Abstract
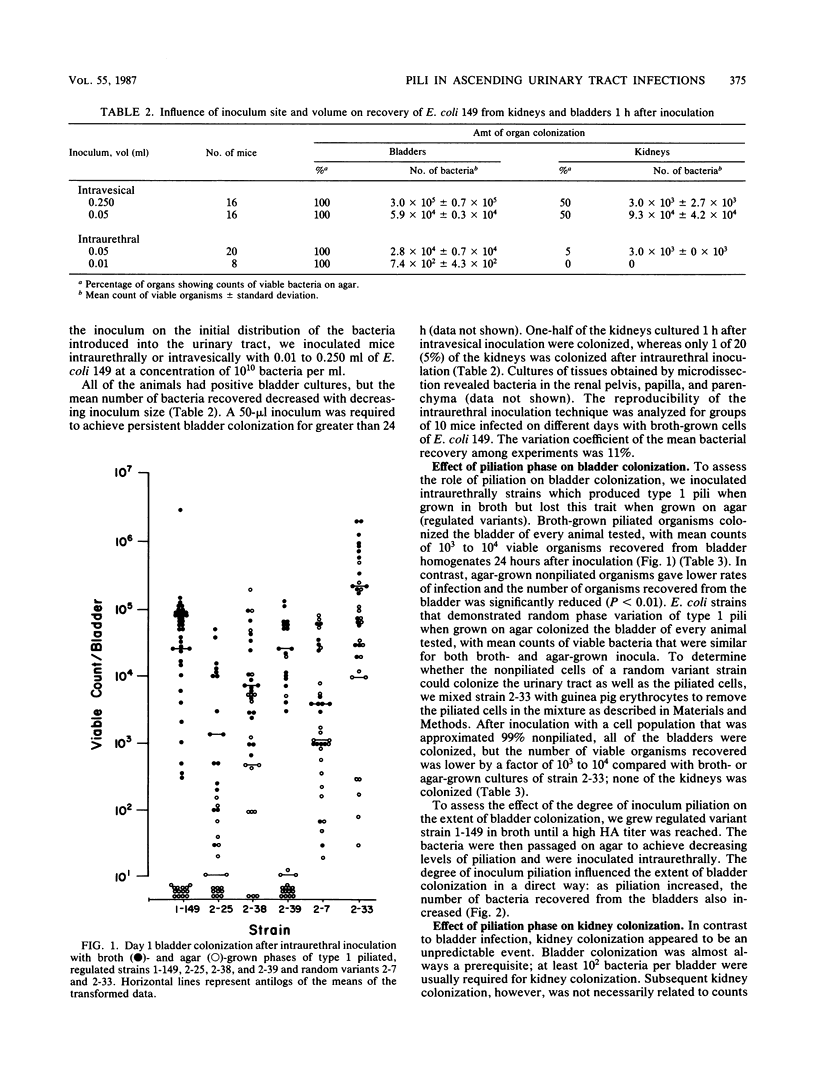
The role of type 1 pili and P adhesins during the in vivo growth of Escherichia coli inoculated into the urethras of BALB/c mice was studied. Strains which produced type 1 pili when grown in broth but lost this trait when grown on agar (regulated variants) were tested. Broth-grown organisms colonized the bladder of every animal tested, with counts of 10(3) to 10(4) viable organisms recovered from bladder homogenates. Agar-grown organisms gave lower rates of infection and the number of viable organisms recovered from bladders was significantly reduced. The degree of inoculum piliation influenced bladder colonization in a direct way: as piliation increased, the number of bacteria recovered from bladders also increased. After intraurethral inoculation, all of the bladders and 44% of the kidneys were colonized on day 1, and by day 5, 94% of the bladders and 16% of the kidneys were positive. Hemagglutination titers remained high for the bladder isolates, but the organisms colonizing the kidneys became significantly less piliated with time. Bacteriuria was unrelated to bladder or renal colonization. Strains that demonstrated random phase variation of type 1 pili during growth on agar produced similar colonizations of the urinary tract with broth- and agar-grown inocula. Strains that produced only P adhesins were less effective in colonizing the urinary tract than were type 1 piliated organisms. Other strains which did not produce pili only minimally colonized the bladder. The results suggest that type 1 pili play an essential role in ascending infections of the urinary tract.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. M., Freitag C. S., Clements J. R., Eisenstein B. I. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5724–5727. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Goldman R., Ofek I., Sharon N., Mirelman D. Mannose-binding activity of Escherichia coli: a determinant of attachment and ingestion of the bacteria by macrophages. Infect Immun. 1980 Aug;29(2):417–424. doi: 10.1128/iai.29.2.417-424.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks H. J., O'Grady F., McSherry M. A., Cattell W. R. Uropathogenic properties of Escherichia coli in recurrent urinary-tract infection. J Med Microbiol. 1980 Feb;13(1):57–68. doi: 10.1099/00222615-13-1-57. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hanson L. A., Jodal U., Lindberg U., Akerlund A. S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet. 1976 Sep 4;1(7984):490–492. [PubMed] [Google Scholar]
- Eisenstein B. I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981 Oct 16;214(4518):337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- Elliott T. S., Reed L., Slack R. C., Bishop M. C. Bacteriology and ultrastructure of the bladder in patients with urinary tract infections. J Infect. 1985 Nov;11(3):191–199. doi: 10.1016/s0163-4453(85)92997-4. [DOI] [PubMed] [Google Scholar]
- Fader R. C., Davis C. P. Klebsiella pneumoniae-induced experimental pyelitis: the effect of piliation on infectivity. J Urol. 1982 Jul;128(1):197–201. doi: 10.1016/s0022-5347(17)52817-7. [DOI] [PubMed] [Google Scholar]
- Guerina N. G., Kessler T. W., Guerina V. J., Neutra M. R., Clegg H. W., Langermann S., Scannapieco F. A., Goldmann D. A. The role of pili and capsule in the pathogenesis of neonatal infection with Escherichia coli K1. J Infect Dis. 1983 Sep;148(3):395–405. doi: 10.1093/infdis/148.3.395. [DOI] [PubMed] [Google Scholar]
- Guze L. B., Silverblatt F., Montgomerie J. Z., Ishida K., Kalmanson G. M. Lack of significance of pili in experimental ascending Escherichia coli pyelonephritis. Scand J Infect Dis. 1983;15(1):57–64. doi: 10.3109/inf.1983.15.issue-1.09. [DOI] [PubMed] [Google Scholar]
- Hagberg L., Engberg I., Freter R., Lam J., Olling S., Svanborg Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983 Apr;40(1):273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Jodal U., Korhonen T. K., Lidin-Janson G., Lindberg U., Svanborg Edén C. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect Immun. 1981 Feb;31(2):564–570. doi: 10.1128/iai.31.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harber M. J., Chick S., Mackenzie R., Asscher A. W. Lack of adherence to epithelial cells by freshly isolated urinary pathogens. Lancet. 1982 Mar 13;1(8272):586–588. doi: 10.1016/s0140-6736(82)91749-4. [DOI] [PubMed] [Google Scholar]
- Hultgren S. J., Porter T. N., Schaeffer A. J., Duncan J. L. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect Immun. 1985 Nov;50(2):370–377. doi: 10.1128/iai.50.2.370-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S. J., Schwan W. R., Schaeffer A. J., Duncan J. L. Regulation of production of type 1 pili among urinary tract isolates of Escherichia coli. Infect Immun. 1986 Dec;54(3):613–620. doi: 10.1128/iai.54.3.613-620.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahi T., Abe Y., Nakao M., Imada A., Tsuchiya K. Role of type 1 fimbriae in the pathogenesis of ascending urinary tract infection induced by escherichia coli in mice. Infect Immun. 1983 Mar;39(3):1307–1315. doi: 10.1128/iai.39.3.1307-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Källenius G., Möllby R., Svenson S. B., Helin I., Hultberg H., Cedergren B., Winberg J. Occurrence of P-fimbriated Escherichia coli in urinary tract infections. Lancet. 1981 Dec 19;2(8260-61):1369–1372. doi: 10.1016/s0140-6736(81)92797-5. [DOI] [PubMed] [Google Scholar]
- Lomberg H., Hanson L. A., Jacobsson B., Jodal U., Leffler H., Edén C. S. Correlation of P blood group, vesicoureteral reflux, and bacterial attachment in patients with recurrent pyelonephritis. N Engl J Med. 1983 May 19;308(20):1189–1192. doi: 10.1056/NEJM198305193082003. [DOI] [PubMed] [Google Scholar]
- Maayan M. C., Ofek I., Medalia O., Aronson M. Population shift in mannose-specific fimbriated phase of Klebsiella pneumoniae during experimental urinary tract infection in mice. Infect Immun. 1985 Sep;49(3):785–789. doi: 10.1128/iai.49.3.785-789.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Lark D., Falkow S., Schoolnik G. Molecular basis of Escherichia coli colonization of the upper urinary tract in BALB/c mice. Gal-Gal pili immunization prevents Escherichia coli pyelonephritis in the BALB/c mouse model of human pyelonephritis. J Clin Invest. 1985 Feb;75(2):347–360. doi: 10.1172/JCI111707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Low D., Romero I., Lark D., Vosti K., Falkow S., Schoolnik G. Gal-Gal binding and hemolysin phenotypes and genotypes associated with uropathogenic Escherichia coli. N Engl J Med. 1985 Aug 15;313(7):414–420. doi: 10.1056/NEJM198508153130704. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein I. J., Grady D., Hamilton-Miller J. M., Brumfitt W. Relationship between adhesion of Escherichia coli to uro-epithelial cells and the pathogenesis of urinary infection: problems in methodology and analysis. J Med Microbiol. 1985 Dec;20(3):335–344. doi: 10.1099/00222615-20-3-335. [DOI] [PubMed] [Google Scholar]
- Schaeffer A. J., Amundsen S. K., Jones J. M. Effect of carbohydrates on adherence of Escherichica coli to human urinary tract epithelial cells. Infect Immun. 1980 Nov;30(2):531–537. doi: 10.1128/iai.30.2.531-537.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Ofek I. Influence of pili on the virulence of Proteus mirabilis in experimental hematogenous pyelonephritis. J Infect Dis. 1978 Nov;138(5):664–667. doi: 10.1093/infdis/138.5.664. [DOI] [PubMed] [Google Scholar]
- TURCK M., PETERSDORF R. G. The epidemiology of nonenteric Escherichia coli infections: prevalence of serological groups. J Clin Invest. 1962 Sep;41:1760–1765. doi: 10.1172/JCI104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen V., Elo J., Tallgren L. G., Siitonen A., Mäkelä P. H., Svanborg-Edén C., Källenius G., Svenson S. B., Hultberg H., Korhonen T. Mannose-resistant haemagglutination and P antigen recognition are characteristic of Escherichia coli causing primary pyelonephritis. Lancet. 1981 Dec 19;2(8260-61):1366–1369. doi: 10.1016/s0140-6736(81)92796-3. [DOI] [PubMed] [Google Scholar]
- van der Bosch J. F., Verboom-Sohmer U., Postma P., de Graaff J., MacLaren D. M. Mannose-sensitive and mannose-resistant adherence to human uroepithelial cells and urinary virulence of Escherichia coli. Infect Immun. 1980 Jul;29(1):226–233. doi: 10.1128/iai.29.1.226-233.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



