximately 1/3 the dose used in routine cardiac perfusion imaging. However, if MBI was to be considered as a regular screening technique, it would be critical to reduce this dose by a factor of 5–10. Various image processing techniques, technical improvements to the MBI system, and the study of alternative radiotracers are under development in our laboratory in order to achieve the use of this lower dose while also maintaining image quality.
In the studies reviewed here, we have found that MBI performed with a small view of field dual head detector can detect small breast cancers with a sensitivity of >90%. Molecular breast imaging appears to have comparable sensitivity for the three primary types of breast cancer, IDC, DCIS, and ILC. This new technique may be of particular value as an adjunct diagnostic tool to mammography, in evaluating extent of disease for treatment planning and in determining response to neoadjuvant therapy.
Acknowledgements
This work was supported by the National Institutes of Health and the Susan G. Komen Foundation. We wish to thank Terry Brinkman, Peggy-Drews Radke, Barb Siem, Lori Johnson, Torey Alabin, Michelle Bartel, and Tracy Decklever for their assistance with this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Kerlikowske K, Grady D, Rubin SM, Sandrock C, Ernster VL. Efficacy of screening mammography: a meta-analysis. JAMA. 1995;273:149–154. [PubMed] [Google Scholar]
- 3.Tabar L, Vitak B, Chen HH, Yen MF, Duffy SW, Smith RA. Beyond randomized controlled trials: organized mammographic screening substantially reduces breast carcinoma mortality. Cancer. 2001;91(9):1724–1731. doi: 10.1002/1097-0142(20010501)91:9<1724::aid-cncr1190>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Humphrey LL, Helfand M, Chan BKS, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:347–360. doi: 10.7326/0003-4819-137-5_part_1-200209030-00012. [DOI] [PubMed] [Google Scholar]
- 5.Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168–175. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 6.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–175. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 7.Leconte I, Feger C, Galant C, et al. Mammography and subsequent whole-breast sonography of nonpalpable breast cancers: The importance of radiologic breast density. Am J Roentgenol. 2003;180:1675–1679. doi: 10.2214/ajr.180.6.1801675. [DOI] [PubMed] [Google Scholar]
- 8.Khalkhali I, Baurn JK, Villanueva-Meyer J, et al. 99mTc-sestamibi breast imaging for the examination of patients with dense and fatty breasts: multicenter study. Radiology. 2002;222:149–155. doi: 10.1148/radiol.2221010237. [DOI] [PubMed] [Google Scholar]
- 9.Khalkhali I, Vargas HI. The role of nuclear medicine in breast cancer detection: functional breast imaging. Radiol Clin North Am. 2001;39:1053–1068. doi: 10.1016/s0033-8389(05)70328-6. [DOI] [PubMed] [Google Scholar]
- 10.Khalkhali I, Villanueva-Meyer J, Edell SL, et al. Diagnostic accuracy of 99mTc-sestamibi breast imaging: multicenter trial results. J Nucl Med. 2000;41:1973–1979. [PubMed] [Google Scholar]
- 11.Majewski S, Kieper D, Curran E, et al. Optimization of dedicated scintimammography procedure using detector prototypes and compressible phantoms. IEEE Trans Nucl Sci. 2001;48:822–829. [Google Scholar]
- 12.McElroy DP, Hoffman EJ, MacDonald L, et al. Evaluation of breast tumor detectability with two dedicated, compact scintillation cameras. IEEE Trans Nucl Sci. 2002;49:794–802. [Google Scholar]
- 13.Pani R, Soluri A, Scafe R, et al. Multi-PSPMT scintillation camera. IEEE Trans Nucl Sci. 1999;46:702–708. [Google Scholar]
- 14.Gruber GJ, Moses WW, Derenzo SE, Wang NW, Beuville E, Ho MH. A discrete scintillation camera module using silicon photodiode readout of CsI(Tl) crystals for breast cancer imaging. IEEE Trans Nucl Sci. 1998;45:1063–1068. [Google Scholar]
- 15.Patt BE, Iwanczyk JS, Rossington Tull C, Wang NW, Tornai MP, Hoffman EJ. High resolution CsI(Tl) / Si-PIN detector development for breast imaging. IEEE Trans Nucl Sci. 1998;45:2126–2131. [Google Scholar]
- 16.Kim JH, Choi Y, Joo KS, et al. Development of a miniature scintillation camera using an NaI(Tl) scintillator and PSPMT for scintimammography. Phys Med Biol. 2000;45:3481–3488. doi: 10.1088/0031-9155/45/11/326. [DOI] [PubMed] [Google Scholar]
- 17.Mueller B, O'Connor MK, Blevis I, et al. Evaluation of a small cadmium zinc telluride detector for scintimammography. J Nucl Med. 2003;44:602–609. [PubMed] [Google Scholar]
- 18.Hruska CB, O’Connor MK, Collins DA. Comparison of small field of view gamma camera systems for scintimammography. Nuc Med Commun. 2005;26:441–445. doi: 10.1097/00006231-200505000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes DJ, O'Connor MK, Phillips SW, et al. Molecular breast imaging: a new technique using technetium Tc 99m scintimammography to detect small tumors of the breast. Mayo Clin Proc. 2005;80:24–30. doi: 10.1016/S0025-6196(11)62953-4. [DOI] [PubMed] [Google Scholar]
- 20.Hruska CB, O’Connor MK. Effect of collimator selection on tumor detection for dedicated nuclear breast imaging systems. IEEE Trans Nuc Sci. 2006;53(5):2680–2689. [Google Scholar]
- 21.O'Connor MK, Phillips SW, Hruska CB, et al. Molecular breast imaging: advantages and limitations of a scintimammographic technique in patients with small breast tumors. Breast J. 2007;13:3–11. doi: 10.1111/j.1524-4741.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 22.American College of Radiology. Illustrated Breast Imaging Reporting and Data System (BIRADS) 4th edn. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 23.Spanu A, Cottu P, Manca A, Chessa F, Sanna D, Madeddu G. Scintimammography with dedicated breast camera in unifocal and multifocal/multicentric primary breast cancer detection: a comparative study with SPECT. Int J Oncol. 2007;31:369–377. [PubMed] [Google Scholar]
- 24.Dibble SL, Israel J, Nussey B, et al. Mammography with breast cushions. Womens Health Issues. 2005;15:55–63. doi: 10.1016/j.whi.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Kuhl C, Kuhn W, Braun M, Schild H. Pre-operative staging of breast cancer with breast MRI: one step forward, two steps back? Breast. 2007 Suppl 16:S34–S44. doi: 10.1016/j.breast.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Ciarmiello A, Del Vecchio S, Silvestro P, et al. Tumor clearance of technetium 99m-sestamibi as a predictor of response to neoadjuvant chemotherapy for locally advanced breast cancer. J Clin Oncol. 1998;16:1677–1683. doi: 10.1200/JCO.1998.16.5.1677. [DOI] [PubMed] [Google Scholar]
- 27.Vecchio SD, Ciarmiello A, Potena MI, et al. In vivo detection of multidrug-resistant (MDR1) phenotype by technetium-99m sestamibi scan in untreated breast cancer patients. Eur J Nucl Med. 1997;24:150–159. doi: 10.1007/BF02439547. [DOI] [PubMed] [Google Scholar]
- 28.Travaini LL, Baio SM, Cremonesi M, et al. Neoadjuvant therapy in locally advanced breast cancer: 99mTc-MIBI mammoscintigraphy is not a reliable technique to predict therapy response. Breast. 2007;16:262–270. doi: 10.1016/j.breast.2006.12.009. [DOI] [PubMed] [Google Scholar]


