Abstract
The reliable assessment of fracture severity plays a critical part in treatment, providing essential information to guide clinical decision-making. However, current classification schemes such as the AO/OTA are constrained by limitations intrinsic to subjective categorical systems. A recently developed objective CT-based assessment methodology quantifies fracture severity by calculating the mechanical energy expended during bony fragmentation. Specifically, fracture energy is determined by comparing the bone free surface area in the fractured limb to that in the intact contralateral limb. Unfortunately, the contralateral limb is not routinely scanned in the course of fracture assessment. Consequently, fracture energy can not be obtained, since there is no datum against which to compare the fractured limb. To facilitate the application of this novel technique to large multi-center and retrospective studies where the intact contralateral CT scan is unavailable, this study aimed to establish a normative, anthropometrically scaled intact bone model to be used as a substitute datum. A mathematical model that estimated free bone surface area along the intact contralateral limb was regressed from a study group of 22 tibial plafond fracture patients. The regressed tibia model provided suitably accurate estimates of the directly measured intact surfaces areas (average error of 15%). The differences between regressed and actual bone surface areas did not ultimately affect the stratification of fracture severity, as fracture energy measures using the regressed model maintained a 0.90 concordance with the original analysis. The results from this study suggest that normative bone surface area can be incorporated into the novel CT-based objective fracture severity assessment technique.
INTRODUCTION
Severe trauma to weight-bearing joints is often followed by eventual joint degeneration and the development of post-traumatic osteoarthritis (PTOA). The resultant chronic pain and decreased joint function impose a severe burden, not only to the patients, but to the US economy as well, costing $12 billion annually.1,2 The path-omechanical determinants that govern the progression of this disease are poorly understood, in part due to the multi-factorial etiology of this disease.
Although factors such as joint instability and residual incongruity have been shown to correlate with degeneration, clinical evidence suggests that the acute injury severity plays a dominant pathologic role. Unfortunately, the fracture severity assessment schemes currently used in clinical care fail to provide the information necessary to make this linkage objectively. Classifications such as the AO/OTA and other categorical schemes are able to reliably group fractures with broadly similar characteristics, but they are unable to formally and reproducibly quantify fracture severity per se.3
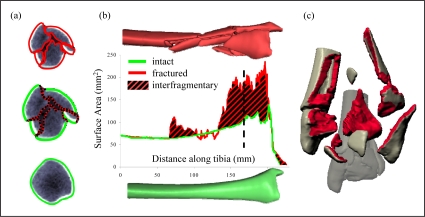
The reliable assessment of fracture severity plays an integral part in clinical decision-making, so improved assessment practices have broad implications for immediate treatment as well as long-term outcome of intra-articular fractures. Until recently, there has been no practical way to objectively measure comminution and fracture severity, which has limited compilation of a suitable body of collective experience to guide care of these patients. A new CT-based methodology was recently developed to objectively quantify fracture severity, working from the principle that mechanical energy is necessarily expended to create new free surface area in a brittle solid. The amount of energy thus expended is proportional to the amount of de novo interfragmentary surface area (Figure 1).
Figure 1.
A sample CT slice from the metaphysis shows the free bone surfaces that are identified by the edge detection algorithm (a). Interfragmentary surface area is the fractured surface area (red) not present in the pre-fractured template (green). Interfragmentary surface area is graphically represented by the area between the intact and fractured curves (b). The dashed line represents the location along the tibia of the sample CT slice. (c) A 3D reconstruction illustrates the total interfragmentary surface area created during the fracture event.
This new objective fracture severity metric has been shown to agree with experienced orthopaedic trauma-tologists’ subjective opinions of injury severity, drawn from standard-of-care plain radiographs.4 The objective metric has also been shown to correlate better with the incidence of PTOA in tibial plafond fracture patients than does clinician opinion (R2= 0.73 vs. 0.47, respectively5). However, since pre-existing and de novo surfaces are both present in fracture fragments, contralateral limb scans have been required to provide datum bone surface areas over a comparable distal segment of the patient’s tibia. With this information in hand, the pre-existing datum bone surface area could be subtracted from the full fractured bone surface area, to yield de novo inter-fragmentary surface area (i.e., the area colored red in Figure 1c).
While feasible in an academic medical center research setting, availability of a contralateral limb scan is problematic in broader orthopaedic practice. Since CT scans of the intact contralateral limb are not routinely obtained during fracture evaluation, this novel energy-based severity assessment methodology can only be used in a limited number of patients. This impedes large multi-center and retrospective studies that could provide high statistical power for establishing the relative efficacy of alternative treatment regimes for these difficult injuries. Toward overcoming this difficulty, a study was designed using CT scan data collected previously from twenty-two different intact tibias. The study aimed to establish a normative anthropometric intact distal tibia model, from which to derive tare bone surface areas, for fracture cases where an intact contralateral CT scan is unavailable. It was hypothesized that an allometrically scaled tibia model would serve as a surrogate datum capable of accurately measuring interfragmentary surface area.
METHODS
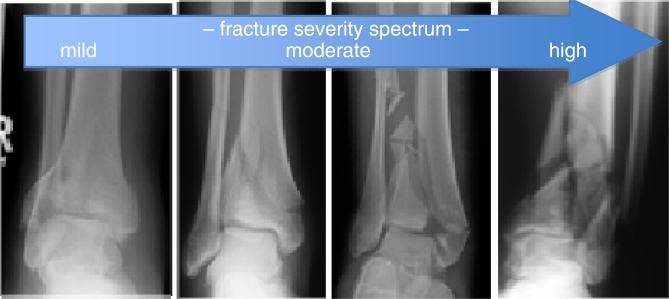
Routine CT studies were obtained retrospectively from the clinical care of twenty-two tibial plafond fractures. The cases chosen represented the spectrum of injury, from simple partial articular fractures to severely comminuted fractures involving the entire tibial plafond (Figure 2). Informed consent was obtained from each patient, under institutional review board approval. Contralateral scans of the unaffected limb provided the anatomy of intact distal tibias, over varying lengths chosen to correspond with the fracture zones. The establishment of an anthropometric distal tibia model required that bone free surface area measurements be made from corresponding locations along the tibia. Subject stature (height) is the dominant factor correlated with limb length.6 Published allometric scaling data were used to estimate the total tibial length, which allowed normalization of surface area measurements based upon subject height.7
Figure 2.
These four tibial plafond fracture radiographs illustrate the fracture severity spectrum. Simple intra-articular fractures are associated with low energy impacts (left hand side), but as energy levels increase, the fracture becomes more complex with greater bony comminution.
A semi-automated edge detection algorithm (coded within MATLAB software) had previously been developed to identify periosteal and endosteal bone surfaces (Figure 1a). In this algorithm, the corresponding bone free surface areas were calculated along the distal tibia at specific increments, according to patient-specific CT scan settings (Figure 1b). Cumulative areas were reported by summing the surface areas over the imaged portion of the tibia. A dataset containing a range of tibial lengths and their corresponding cumulative areas was assembled from these twenty-two studies. Distal tibial lengths were then normalized to an overall stature-regressed tibial length to regress height-dependent variation in bone lengths within this cohort.
The patients’ predicted tibial lengths (l) were calculated as a function of their height (h) using the anthropometric equation, l=0.246h.7 In addition to length normalization, the edge-detected cumulative surface area measurements were scaled by a patient height-dependent function. This function was determined by maximizing the correlation coefficient of a second order polynomial relating the scaled surface area measurements to normalized spatial locations within the tibia. The resultant polynomial yielded an equation predictive of the tibia’s total free surface area, based upon relative tibial lengths measured proximally from the base of the medial malleolus.
The regression model’s capability to predict datum surface area was subsequently investigated, by comparing model-estimated measurements of surface area to the original CT-based measurements. The datum cumulative free bone surface area for the imaged portion of each patient’s intact contralateral distal tibia was predicted and compared to the directly measured total cumulative areas over that same range. Since the fractured scans extended less proximally than their intact contralaterals, errors in interfragmentary surface area calculations were not linearly proportional to the differences found between modeled and directly measured contralateral datum surface areas. Therefore, interfragmentary surface areas based upon actual versus regressed surfaces areas were also compared. The concordance between rankings of interfragmentary area derived from the actual (contralateral limb-measured) versus normatively regressed datum surface areas was evaluated to further establish the model’s accuracy.
RESULTS
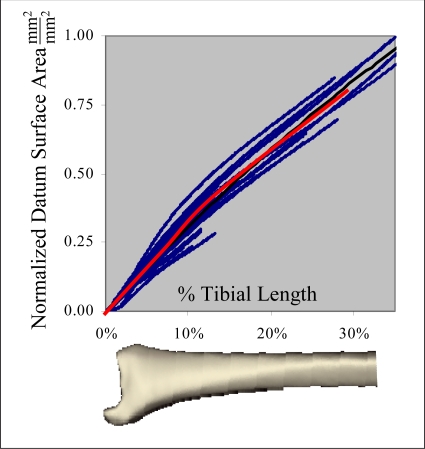
There was large variability as to the proximal extent of CT scans obtained for the intact distal tibias, where the extent of scanned segments ranged from 4.2 to 15.4 (8.5 ± 3.7) cm proximal from the base of the medial malleolus. This was because CT scans were typically obtained over only the portion of each intact tibia corresponding to the fracture zone in the injured contralateral tibia. only the portion of each intact tibia corresponding to the fracture zone in the injured contralateral. The patients included in this study ranged in height from 160 to 190 cm (175 ± 9.3). The observed relationship between intact contralateral tibia segment length (x, normalized to tibia length, l) and datum cumulative bone free surface area (SA) was reasonably well fit by a second-order polynomial. The optimal cumulative surface area scaling function was determined to be the square of the patient’s height, h, from correlation maximization (Figure 3).
| [1] |
Figure 3.
A second order polynomial (red line) of the normalized tibial length was fit to the normalized cumulative free bone surface areas (scaled by h−2) for all 22 cases.
Re-arrangement of equation [1] enables calculation of the cumulative surface area, given simply a patient’s height (h) and the physical distance of interest along the tibia (x):
| [2] |
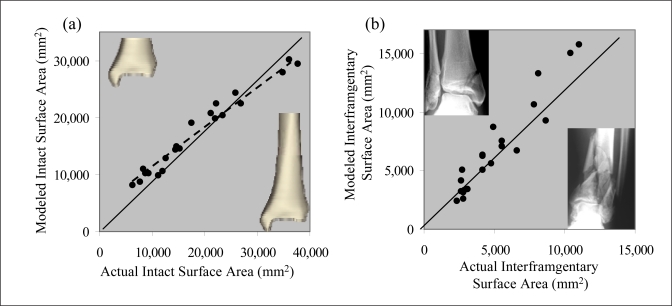
The anthropometric distal tibia model provided suitably accurate estimates of the directly measured intact surfaces areas (average error of 15%). From linear regression, the slope relating actual to modeled intact surface areas was approximately 0.75 (Figure 4a). The difference between actual and modeled intact data correspondingly changed the absolute interfragmentary surface area magnitudes (Figure 4b). However, when the actual rank ordering of fracture severity was considered, rather than just magnitude, measurements based on predicted intact area agreed very well with the original results (concordance = 90%).
Figure 4.
(a) The total intact cumulative areas, at different segmental lengths, from previous direct measurement were compared to those predicted by the model. (b) The interfragmentary surface areas using the actual datum areas were compared to those calculated using the regressed datum areas. Points along the diagonal indicate perfect agreement between the actual and regressed data. Representative cases illustrate low (inset upper left) and high-energy fractures (inset, lower right).
DISCUSSION
Methods to facilitate objective assessment of fracture severity in large series of cases would provide unifying information helpful to systematically improve the treatment of these complex injuries. Presently, treatment decision-making depends largely on subjective visual assessment, which has questionable reliability and documented poor inter-observer agreement. The results from this study show that reasonably accurate, objective fracture severity metrics can be obtained in distal tibia fracture cases for which an intact contralateral CT scan is not available, through use of allometrically scaled normative surface area.
Using patient height as the sole scaling parameter, the allometric model was able to predict cumulative intact surface areas with reasonable accuracy. Differences between the modeled and actual data were inevitable, since height is only a partial predictor for total tibial length, and given that distal tibia datum bone surface area is somewhat variable. The model’s performance could likely be improved by including additional shape parameters and/or actual tibial lengths. Future models might draw from nearby unaffected anatomic structures such as the talus to provide that additional scaling information, however, an attraction of the present approach is that only patient height is required in order to formulate the model. And, results from the present study suggest that acquiring such additional information may add unnecessary complexity, since the current model reproduces fracture energy assessments with a 90% concordance.
The tibial plafond fracture is useful as an intra-articular model for investigating PTOA, since the ankle rarely degenerates in the absence of trauma. However, the ankle is by no means the only joint subject to the limitations of subjective injury severity assessments. Since side-to-side symmetry exists between contralateral limbs, this technique is equally well suited for other joint injuries, such as the much more frequently occurring tibial plateau fracture. Not only does this modified technique support other injuries, but avoidance of the need for an intact contralateral scan enables the analysis of patients with concomitant injuries or contralateral deformities. Patients with bilateral fractures or with developmental abnormalities are not compatible with a methodology that relies on a healthy contralateral for reference measurements, whereas fracture severity measures using allometric models are free from such limitations.
In addition to being a valuable clinical tool for assessing trauma, this technique could help answer other interesting research questions. For some applications such as correlating cell death to impact energy, parity of fracture energy magnitudes is critical for acquiring meaningful data. In these instances, an allometric methodology may not be appropriate due to errors introduced by anatomic variation. However, absolute magnitude becomes less important for this methodology’s principal purpose, the stratification of an injury within a spectrum of fracture severity. For such purpose, it suffices to reproduce the relative energy magnitudes, so that the ordering of severities is not disturbed. The results from the present study show a level of accuracy in predicting the distal tibia’s cumulative surface area that is very satisfactory for incorporation into the objective CT-based injury severity methodology.
CONCLUSION
It has been shown that a distal tibia’s cumulative surface area can be accurately modeled using a second order polynomial, for a diverse group of ankle fractures. The developed mathematical model based upon this finding not only aids research of OA treatment in the clinical setting, but it also opens new research possibilities pertaining to related anatomic pathologies of the distal tibia.
Acknowledgments
Financial support was provided by grants from the National Institutes of Health (AR55533 and AR48939), the AO Research Fund and the Orthopaedic Trauma Association.
REFERENCES
- 1.Borrelli J., Jr., Ricci W.M. Acute Effects of Cartilage Impact. Clinical Orthopaedics & Related Research. 2004;1(423):33–39. doi: 10.1097/01.blo.0000132627.13539.02. [DOI] [PubMed] [Google Scholar]
- 2.Marsh J.L., Weigel D.P., Dirscxhl D.R. Tibial Plafond Fractures: How Do These Ankles Function Over Time? Journal of Bone and Joint Surgery. 2003;85(2):287. [PubMed] [Google Scholar]
- 3.Swiontkowski M.F., et al. Interobserver variation in the AO/OTA fracture classification system for pilon fractures: Is there a problem. J Orthop Trauma. 1997;11(7):467–70. doi: 10.1097/00005131-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DD, Mosqueda T, Thomas T, Hermanson EL, Brown TD, Marsh J. Quantifying tibial plafond fracture severity: Absorbed energy and fragment displacement agree with clinical rank ordering. J Orthop Res. 2008 Mar 7; doi: 10.1002/jor.20550. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas T.P. Biomedical Engineering. Iowa City, IA: University of Iowa; 2007. Development and Implentation of CT-Based Measures for Objective Fracture Severity Assessment; p. 105. [Google Scholar]
- 6.Drillis R C.R. Technical Report 1166.03. New York NY: US National Health Survey; 1966. Body Segment Parameters. [Google Scholar]
- 7.Associates W. Anthropometric Source Book, Vol. I: Anthropometry for Designers. Washington DC: National Aeronautics and Space Administration; 1978. [Google Scholar]