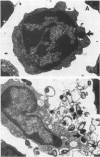
Abstract
We have reported previously that bovine neutrophils are unable to kill the bovine respiratory pathogen "Haemophilus somnus." In the present study we expanded our efforts and examined the interaction of bovine mononuclear phagocytes with this important veterinary pathogen. Bovine alveolar macrophages and blood monocytes ingested but did not kill opsonized "Haemophilus somnus" in vitro, whereas these same cells ingested and killed opsonized Escherichia coli. Because this suggested that "H. somnus" was a facultative intracellular pathogen, we developed an assay to monitor the intracellular fate of ingested "H. somnus" within bovine monocytes. Our results indicated that ingested "H. somnus" multiplied within bovine monocytes (1- to 2-log10 increase in 4 h); equivalent intracellular growth was noted for both a laboratory strain and a recent field isolate of "H. somnus." Bovine monocytes killed ingested E. coli (1- to 2-log10 decrease in 4 h) under the same assay conditions that were used to follow intracellular growth of "H. somnus," thus indicating that the assay conditions did not induce a generalized defect in monocyte antibacterial activity. Light and electron microscopic examination of "H. somnus"-infected monocytes confirmed that intracellular growth had occurred. We did not observe an obvious correlation between the release of superoxide anion from bovine mononuclear phagocytes that had ingested opsonized "H. somnus" and E. coli and the subsequent intracellular survival of the bacteria. The results of this study suggest that infected mononuclear phagocytes sustain "H. somnus" infections in cattle and thus contribute to the subacute to chronic clinical course that has been reported.
Full text
PDF

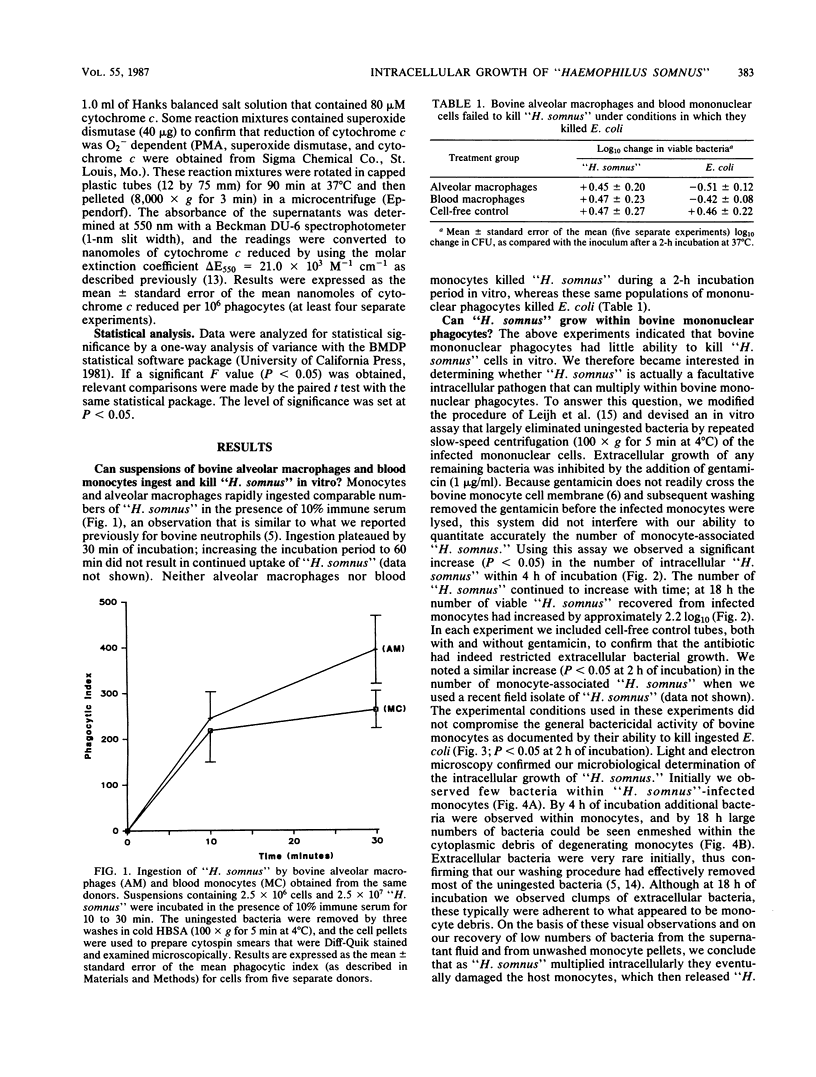
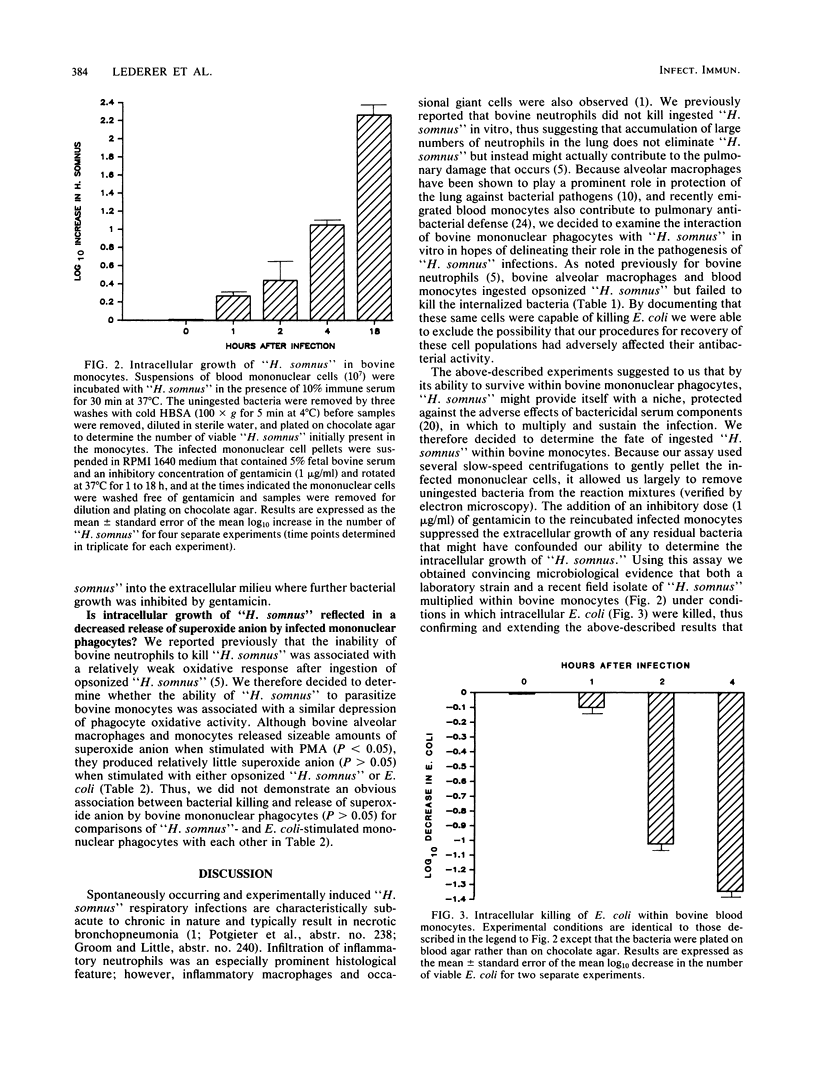
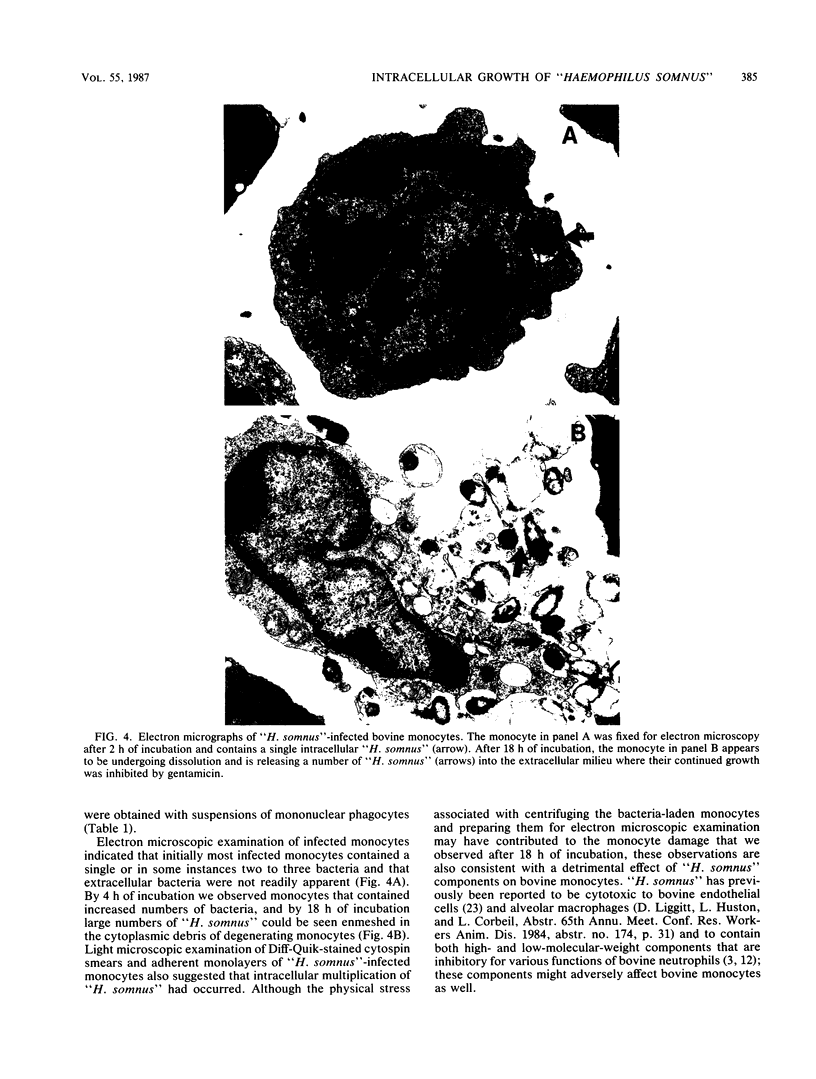


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J. J., Anderson T. D., Slife L. N., Stevenson G. W. Microscopic lesions associated with the isolation of Haemophilus somnus from pneumonic bovine lungs. Vet Pathol. 1985 Mar;22(2):131–136. doi: 10.1177/030098588502200206. [DOI] [PubMed] [Google Scholar]
- CAVANAUGH D. C., RANDALL R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J Immunol. 1959 Oct;83:348–363. [PubMed] [Google Scholar]
- Chiang Y. W., Kaeberle M. L., Roth J. A. Identification of suppressive components in "Haemophilus somnus" fractions which inhibit bovine polymorphonuclear leukocyte function. Infect Immun. 1986 Jun;52(3):792–797. doi: 10.1128/iai.52.3.792-797.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Hamilton H. L. Bovine neutrophils ingest but do not kill Haemophilus somnus in vitro. Infect Immun. 1985 Nov;50(2):431–436. doi: 10.1128/iai.50.2.431-436.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dees C., Fountain M. W., Taylor J. R., Schultz R. D. Enhanced intraphagocytic killing of Brucella abortus in bovine mononuclear cells by liposomes-containing gentamicin. Vet Immunol Immunopathol. 1985 Jan;8(1-2):171–182. doi: 10.1016/0165-2427(85)90120-5. [DOI] [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Phagocyte strategy vs. microbial tactics. Rev Infect Dis. 1980 Sep-Oct;2(5):817–838. doi: 10.1093/clinids/2.5.817. [DOI] [PubMed] [Google Scholar]
- FURNESS G. Interaction between Salmonella typhimurium and phagocytic cells in cell culture. J Infect Dis. 1958 Nov-Dec;103(3):272–277. doi: 10.1093/infdis/103.3.272. [DOI] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharam P. R., Edwards C. K., 3rd Release of superoxide anion from resident and activated mouse peritoneal macrophages infected with Mycobacterium intracellulare. Am Rev Respir Dis. 1984 Nov;130(5):834–838. doi: 10.1164/arrd.1984.130.5.834. [DOI] [PubMed] [Google Scholar]
- Hazlett M. J., Little P. B., Barnum D. A., Maxie M. G., Leslie K. E., Miller R. B. Haemophilus somnus: investigations of its potential role in bovine mastitis. Am J Vet Res. 1985 Nov;46(11):2229–2234. [PubMed] [Google Scholar]
- Hubbard R. D., Kaeberle M. L., Roth J. A., Chiang Y. W. Haemophilus somnus-induced interference with bovine neutrophil functions. Vet Microbiol. 1986 Jun;12(1):77–85. doi: 10.1016/0378-1135(86)90043-x. [DOI] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Zwet T. L., Daha M. R., van Furth R. Requirement of extracellular complement and immunoglobulin for intracellular killing of micro-organisms by human monocytes. J Clin Invest. 1979 Apr;63(4):772–784. doi: 10.1172/JCI109362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie M. B., Collaboration of Peter Zappasodi STUDIES ON THE MECHANISM OF IMMUNITY IN TUBERCULOSIS : THE FATE OF TUBERCLE BACILLI INGESTED BY MONONUCLEAR PHAGOCYTES DERIVED FROM NORMAL AND IMMUNIZED ANIMALS. J Exp Med. 1942 Mar 1;75(3):247–268. doi: 10.1084/jem.75.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riches D. W., Watkins J. L., Henson P. M., Stanworth D. R. Regulation of macrophage lysosomal secretion by adenosine, adenosine phosphate esters, and related structural analogues of adenosine. J Leukoc Biol. 1985 May;37(5):545–557. doi: 10.1002/jlb.37.5.545. [DOI] [PubMed] [Google Scholar]
- SMITH H., FITZGEORGE R. B. THE CHEMICAL BASIS OF THE VIRULENCE OF BRUCELLA ABORTUS. V. THE BASIS OF INTRACELLULAR SURVIVAL AND GROWTH IN BOVINE PHAGOCYTES. Br J Exp Pathol. 1964 Apr;45:174–186. [PMC free article] [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson R. R., Maheswaran S. K. Host humoral factors in natural resistance to Haemophilus somnus. Am J Vet Res. 1982 Jul;43(7):1160–1164. [PubMed] [Google Scholar]
- Stephens L. R., Little P. B., Wilkie B. N., Barnum D. A. Infectious thromboembolic meningoencephalitis in cattle: a review. J Am Vet Med Assoc. 1981 Feb 15;178(4):378–384. [PubMed] [Google Scholar]
- Thompson K. G., Little P. B. Effect of Haemophilus somnus on bovine endothelial cell in organ culture. Am J Vet Res. 1981 May;42(5):748–754. [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbrick B. G., Hamilton H. L., Czuprynski C. J. Cultured bovine monocytes exhibit decreased release of superoxide anion and increased levels of lysosomal enzymes but do not secrete detectable lysozyme activity. Vet Immunol Immunopathol. 1986 Sep;13(1-2):85–95. doi: 10.1016/0165-2427(86)90051-6. [DOI] [PubMed] [Google Scholar]