Abstract
Quadriceps muscle strength is often used as a criterion for functional progression and return to activity after knee joint injury or surgery. Previous research has demonstrated that noteworthy antagonist activity is present during knee strength testing. the countermoment associated with this antagonist muscle activity may lead to an underestimation of knee strength. the burst superimposition method of strength testing is considered by some to be the current gold standard. the effect of burst super-imposition on antagonist activity is unknown. the purpose of this study was to test the hypothesis that burst superimposition diminishes antagonistic hamstrings activity during knee extensor strength testing. Isometric knee strength testing was performed in 22 (11 males, 11 females) active young people with no history of serious lower extremity injuries using the burst superimposition method. the magnitude of hamstrings muscle activity was assessed just before and after burst superimposition. contrary to our hypothesis, a small, but statistically significant increase in antagonistic medial hamstrings activity was observed with burst superimposition (7.23 vs. 9.62; P < 0.001). Higher lateral hamstrings activity was also observed, but this did not reach statistical significance (15.03 vs. 13.50; P = 0.087). though statistically significant, the small increase in hamstrings activity is unlikely to be clinically meaningful.
INTRODUCTION
A primary goal of rehabilitation after knee joint injury or surgery is to safely return individuals to activities they desire to participate in. Quadriceps muscle strength has been shown to correlate strongly with knee function and is postulated to be a critical factor in knee joint health.1–5 Accordingly, clinicians often use quadriceps muscle strength as a criterion for return to activity, especially sports participation. Research from our lab and that of others has shown that noteworthy antagonist muscle activity is present during knee strength testing.6–8 This antagonist muscle activity may be problematic as the associated moments may lead to measurement error resulting in an underestimation of the knee strength, which could impact clinical decision making.
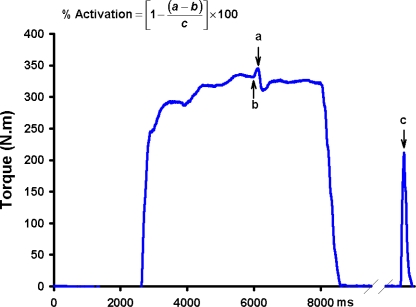
Muscle force generation capacity is a product of both the size of a muscle’s fibers and the ability to fully activate the fibers within a muscle. Traditional isokinetic, isometric, and isotonic strength testing provide meaningful information about one’s ability to voluntarily produce joint torque, but may underestimate the true strength of a muscle group due to the inability of these methods to account for activation failure. For this reason, scientists interested in quantifying knee extensor strength often superimpose an electrical stimulus on the quadriceps muscle during maximal voluntary contraction (burst superimposition or twitch interpolation method) to obtain a more complete picture of strength.1,5,9–11 If the muscle is fully activated the superimposed electrical stimulus will not augment torque, whereas when activation failure is present the supramaximal electrical stimulus will cause the inactive muscle to become active resulting in a torque increment (Figure 1).11–13 Thus, burst superimposition and twitch interpolation methods allow assessment of voluntary strength, activation failure, and theoretically, true muscle strength. Consequently, this approach is considered by many to be the current “gold standard” for assessing knee extensor strength. The effect of burst superimposition on antagonistic hamstrings muscle activity during knee extensor strength testing is unknown. There is evidence that peripheral electric stimulation can affect spinal and cortical excitability.14 Hence, it is reasonable that the burst superimposition method of strength testing may alter antagonistic muscle activity and thereby affect the accuracy of strength testing. The purpose of this study was to evaluate the effect of burst superimposition on antagonistic hamstrings muscle activity during isometric knee extensor strength testing. We hypothesized that burst superimposition would decrease antagonistic hamstrings muscle activity.
Figure 1.
Schematic representation of the burst interpolated twitch technique. Incomplete activation of the quadriceps muscle is visualized by the increment in torque associated with the superimposed pulse train. Voluntary activation failure of the quadriceps muscle is estimated by expressing the stimulus evoked torque during contraction as a percentage of stimulus evoked torque during rest (a = torque evoked due to the superimposition of the electrical pulses, b = voluntary torque just prior to the stimulus, and c = torque produced by the pulse train at rest).
METHODS
Twenty-two (11 males, 11 females) uninjured young people volunteered to participate in this study. All subjects were regular participants in fitness activities or sports (Tegner Activity Score ≥ 4).15 Exclusion criteria included a history of significant lower extremity muscle injury, major knee ligament injury, signs and symptoms of patellofemoral joint dysfunction, abnormal KT-2000™ evaluation (≥ 3 mm side-to-side difference in laxity), history of lower extremity surgery, lower extremity nerve injuries, or an abnormal gait pattern. Subjects were asked to refrain from any strenuous physical activity for 24 hours prior to participation. All subjects provided written informed consent that was approved by the University of Iowa Human Subjects Research Institutional Review Board prior to their participation.
TESTING PROCEDURES
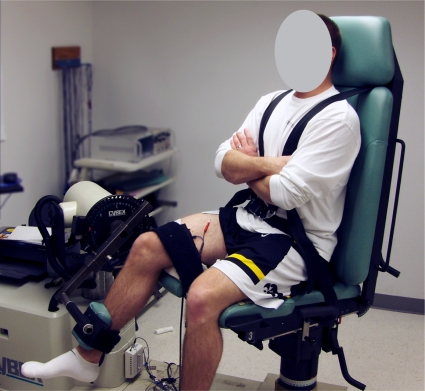
Subjects performed a five minute warm-up on a cycle ergometer followed by self-directed stretching of the quadriceps, hamstrings, and gastrocnemius muscles prior to testing. The order of limb testing was randomized a prioriusing a computer-based random number generator in order to minimize any effects associated with the order of testing. Electromyographic preamplifiers (model 544, Therapeutic Unlimited, Iowa City, IA; 35x differential gain, 87 dB common-mode rejection at 60 Hz, input impedance > 25 Mω, noise < 2 ΩV RMS) were applied over the bellies of the semitendinosus and biceps femoris longus muscles. Electrode placements were standardized according to the recommendations of Perotto.16 Self-adhesive stimulating electrodes (2.75 × 5.00 inches, Dura-Stick II, Chattanooga Group, Hixson, TN, USA) were placed over the proximal and distal surface of the quadriceps muscles and connected to a high voltage constant current stimulator (model DS7AH, Digitimer Ltd., Hertfordshire, England) that was used to provide electrical stimuli during testing. Subjects sat on a small platform placed on the HUMAC NORM Testing and Rehabilitation System’s (Computer Sports Medicine, Inc., Stoughton, MA, USA) chair during testing in order to limit noise associated with pressure on the EMG preamplifers. Subjects were tightly secured to the test system’s chair using a waist strap, chest straps, and a thigh strap according to the manufacturer’s guidelines. The lateral epicondyle of the femur (theoretical axis of motion) was aligned with the axis of the dynamometer (Figure 2). The knee was positioned in 60° of flexion and the hip in 90° of flexion. The test system’s torque arm pad was fixed to the shank approximately 7.5 cm proximal to the medial malleolus.
Figure 2.
Experimental setup to evaluate the effects of burst superim-position on hamstrings muscle activity. subject positioned for testing (hip at ∼ 90° of flexion and knee at 60° of flexion) with stimulation electrodes on the quadriceps muscles and surface EMG electrodes on the medial and lateral hamstrings muscles.
In preparation for testing, brief electrical pulses of submaximal intensity were then delivered to familiarize the subjects to the electrical pulses. The intensity of electrical stimuli used during testing was determined while the subjects were seated at rest by sequentially stimulating the quadriceps muscle with pulse trains (10-pulses, frequency:100 Hz, pulse duration: 200 μs, 400V) in current steps of 100 mA until the torque associated with the stimulation-induced contractions no longer increased, but decreased. Current was then reduced by 50 mA and a final stimulus was provided. The current that produced the greatest torque was selected for use during testing.
Four submaximal isometric knee extension and flexion trials (approximately 50% to 85% maximum effort) and one five second maximal voluntary isometric contraction (MVIC) were then performed to familiarize the subjects with performing the isometric test and potentiate their quadriceps muscles. Just prior to maximal testing, three baseline EMG recordings were obtained while subjects sat at rest. The results of these trials were later used to remove baseline noise from the signals in post-processing. After a two minute rest period, subjects performed three knee extensor and three knee flexor MVICs (five second duration) in an alternating order. Loud verbal encouragement and visual feedback of the real-time torque was provided during maximal contractions to facilitate maximal effort. Approximately three seconds after the onset of each MVIC trial, a supramaximal train of electrical pulses at the predetermined stimulus intensity was superimposed on the subject’s maximal effort. Each maximal trial in extension or flexion was separated by three minutes to minimize fatigue. When testing was completed on the first side, subjects were positioned for testing of the opposite side. The testing procedures for the opposite leg were identical to those used when testing the first side.
DATA MANAGEMENT AND ANALYSIS
A custom-written LabVIEW program (version 7.0, National Instruments Corporation, Austin, TX, USA) was used to trigger the stimulator and collect EMG and torque data during testing. The EMG signals were low pass filtered at 500 Hz using an 8th order analog Butterworth filter and torque signals were low pass filtered at 4 Hz using a 3rd order analog Butterworth filter. All signals were sampled at 1000 Hz. Torque signals were converted to torque values (N·m) using calibrated conversion factors that were validated onsite prior to testing. After removing the recorded baseline values, the EMG signals were full-wave rectified. The average hamstrings muscle activities observed in the 200 ms epoch immediately prior to and after burst superimposition were used in analysis. Hamstrings activity during knee extension trials was normalized using the average hamstrings activity recorded during the 200 ms epoch preceding peak torque in the maximal knee flexion trials. The average of the values recorded during the three test trials was used in analysis. Quadriceps voluntary activation was determined for each leg using the following formula:
All statistical analyses were performed using SPSS for Windows version 15.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were calculated for subject demographics, voluntary activation values, and hamstrings activity during knee extension. Gender, time epoch (before and after stimulation), and side were the independent variables. Quadriceps voluntary activation values and magnitude of medial and lateral hamstrings activity were the dependent variables. A one-way analysis of variance (ANOVA) was used to assess differences in demographics by sex. Repeated measures ANOVA with two within-subjects factors (time, side) and one between-subjects factor (sex) was used to evaluate the effects of burst superimposition on the hamstrings muscle activity. Repeated measures ANOVA with side as within-subjects factor and sex as a between-subjects factor was used to evaluate side-to-side and sex differences in the estimates of voluntary activation. A significance level of α = 0.05 was set for all statistical analyses.
RESULTS
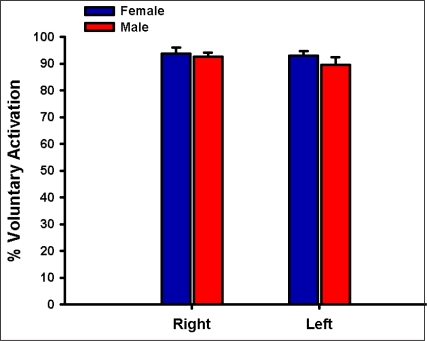
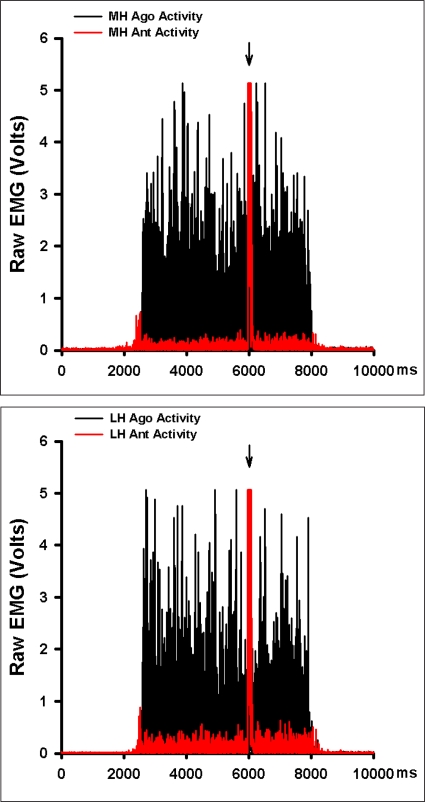
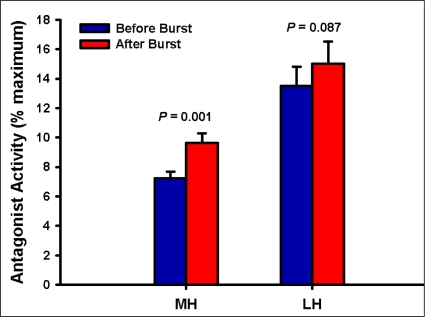
As expected males and females differed significantly in their height and weight; their age and activity-level were similar, however (Table 1). The mean estimates of voluntary activation of the quadriceps muscles were similar by sex (P = 0.353, Figure 3). No side-to-side differences in voluntary activation values were observed (P = 0.318, Figure 3). Burst superimposition did not reduce the magnitude of antagonistic hamstrings muscle activity observed (Figure 4). Conversely, antagonistic medial hamstrings activity was significantly higher after burst superimposition (P < 0.001, Figure 5). Antagonistic lateral hamstrings activity was also higher after burst superimposition, but did not reach statistical significance (P = 0.087, Figure 5).
TABLE 1.
Subject Demographics
| Variable | Female | Male | P value |
|---|---|---|---|
| Age | 24.36 ± 2.84 | 24.00 ± 2.19 | 0.74 |
| Height | 1.65 ± 0.06 | 1.80 ± 0.07 | < 0.001* |
| Weight | 63.63 ± 4.96 | 80.24 ± 8.40 | < 0.001* |
| BMI | 23.20 ± 2.17 | 24.57 ± 2.20 | 0.157 |
| Tegner Score | 6.00 ± 1.00 | 6.45 0.69 | 0.229 |
Figure 3.
Mean estimates of voluntary activation of the quadriceps muscles for both sides in males and females. There was no significant difference in voluntary activation values between sides or across sexes. Error bars represent the standard error of the mean.
Figure 4.
Example of the hamstrings activity during burst superim-position knee extensor strength testing (red) overlaid on hamstrings activity collected during a maximal isometric knee flexion testing (black) from a subject tested in this study. Arrows indicate the time of burst superimposition. MH = medial hamstrings, LH = lateral hamstrings, ant activity = antagonist activity, & ago activity = agonist activity.
Figure 5.
Mean antagonist activity of the medial (MH) and lateral (LH) hamstrings muscles during the 200 ms epoch immediately before and after burst superimposition during maximal isometric knee extensor strength testing.
DISCUSSION
The aim of this study was to evaluate the effects of the burst superimposition method of knee extensor strength testing on hamstrings muscle activity during testing. Consistent with previous findings, noteworthy hamstrings activity was observed during voluntary knee extensor MVICs.6,17 Males and females were able to activate their quadriceps muscles to a similar extent. This finding and the mean voluntary activation values obtained using the burst interpolated twitch technique are consistent with those reported in the literature.18,19 The results of the study indicate that hamstrings activity is not minimized in burst superimposition strength testing. Contrary to our hypothesis, we observed a small (approximately 2%), but statistically significant increase in hamstrings activity with burst superimposition. A practical mathematical model previously described in work from our laboratory predicted that the small increase in hamstrings activity after burst superimposition only increases the error in strength testing by approximately 0.84%.8 Hence, we do not believe that the small increase in antagonist activity is clinically meaningful.
Restoring quadriceps muscle strength and function is a primary focus for patients and therapists after knee joint injury and surgery. Knee extensor strength tests are advocated in the clinical management of patients who sustain serious knee injuries or undergo knee surgery because a growing body of evidence indicates that quadriceps strength and control are important to knee health.20,21 Accordingly, strength testing accuracy is important. Previous research from our laboratory and other researchers suggests that antagonist activity is a source of measurement error in knee strength testing.6,8,17 Minimizing antagonist activity, therefore, may lead to more accurate strength tests. We hypothesized that superimposing an electrical stimulus on the quadriceps MVIC, a method already considered to be the “gold-standard” for knee strength testing by some, may reduce antagonistic hamstrings activity based on evidence that cortical reciprocal inhibition (reduced cortical excitability of antagonist muscles) occurs with peripheral electrical stimulation.14 Our results, however, indicate that hamstrings activity increased to a small degree.
There are several possible explanations for the observed increase in hamstrings activity with burst super-imposition. It is plausible that the sudden increase in the rate of motor unit discharge with burst superimposition excited Renshaw cells and resulted reciprocal excitation as Renshaw cells are known to have an inhibitory effect on Ia inhibitory interneurons that mediate reciprocal inhibition.22 Another possible explanation relates to the so-called “common drive phenomenon”, which states that the agonist and antagonist motor neuron pools are governed as if they belong to the same motor neuron pool.23 Accordingly, the increased activation of quadriceps muscle with burst superimposition may have lead to a parallel increase in antagonist hamstrings muscle activity. The rather small increase in hamstrings activity after burst superimposition is likely due to the fact that most of our subjects had near complete quadriceps activation (Figure 3). One of the limitations of this study is that we are unable to evaluate muscle activity during the period when the burst was being delivered due to the large stimulus artifact associated with the pulse train (Figure 4). It is possible that there was a brief, but significant increase in hamstrings activity as a result of volume conduction of the pulse train during this epoch. Such an increase in hamstrings activity could have potentiated and increased the excitability of the hamstrings motor neurons. Finally, it is possible that the increase in knee extensor torque resulting from burst superimposition increased the strain in the connective tissues of the knee and thereby provoked reflexive hamstrings excitation. Though plausible, we believe that this mechanism is unlikely when the magnitude of change in knee extensor torque, the hamstrings activity patterns, and the angle of testing are considered.
It should be noted that a selection bias was introduced by only including active young people with no history of serious lower extremity injuries. We selected this population because prior research had clearly demonstrated that noteworthy antagonistic hamstrings activity could be expected in active young people. The population used, therefore, was appropriate for our research question. We excluded people with a history of knee injuries because we believe it was advantageous to minimize the potential confounding effects and the variability associated with strength testing after injury. Yet, we acknowledge that the generalizability of the results was limited by using this approach.
CONCLUSION
Our results indicate that the burst superimposition knee extensor strength testing method results in a small increase in hamstrings activity rather than more complete reciprocal inhibition. This increased antagonistic activity, however, is not believed to be clinically meaningful as practical mathematical modeling suggests the associated error is approximately 1%.
REFERENCES
- 1.Fitzgerald G.K., Piva S.R., Irrgang J.J., Bouzubar F., Starz T.W. Quadriceps activation failure as a moderator of the relationship between quadriceps strength and physical function in individuals with knee osteoarthritis. Arthritis Rheum. 2004;51(1):40–48. doi: 10.1002/art.20084. [DOI] [PubMed] [Google Scholar]
- 2.Herzog W., Longino D., Clark A. The role of muscles in joint adaptation and degeneration. Langenbecks Arch Surg. 2003;388(5):305–15. doi: 10.1007/s00423-003-0402-6. [DOI] [PubMed] [Google Scholar]
- 3.Hurley M.V. Quadriceps weakness in osteoarthritis. Curr Opin Rheumatol. 1998;10(3):246–250. doi: 10.1097/00002281-199805000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Lewek M., Rudolph K., Axe M., Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 2002;17(1):56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 5.Lewek M.D., Rudolph K.S., Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22(1):110–115. doi: 10.1016/S0736-0266(03)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnan C., Williams G.N. Antagonist muscle activity during maximal isometric knee strength testing. J Orthop Sports Phys Ther. 2007;37(1):A72–A73. [Google Scholar]
- 7.Aagaard P., Simonsen E.B., Andersen J.L., Agnusson S.P., Bojsen-Moller F., Dyhrepoulsen P. Antagonist muscle coactivation during isokinetic knee extension. Scand J Med Sci Sports. 2000;10(2):58–67. doi: 10.1034/j.1600-0838.2000.010002058.x. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan C., Williams G.N. Error associated with antagonist muscle activity during isometric knee strength testing. J Orthop Sports Phys Ther. 2008;38(1):A74–A75. doi: 10.1007/s00421-010-1391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurley M.V., Jones D.W., Newham D.J. Arthrogenic quadriceps inhibition and rehabilitation of patients with extensive traumatic knee injuries. Clin Sci (Lond) 1994;86(3):305–310. doi: 10.1042/cs0860305. [DOI] [PubMed] [Google Scholar]
- 10.Stevens J.E., Mizner R.L., Snyder-Mackler L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J Orthop Res. 2003;21(5):775–779. doi: 10.1016/S0736-0266(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 11.Williams G.N., Buchanan T.S., Barrance P.J., Axe M.J., Snyder-Mackler L. Quadriceps weakness, atrophy, and activation failure in predicted noncopers after anterior cruciate ligament injury. Am J Sports Med. 2005;33(3):402–407. doi: 10.1177/0363546504268042. [DOI] [PubMed] [Google Scholar]
- 12.Kent-Braun J.A., Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996;19(7):861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Shield A., Zhou S. Assessing voluntary muscle activation with the twitch interpolation technique. Sports Med. 2004;34(4):253–267. doi: 10.2165/00007256-200434040-00005. [DOI] [PubMed] [Google Scholar]
- 14.Bertolasi L, Priori A, Tinazzi M, Bertasi V, rothwell J.C. Inhibitory action of forearm flexor muscle afferents on corticospinal outputs to antagonist muscles in humans. J Physiol. 1998;511(Pt 3):947–956. doi: 10.1111/j.1469-7793.1998.947bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tegner Y., Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43–49. [PubMed] [Google Scholar]
- 16.Perotto A. Anatomical guide for the electromyographer. 3rd. Springfield, Illinois: Charles C. Thomas; 1994. [Google Scholar]
- 17.Kellis E., Katis A. Hamstring antagonist moment estimation using clinically applicable models: Muscle dependency and synergy effects. J Electromyogr Kinesiol. 2008;18(1):144–153. doi: 10.1016/j.jelekin.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Behm D., Power K., Drinkwater E. Comparison of interpolation and central activation ratios as measures of muscle inactivation. Muscle Nerve. 2001;24(7):925–934. doi: 10.1002/mus.1090. [DOI] [PubMed] [Google Scholar]
- 19.Miller M., Holmback A.M., Downham D., Lexell J. Voluntary activation and central activation failure in the knee extensors in young women and men. Scand J Med Sci Sports. 2006;16(4):274–281. doi: 10.1111/j.1600-0838.2005.00479.x. [DOI] [PubMed] [Google Scholar]
- 20.Kvist J. Rehabilitation following anterior cruciate ligament injury: current recommendations for sports participation. Sports Med. 2004;34(4):269–280. doi: 10.2165/00007256-200434040-00006. [DOI] [PubMed] [Google Scholar]
- 21.Myer G.D., Paterno M.V., Ford K.R., Quatman C.E., Hewett T.E. Rehabilitation after anterior cruciate ligament reconstruction: criteria-based progression through the return-to-sport phase. J Orthop Sports Phys Ther. 2006;36(6):385–402. doi: 10.2519/jospt.2006.2222. [DOI] [PubMed] [Google Scholar]
- 22.Smith A.M. The coactivation of antagonist muscles. Can J Physiol Pharmacol. 1981;59(7):733–747. doi: 10.1139/y81-110. [DOI] [PubMed] [Google Scholar]
- 23.De Luca C.J., Mambrito B. Voluntary control of motor units in human antagonist muscles: co-activation and reciprocal activation. J Neurophysiol. 1987;58(3):525–542. doi: 10.1152/jn.1987.58.3.525. [DOI] [PubMed] [Google Scholar]