Abstract
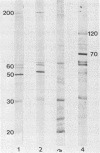
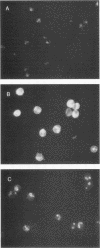
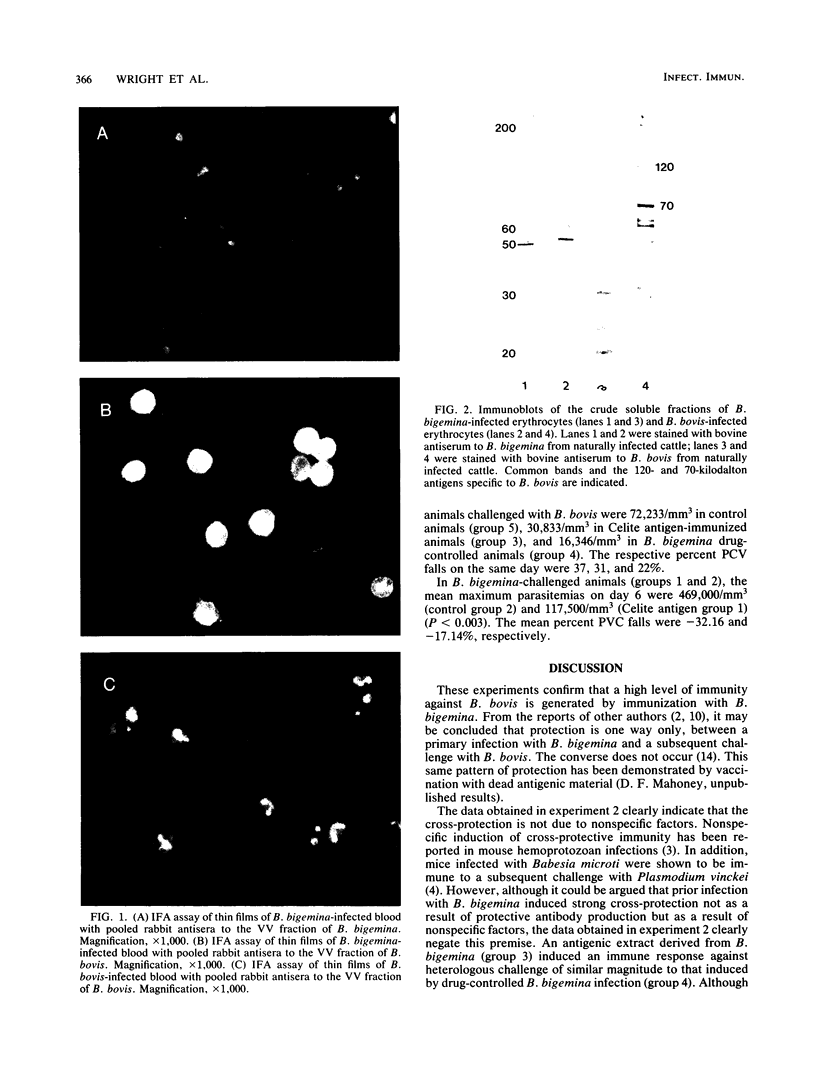
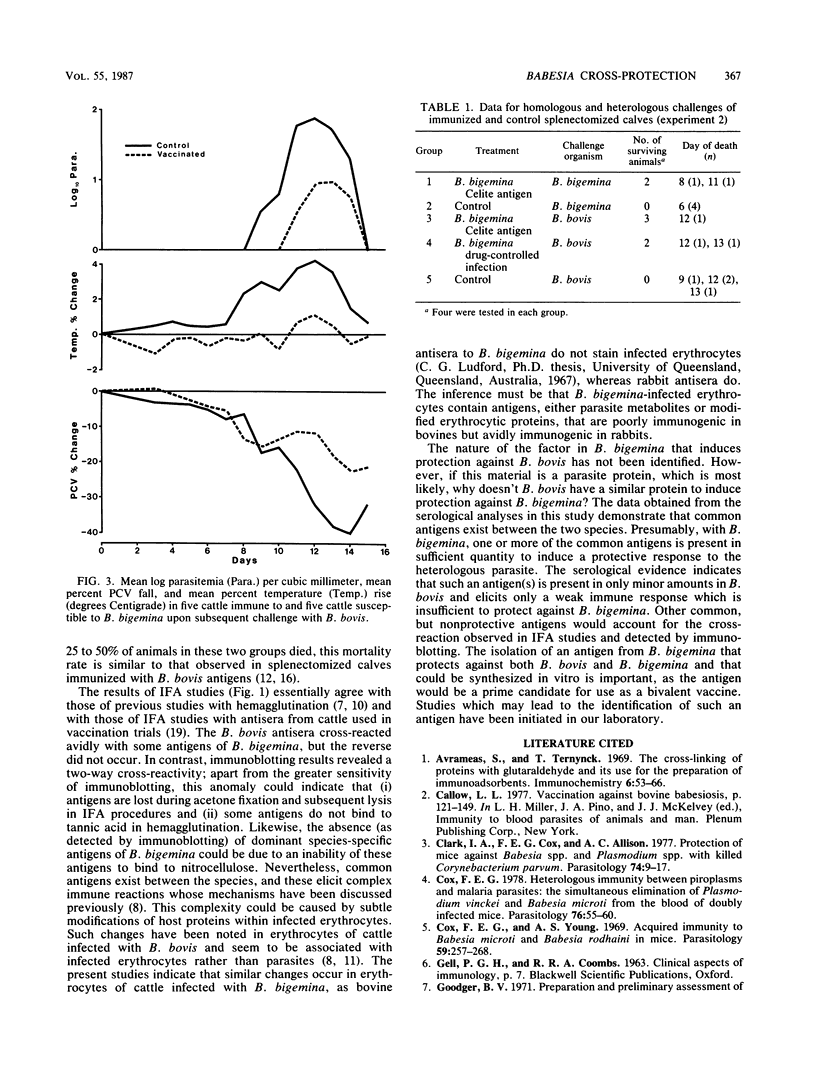
Two groups of cattle, one previously exposed to Babesia bigemina and one not, were challenged with Babesia bovis. The group previously infected with Babesia bigemina was only mildly affected upon challenge with B. bovis, whereas four of five of the other group were severely affected. Immunoblotting studies performed in both homologous and heterologous systems showed that there were polypeptides of similar molecular weight in both species, but species-specific polypeptides were demonstrated only in B. bovis by the homologous B. bovis reaction. B. bovis antisera reacted avidly with B. bigemina-infected erythrocytes in fluorescent-antibody assays. In contrast, B. bigemina antisera did not cross-react with B. bovis-infected erythrocytes. Two groups of splenectomized calves were immunized with an enriched antigen fraction of B. bigemina. A third group was immunized by infection with B. bigemina and treatment with a drug. One of the groups of calves immunized with the antigenic fraction of B. bigemina, the group immunized by infection with B. bigemina, and a control group were challenged with B. bovis. All control calves died, whereas 50% of the calves immunized by infection with B. bigemina and 75% of the animals immunized with the B. bigemina antigen survived. The second group immunized with the B. bigemina antigen and a control group were challenged with B. bigemina. All control animals died by day 6, whereas 50% of the vaccinates survived, the deaths occurring on days 8 and 11. The nature of the probable protective mechanism is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Callow L. L. Vaccination against bovine babesiosis. Adv Exp Med Biol. 1977;93:121–149. doi: 10.1007/978-1-4615-8855-9_9. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cox F. E., Allison A. C. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology. 1977 Feb;74(1):9–18. doi: 10.1017/s003118200004748x. [DOI] [PubMed] [Google Scholar]
- Cox F. E. Heterologous immunity between piroplasms and malaria parasites: the simultaneous elimination of Plasmodium vinckei and Babesia microti from the blood of doubly infected mice. Parasitology. 1978 Feb;76(1):55–60. doi: 10.1017/s0031182000047387. [DOI] [PubMed] [Google Scholar]
- Cox F. E., Young A. S. Acquired immunity to Babesia microti and Babesia rodhaini in mice. Parasitology. 1969 Feb;59(1):257–268. doi: 10.1017/s0031182000069997. [DOI] [PubMed] [Google Scholar]
- Goodger B. V. Babesia argentina: intraerythrocytic location of babesial antigen extracted from parasite suspensions. Int J Parasitol. 1973 May;3(3):387–391. doi: 10.1016/0020-7519(73)90118-5. [DOI] [PubMed] [Google Scholar]
- Goodger B. V. Babesia argentina: studies on the nature of an antigen associated with infection. Int J Parasitol. 1976 Jun;6(3):213–216. doi: 10.1016/0020-7519(76)90036-9. [DOI] [PubMed] [Google Scholar]
- Goodger B. V., Commins M. A., Wright I. G., Mirre G. B. Babesia bovis: vaccination of cattle against heterologous challenge with fractions of lysate from infected erythrocytes. Z Parasitenkd. 1984;70(3):321–329. doi: 10.1007/BF00927818. [DOI] [PubMed] [Google Scholar]
- Goodger B. V. Further studies of haemagglutinating antigens of Babesia bigemina. Aust Vet J. 1973 Feb;49(2):81–84. doi: 10.1111/j.1751-0813.1973.tb09321.x. [DOI] [PubMed] [Google Scholar]
- Goodger B. V., Wright I. G., Waltisbuhl D. J. The lysate from bovine erythrocytes infected with Babesia bovis. Analysis of antigens and a report on their immunogenicity when polymerized with glutaraldehyde. Z Parasitenkd. 1983;69(4):473–482. doi: 10.1007/BF00927703. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Smith R. D., Molinar E., Larios F., Monroy J., Trigo F., Ristic M. Bovine babesiosis: pathogenicity and heterologous species immunity of tick-borne Babesia bovis and B bigemina infections. Am J Vet Res. 1980 Dec;41(12):1957–1965. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]