Abstract
Objectives
Oxygen is an essential component for many aspects of tissue repair. However, the effect of oxygen levels on differentiation of stem cells into osteoblasts and chondrocytes during fracture healing is unknown, in part because of the difficulty in measuring oxygen during fracture healing. In this study we tested the feasibility of using electron paramagnetic resonance (EPR) oximetry to assess tissue oxygen partial pressure (pO2) after tibial fractures in mice.
Methods
Transverse tibia fractures were created by three-point bending in adult mice. Paramagnetic material, lithium phthalocyanine (LiPc), was implanted into the fracture site or adjacent to the periosteum in the contralateral leg immediately after fracture. Tissue pO2 was assessed by EPR 90-110 minutes after implantation of the crystals. in a second experiment, LiPc was implanted into the fracture site and fracture repair and the bio-compatibility of LiPc were assessed at 14 and 28 days after injury.
Results
At the very early stage after fracture, injury significantly decreased tissue oxygenation at the fracture site. When animals were breathing 21% oxygen, pO2 at the fracture site ((30.6 ± 12.7mmhg, n=7) was lower than that in contralateral legs (45.5 ± 15.3mmhg, n=7, p<0.01). breathing 100% inspired oxygen increased the pO2 in both the fractured (72.8 ± 28.2mmhg; n=7) and contralateral legs (148.4 ± 59.2mmhg; n=7, p<0.01). in addition, LiPc crystals implanted into fracture sites did not interfere with normal fracture healing at 10 and 28 days post-injury.
Conclusions
EPR oximetry is a valuable tool for monitoring oxygen levels during fracture repair in mice.
INTRODUCTION
Fractures are usually accompanied by local tissue hypoxia due to the disruption of vasculature. Oxygen levels at fracture sites are initially very low, and they gradually increase as re-vascularization occurs.1,2,3 During wound repair, oxygen is required for cell survival, collagen and extracellular matrix deposition,4,5 angiogenesis,6,7 epithelialization,8 and production of the oxidative burst used for intracellular bacterial killing.9,10 However, the role of oxygen in fracture healing has not been extensively studied. Previous studies have shown that chronic systemic hypoxia inhibits11 and oxygen supplementation enhances bone repair.12,13 These studies suggest that oxygen is important for fracture healing. Therefore studying the role of oxygen during bone repair could greatly increase our understanding of mechanisms that regulate skeletal regeneration.
In order to examine the role of oxygen during fracture healing in detail a reliable method of measuring tissue oxygen levels in vivo is required. Oxygen-sensing electrodes are widely used to measure tissue PO2 directly.1,2,14 However, this technique has several disadvantages when it is used to measure oxygenation at fracture sites. First, inserting an electrode into tissue is invasive and affects O2 measurement immediately after injury. Second, microelectrodes are fragile and may be damaged during insertion into the fracture callus. Last, measuring tissue O2 repeatedly at the same location in the same animal is not possible. An alternate approach of measuring oxygen concentration in vivo utilizes spectroscopic relaxation methods (reviewed by Vanderkooi et al.15). Oxygen affects the relaxation times of excited species such as luminescent or magnetic probes. These relaxation times can be measured optically or magnetically and used to determine pO2 directly. Among all spectroscopic relaxation methods, electron paramagnetic resonance (EPR)15,16,17 is a particularly promising method to use for studying tissue oxygenation in murine fracture models. In vivo EPR oximetry is non-invasive after the initial implantation of the oxygen-sensitive paramagnetic probe into the tissue of interest.16,17,18 This procedure causes only minimal injury as materials such as lithium phthalocyanine (LiPc) can be placed with a 25-gauge needle, and the amount deposited is less than 50 micrograms. Furthermore, these paramagnetic probes are biocompatible and induce minimal or no tissue reaction.18 In the brain, repetitive and reproducible measurements of pO2 using EPR have been achieved for more than 30 days.18 Importantly, the location of paramagnetic material in the tissue can be determined on histological sections, allowing the exact site of pO2 measurement to be determined. In this study, we tested the feasibility of using EPR oximetry to assess tissue oxygenation at the fracture site in mice.
MATERIALS AND METHODS
Animals and creation of tibia fractures
All procedures in this work were approved by IACUC (Institutional Animal Care and Use Committee) at Dart-mouth Medical School, Hanover, NH or at the University of California at San Francisco, San Francisco, CA. Male C57B6 mice (8 weeks old) were purchased from Charles River Laboratories, Inc. and accommodated in a conventional animal facility for 1 week before initiating experiments. Animals were anesthetized with 2% isoflurane in 100% oxygen. A transverse closed fracture was created by three-point bending at the mid-shaft of right tibia.19
To create ischemic tibial fractures, the right femoral artery and its branches were ligated and removed immediately prior to fracture.20 Briefly, using aseptic technique, a small incision was made over the right groin to expose the femoral vessels. The femoral nerve and vein were separated from the femoral artery, and the femoral artery and its branches between the inguinal ligament and the popliteal bifurcation were ligated. Then the femoral artery was removed. Immediately after resection of the femoral artery, a transverse closed fracture was created in the mid-shaft of the right tibia.
EPR oximetry
Immediately after the creation of tibia fractures, LiPc cr ystals (20 -30 micrograms) were implanted into the fracture site and, as controls, adjacent to the periosteum in the contralateral legs using a 25 gauge needle. Tissue pO2 was then sequentially assessed by EPR oximetry using an in vivo EPR spectrometer, with a custom made low-frequency microwave bridge operating at 1.1 GHz and an extended loop resonator (11 mm diameter).18,21 Animals were placed in the magnetic field and maintained under general anesthesia (1.8% inspired isoflurane). Euthermia (37.5°C ± 0.5°C) was monitored with a rectal temperature probe and maintained with a circulating water blanket. The leg of interest was positioned under the extended loop resonator. The EPR spectrum was then acquired with the spectrometer. Although spectrometer settings varied slightly from scan to scan, typical settings were: incident microwave power-10mW, magnetic field center-425G, scan range 1G. Modulation amplitude was set less than one third of the EPR line width. Scan time was approximately 2 to 3 minutes and 3 to 5 individual scans were averaged to achieve a better signal to noise ratio. The line-width was determined from the EPR spectra, and the partial pressure of tissue oxygen was calculated from an existing calibration curve specific to the given batch of LiPc.16,18
Determining the time course of tissue pO2 change after injury
The length of time required to obtain a steady state of pO2 after fracture was determined in 4 animals with tibia fracture and intact femoral artery, 1 animal with femoral artery resection without fracture (ischemia only), and 1 animal with femoral artery resection with a tibia fracture (ischemic fracture). Animals were administered 100% oxygen and pO2 at the fracture sites was measured every 15 minutes after injury for 90 minutes. The time required to reach pO2 equilibration after changing the inspired oxygen level was also determined using one animal with a tibia fracture and an intact femoral artery. This animal was switched to 21% oxygen and pO2 was measured every 5 minutes for 20 minutes. Inspired oxygen for this same animal was again changed back to 100% and pO2 was monitored for another 20 minutes.
Determining the effects of fracture on tissue pO2
Tibia fractures were created in 7 animals and LiPc crystals were implanted into the fracture sites and adjacent to the periosteum in the contralateral legs. Following randomization, animals were initially administered either 100% or 21% oxygen for 90 minutes and pO2 at the fracture sites or periosteum in the contralateral legs was measured. The inspired oxygen level was then changed to 21% or 100% respectively and pO2 measurements were performed again 20 minutes later.
Determining the effects of femoral artery resection on tissue pO2 at fracture sites
Tibia fractures with femoral artery resection were created in 4 animals and LiPc crystals were implanted into the fracture sites. Animals were administered 100% O2 and oxygen concentration was measured at 90 minutes after injury. No measurements were made in 21% oxygen.
Histological analysis of crystal placement
To correlate the position of crystals with measurements of pO2, animals from the above experiments were sacrificed immediately after the completion of oximetry and both the fractured and contralateral tibiae were collected. Tissues were processed to decalcified paraffin sections as described above. Sections were stained with Hemotoxylin and eosin.
Assessing the long term biocompatibility of LiPc
The long-term biocompatibility of LiPc was firstly assessed by implanting LiPc crystals (20-30 micrograms) with a 25 gauge needle into fracture sites right after injury and analyzing fracture healing at 10 (n=4) and 28 (n=3) days post-fracture. Fractured tibiae were collected, fixed in 4% paraformaldehyde, decalcified in 19% EDTA, and then dehydrated and embedded in paraffin. Longitudinal sections (10μm) were prepared. Sections were stained with Hall and Brunt’s Quadruple stain (HBQ22,23) to stain cartilage blue and bone red.
Statistical analyses
A one-sided paired (one-sample) t-test was used to determine whether fracture and inspired oxygen altered the pO2 in vivo. The effect of femoral artery resection on the pO2 level at fracture sites was determined using a t-test. Data are presented as mean ± one standard deviation. A power analysis on current data demonstrated that a sample size of 7 in each group is sufficient to detect a 30% decrease in pO2 in fractured legs compared to contralateral legs, assuming a power of 80% and a significance level of 5%.
RESULTS
Assessing equilibration of pO2 after fracture and after changing inspired oxygen
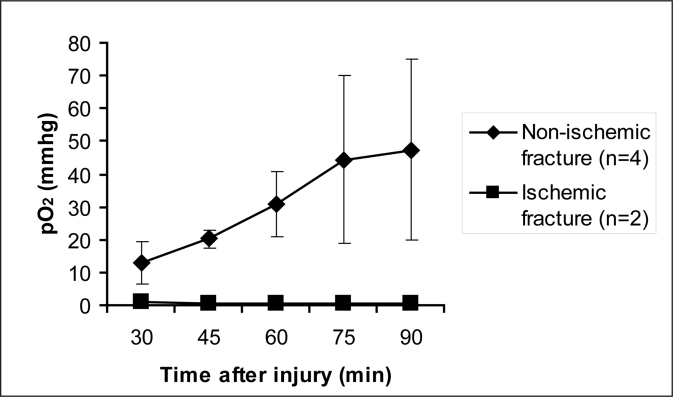
The length of time required to reach a steady state of pO2 after fracture and after changing the inspired oxygen level was determined in a small group of animals. In fractures without femoral artery resection (n=4), tissue pO2 equilibrium was achieved in 90 minutes after fracture with animals breathing 100% oxygen (Figure 1). After changes in the inspired oxygen concentration, pO2 equilibrated within 20 minutes (data not shown). In the animals with femoral artery resection (n=2), pO2 in the hind limb remained low and unchanged during the first 90 minutes after injury (Figure 1). Therefore all measurements throughout this work were made at 90 minutes after fracture with constant administration of the same concentration of oxygen and 20 minutes after changing the inspired oxygen level (i.e., 110 minutes after injury).
Figure 1.
Time course of pO2 change at the fracture site. When animals breathed 100% oxygen, tissue oxygen equilibrium was achieved in 90 minutes in fractures with intact femoral artery. after femoral artery resection, very low tissue pO2 was detected at fracture sites, which remained low even after 90 minutes of inspiring 100% oxygen.
Injury decreases tissue oxygenation at fracture sites
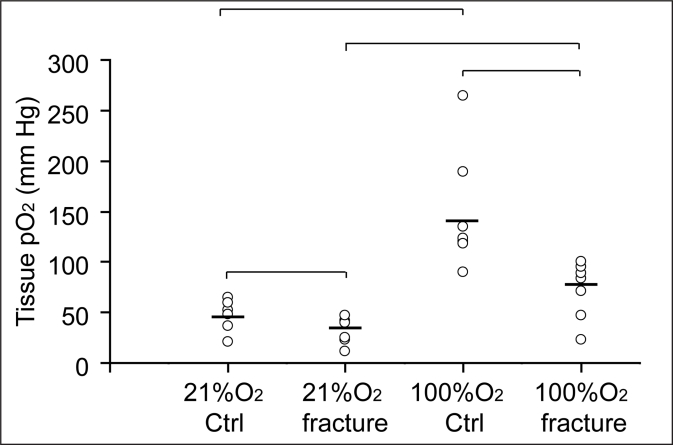
Our next objective was to determine the immediate effects of traumatic injury on tissue oxygen levels at the fracture site. At the fracture site in animals breathing 21% oxygen, the mean pO2 (30.6 ± 12.7 mmHg; n=7) was significantly lower than that at the periosteum in contralateral limbs (45.5 ± 15.3 mmHg; n=7; p<0.01. Figure 2). pO2 was significantly increased in both the fractured limbs (72.8 ± 28.2 mm Hg, p<0.01) and contralateral legs (148.4 ± 59.2 mmHg, p<0.01) during 100% oxygen administration (Figure 2). However, the pO2 at fracture sites still remained significantly lower than that in the control tibial periosteum (Figure 2, p<0.01), suggesting that disruptions to the vasculature had occurred that limited the functional capacity of the blood supply. To further assess the ability of EPR to measure vascular oxygen delivery after skeletal injury, we measured fracture and periosteal pO2 levels in animals breathing 100% oxygen, that had undergone femoral artery resection as an additional ischemic insult; this injury is sufficiently severe to delay fracture healing.20 Femoral artery resection significantly reduced pO2 at the fracture site (1.4 ± 0.7 mmHg; n=4) compared to 72.8 ± 28.2 mm Hg in fractures with intact femoral artery (n=7; p<0.01).
Figure 2.
Fracture and inspired oxygen level significantly affect tissue pO2. When animals (n=7) were breathing 21% oxygen, the mean pO2 was 45.5 ± 15.3 mmhg in contralateral limbs and 30.6 ± 12.7 mmhg at fracture sites. When animals were breathing 100% oxygen, the mean pO2 was 148.4 ± 59.2 mmhg in contralateral legs and 72.8 ± 28.2 mm hg in fractured limbs. ctrl = contralateral limbs.——mean of tissue pO2· |¯¯¯| p<0.01.
Crystal placement
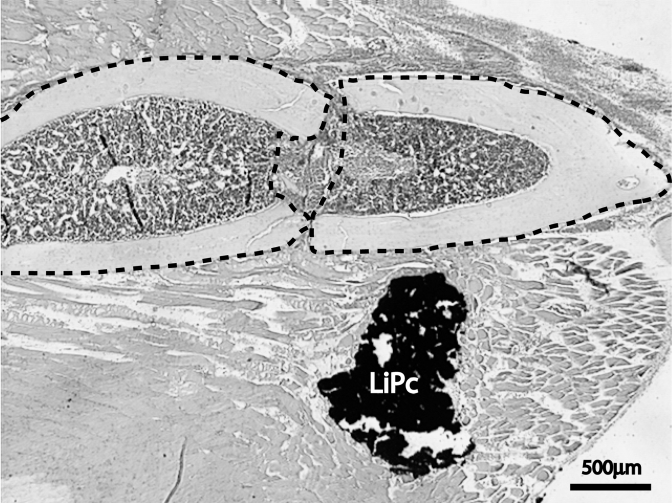
In the majority of limbs with skeletal injury, LiPc crystals were located within the injured muscle near the fracture site (Figure 3). The location of LiPc crystals does not appear to correlate with the variation of pO2 measurements, suggesting that extensive muscle and vascular injury was present around fracture sites.
Figure 3.
LiPc crystals were located within muscle near the fracture site in the majority of animals. H&E staining. outlined area = fracture ends.
LiPc does not interfere with normal fracture healing
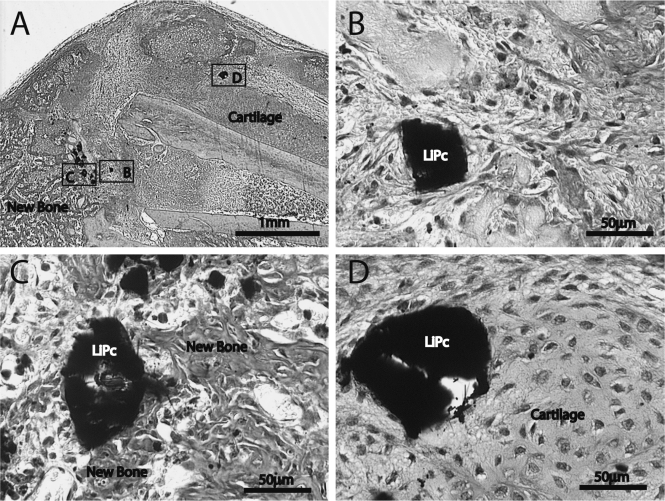
Our last goal was to examine the biocompatibility of LiPc crystals during fracture repair. LiPc crystals were placed at the fracture site and then the injuries were allowed to heal for 10 days to assess endochondral ossification and for 28 days to assess union of the fractured bone ends. At 10 days after injury (n=4), hypertrophic cartilage and newly formed bone were observed in fracture calluses indicating endochondral ossification was under way (Figure 4A). LiPc crystals were embedded in granulation tissue (Figure 4B) or were in direct contact with newly formed bone (Figure 4C) and cartilage (Figure 4D). At 28 days (data not shown), all fractures (n=3) had healed by bony bridging. LiPc crystals were observed in direct contact with bone or in surrounding soft tissue. A thin layer of fibrous tissue was occasionally observed around LiPc crystals at this time. Compared to previously published data,19,20,24 LiPc crystals did not significantly alter the normal progress of tibia fracture healing in mice. Mice typically heal experimental tibia fractures by day 28 post-injury and this was the case in our current study. Furthermore, the crystals were in direct contact with cartilage and bone, and there was no evidence of a large population of inflammatory cells in adjacent space. Thus, LiPc crystals do not induce much inflammatory response and they have excellent biocompatibility during fracture healing.
Figure 4.
Histological analysis of fracture healing with the presence of LiPc crystals. (A) At 10 days after injury, hypertrophic cartilage and newly formed bone were observed in fracture calluses. (B) High magnification of box B in (A) shows a LiPc crystal is embedded in granulation tissue with minimal fibrosis. (C) High magnification of box C in (A) shows a LiPc crystal is in direct contact with newly formed bone. (D) High magnification of box D in (A) shows a LiPc crystal is in direct contact with cartilage.
DISCUSSION
The results of this study indicate that EPR oximetry will be a valuable tool for monitoring oxygen levels during fracture repair in mice. EPR oximetry detected significantly lowered tissue oxygen levels at fracture sites after injury and it also successfully detected the expected changes of tissue oxygen levels after switching the breathing oxygen concentration from 21% to 100%. These data suggest that the sensitivity of EPR oximetry is adequate to assess tissue pO2 during fracture healing. In addition, we confirmed that the paramagnetic probe used in current study, LiPc, is biocompatible. LiPc crystals did not induce obvious inflammatory response and interfere with normal fracture healing. Further, LiPc crystals gave us the ability to make repeated measurements in the same location and to assess tissue formation at each site, providing a unique opportunity to examine the effect that oxygen has on stem cell differentiation and function. For instance, this technique will allow us to assess pO2 at a particular site and then at a later time point we can examine what type of tissue has formed in this region using histologic methods. Developing and employing these technologies for studying fracture repair in mice is of great importance, because compared to other rodent models, a murine model offers the advantage of using a multitude of genetically engineered strains, and there is an abundance of reagents for molecular and cellular analyses available.
The level of tissue oxygenation may affect fracture repair. Decreasing tissue oxygenation by inducing chronic systemic hypoxia11 or by ligating femoral artery20 delays fracture healing. Increasing tissue oxygenation by providing hyperbaric oxygen, on the contrary, can accelerate bone repair.25 Understanding the mechanisms underlying these effects is of great important. There is evidence suggesting that oxygen levels may have direct effects on stem cells. For example, the function and differentiation of chondrocytes and osteoblasts is affected by oxygen levels in vitro.26,27,28 Under experimental conditions that induce chronic hypoxia, fractured femurs did not exhibit signs of bone formation. Rather the calluses were comprised of abundant cartilage11 which indicates that chondrocyte differentiation can occur in a more hypoxic environment than osteoblast differentiation. In addition to the effect of hypoxia on stem cells, tissue oxygen levels may play other important roles during bone repair. Studies on wound healing have demonstrated that oxygen is involved in multiple processes, including cell survival, collagen and extracellular matrix deposition,4,5 angiogenesis,6,7 epithelialization,8 and production of the oxidative burst used for intracellular bacterial killing.9,10 When tissue pO2 falls below 40 mmHg, angiogenesis, extracellular matrix formation, and resistance to infection are all impaired.29 In current study, we detected that tissue oxygen level at fracture sites dropped below this level after injury, especially after femoral artery ligation, warranting further studies on the effects of oxygen on cell death, angiogenesis, and infection during fracture healing.
In summary, these data illustrate that EPR oximetry is a promising technique to assess tissue oxygenation in murine fracture models. We have used EPR to examine the effect that tibial fracture has on pO2 during fracture repair. We have demonstrated that these fractures generate vascular damage that significantly reduces tissue oxygen levels to levels that could impair healing. However, mice exhibit exceptional regenerative capacity. Normally no difficulties in skeletal healing are detected without the inclusion of a major traumatic insult such as cautery of the periosteum30 or resection of the femoral artery.20 Even in the latter situation the mice salvage their limbs, and eventually the skeletal injury heals.20 These results suggest that tissue oxygenation returns to normoxic levels rapidly after injury. Thus, understanding this time course and evaluating the role that oxygen plays during fracture repair are important parameters for elucidating and exploiting the mechanisms that underlie the regenerative potential during fracture repair in mice.
Acknowledgments
This work was supported by grants from the NIH (R01 to T.M., P01 to H.S.), the Orthopaedic Trauma Association (research grants to R.M. and C.L.), and Zimmer Corporation (a grant to R.M.). The EPR Center for the Study of Viable Systems at Dartmouth Medical School, Hanover, NH is supported by NIBIB (P41 to H.S.).
Footnotes
The authors have no conflicts of interest.
REFERENCES
- 1.Brighton CT, Krebs AG. Oxygen tension of healing fractures in the rabbit. J Bone Joint Surg Am. 1972;54:323–32. [PubMed] [Google Scholar]
- 2.Heppenstall RB, Grislis G, Hunt TK. Tissue gas tensions and oxygen consumption in healing bone defects. Clin Orthop Relat Res. 1975;6:357–65. doi: 10.1097/00003086-197501000-00048. [DOI] [PubMed] [Google Scholar]
- 3.Aro H, Eerola E, Aho AJ, Niinikoski J. Tissue oxygen tension in externally stabilized tibial fractures in rabbits during normal healing and infection. J Surg Res. 1984;37:202–207. doi: 10.1016/0022-4804(84)90181-1. [DOI] [PubMed] [Google Scholar]
- 4.Uitto J, Prockop DJ. Synthesis and secretion of under-hydroxylated procollagen at various temperatures by cells subject to temporary anoxia. Biochem Biophys Res Commun. 1974;60:414–23. doi: 10.1016/0006-291x(74)90220-4. [DOI] [PubMed] [Google Scholar]
- 5.Myllyla R, Tuderman L, Kivirikko KI. Mechanism of the prolyl hydroxylase reaction. 2 Kinetic analysis of the reaction sequence. Eur J Biochem. 1977;80:349–57. doi: 10.1111/j.1432-1033.1977.tb11889.x. [DOI] [PubMed] [Google Scholar]
- 6.Knighton DR, Silver IA, Hunt TK. Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration. Surgery. 1981;90:262–70. [PubMed] [Google Scholar]
- 7.Hopf HW, Gibson JJ, Angeles AP, Constant JS, Feng JJ, Rollins MD, Zamirul Hussain M, Hunt TK. Hyperoxia and angiogenesis. Wound Repair Regen. 2005;13:558–64. doi: 10.1111/j.1524-475X.2005.00078.x. [DOI] [PubMed] [Google Scholar]
- 8.Medawar PS. The behavior of mammalian skin epithelium under strictly anaerobic conditions. A J Microsc Sci. 1947;88:27. [PubMed] [Google Scholar]
- 9.Babior BM. Oxygen-dependent microbial killing by phagocytes (first of two parts) N Engl J Med. 1978;298:659–68. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 10.Allen DB, Maguire JJ, Mahdavian M, Wicke C, Marcocci L, Scheuenstuhl H, Chang M, Le AX, Hopf HW, Hunt TK. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg. 1997;132:991–6. doi: 10.1001/archsurg.1997.01430330057009. [DOI] [PubMed] [Google Scholar]
- 11.Heppenstall RB, Goodwin CW, Brighton CT. Fracture healing in the presence of chronic hypoxia. J Bone Joint Surg Am. 1976;58:1153–6. [PubMed] [Google Scholar]
- 12.Coulson DB, Ferguson AB, jr., Diehl RC., jr. Effect of hyperbaric oxygen on the healing femur of the rat. Surg Forum. 1966;17:449–50. [PubMed] [Google Scholar]
- 13.Ueng SW, Lee SS, Lin SS, Wang CR, Liu SJ, Tai CL, Shih CH. Hyperbaric oxygen therapy mitigates the adverse effect of cigarette smoking on the bone healing of tibial lengthening: An experimental study on rabbits. J Trauma. 1999;47:752–9. doi: 10.1097/00005373-199910000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Brighton CT, Heppenstall RB, Labosky DA. An oxygen microelectrode suitable for cartilage and cancellous bone. Clin Orthop Relat Res. 1971;80:161–6. doi: 10.1097/00003086-197110000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Vanderkooi JM, Erecinska M, Silver IA. Oxygen in mammalian tissue: Methods of measurement and affinities of various reactions. Am J Physiol. 1991;260:C1131–50. doi: 10.1152/ajpcell.1991.260.6.C1131. [DOI] [PubMed] [Google Scholar]
- 16.Hou H, Grinberg OY, Grinberg SA, Demidenko E, Swartz HM. Cerebral tissue oxygenation in reversible focal ischemia in rats: Multi-site epr oximetry measurements. Physiol Meas. 2005;26:131–41. doi: 10.1088/0967-3334/26/1/012. [DOI] [PubMed] [Google Scholar]
- 17.Hou H, Khan N, O’hara JA, Grinberg OY, Dunn JF, Abajian MA, Wilmot CM, Demidenko E, Lu S, Steffen RP, Swartz HM. Increased oxygenation of intracranial tumors by efaproxyn (efaproxiral), an allosteric hemoglobin modifier: In vivo epr oximetry study. Int J Radiat Oncol Biol Phys. 2005;61:1503–9. doi: 10.1016/j.ijrobp.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 18.Swartz HM, Walczak T. Developing in vivo epr oximetry for clinical use. Adv Exp Med Biol. 1998;454:243–52. doi: 10.1007/978-1-4615-4863-8_29. [DOI] [PubMed] [Google Scholar]
- 19.Lu C, Miclau T, Hu D, Hansen E, Tsui K, Puttlitz C, Marcucio RS. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23:1300–7. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu C, Miclau T, Hu D, Marcucio RS. Ischemia leads to delayed union during fracture healing: A mouse model. J Orthop Res. 2007;25:51–61. doi: 10.1002/jor.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirata H, Walczak T, Swartz HM. Electronically tunable surface-coil-type resonator for l-band epr spectroscopy. J Magn Reson. 2000;142:159–67. doi: 10.1006/jmre.1999.1927. [DOI] [PubMed] [Google Scholar]
- 22.Hall BK. The role of movement and tissue interactions in the development and growth of bone and secondary cartilage in the clavicle of the embryonic chick. J Embryol Exp Morphol. 1986;93:133–52. [PubMed] [Google Scholar]
- 23.Eames BF, Sharpe PT, Helms JA. Hierarchy revealed in the specification of three skeletal fates by sox9 and runx 2. Dev Biol. 2004;274:188–200. doi: 10.1016/j.ydbio.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture healing. J Orthop Res. 2002;20:1091–8. doi: 10.1016/S0736-0266(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 25.Ueng SW, Lee SS, Lin SS, Wang CR, Liu SJ, Yang HF, Tai CL, Shih CH. Bone healing of tibial lengthening is enhanced by hyperbaric oxygen therapy: A study of bone mineral density and torsional strength on rabbits. J Trauma. 1998;44:676–81. doi: 10.1097/00005373-199804000-00020. [DOI] [PubMed] [Google Scholar]
- 26.D’Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human miami cells. Bone. 2006 doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 27.Tuncay OC, Ho D, Barker MK. Oxygen tension regulates osteoblast function. Am J Orthod Dentofacial Orthop. 1994;105:457–63. doi: 10.1016/S0889-5406(94)70006-0. [DOI] [PubMed] [Google Scholar]
- 28.Salim A, Nacamuli RP, Morgan EF, Giaccia AJ, Longaker MT. Transient changes in oxygen tension inhibit osteogenic differentiation and runx2 expression in osteoblasts. J Biol Chem. 2004;279:40007–16. doi: 10.1074/jbc.M403715200. [DOI] [PubMed] [Google Scholar]
- 29.Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg. 2003;186:259–63. doi: 10.1016/s0002-9610(03)00211-3. [DOI] [PubMed] [Google Scholar]
- 30.Kokubu T, Hak DJ, Hazelwood SJ, Reddi AH. Development of an atrophic nonunion model and comparison to a closed healing fracture in rat femur. J Orthop Res. 2003;21:503–10. doi: 10.1016/S0736-0266(02)00209-7. [DOI] [PubMed] [Google Scholar]